Homemade Food Allergen Extracts for Use in Skin Prick Tests in the Diagnosis of IgE-Mediated Food Allergy: A Good Alternative in the Absence of Commercially Available Extracts?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Skin Prick Tests
2.3. HM Food Allergen Extracts
2.4. Serum-Specific IgE
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Skin Prick Tests
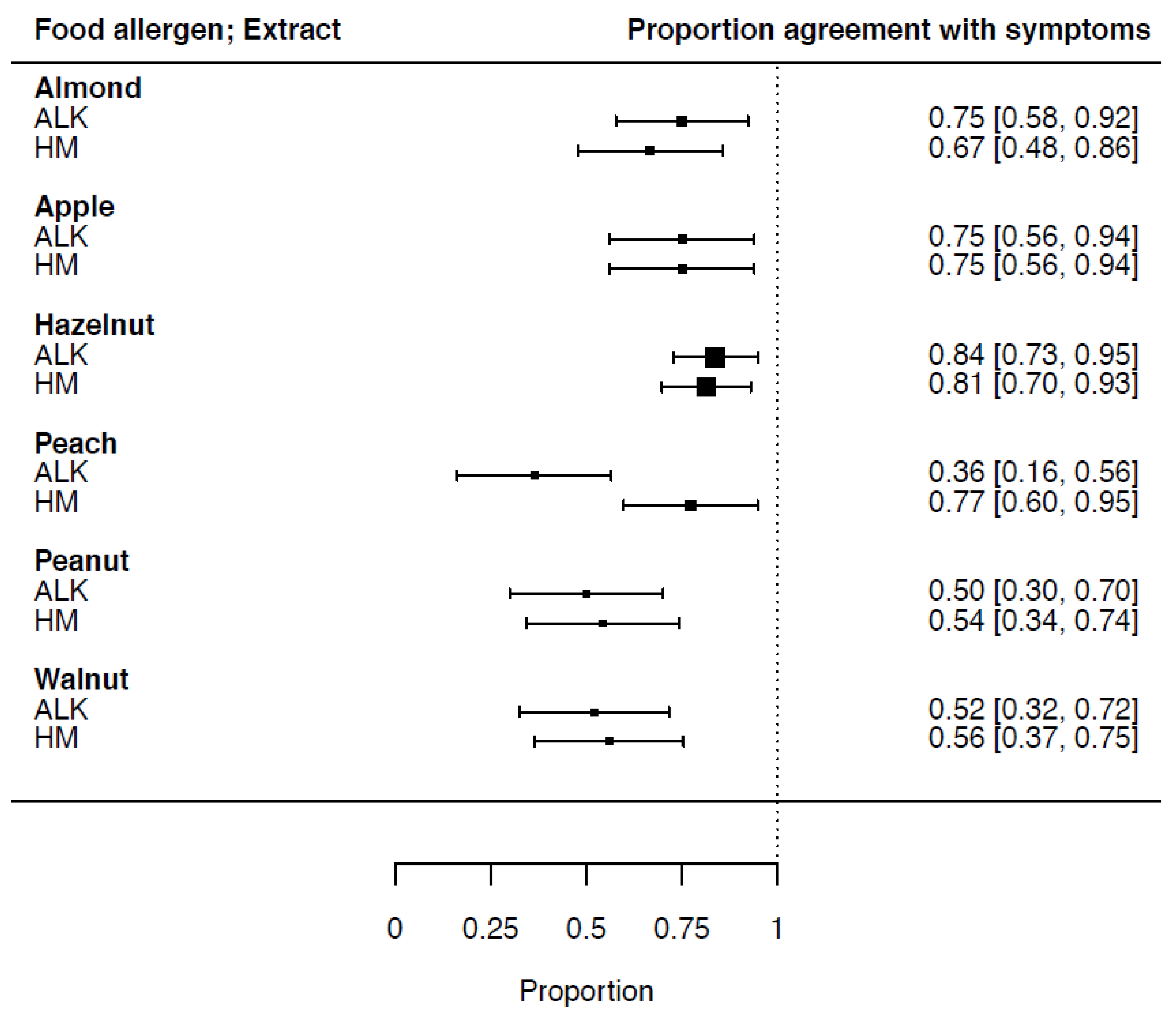
3.3. Proportion of Agreement of SPT-HEP Results with Symptoms
3.4. Serum-Specific IgE Measurements
3.5. Accuracy of Sensitization Measurements in Relation to Reported Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Academic Center of Excellence |
| ALK | Allergy Laboratories Kopenhagen |
| CI | confidence interval |
| DBPCFC | double blind placebo controlled food challenge |
| EAACI | European Academy of Allergy and Clinical Immunology |
| e.g., | exempli gratia |
| F | female |
| FN | false negative |
| FP | false positive |
| HEP | Histamine Equivalent Prick test |
| HM | homemade |
| ICC | intra-class correlation coefficient |
| IgE | immunoglobulin E |
| ISAC | immuno-solid phase allergen chip |
| LR | likelihood ratio |
| LTP | lipid transfer proteins |
| M | male |
| Mm | millimetre |
| n | number |
| NA | not applicable |
| NPV | negative predictive value |
| Nr | number |
| OAS | oral allergy syndrome |
| OFC | oral food challenge |
| PPV | positive predictive value |
| Resp | respectively |
| Sens | sensitivity |
| sIgE | specific immunoglobulin E |
| Spec | specificity |
| SPT | skin prick test |
| TN | true negative |
| TP | true positive |
| vs. | versus |
References
- Grabenhenrich, L.B. Epidemiologische Daten zur Nahrungsmittelallergie in Europa [The epidemiology of food allergy in Europe]. Bundesgesundheitsblatt Gesundh. Gesundh. 2016, 59, 745–754. [Google Scholar] [CrossRef]
- Sampson, H.A.; van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology—European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Belo, J.; Hannachi, F.; Swan, K.; Santos, A. Advances in Food Allergy Diagnosis. Curr. Pediatr. Rev. 2018, 14, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Hoffmann, H.J.; Kalpaklioglu, A.F.; Demoly, P.; Agache, I.; Popov, T.A.; Muraro, A.; Schmid-Grendelmeier, P.; Bonini, S.; Bonertz, A.; et al. In-vivo diagnostic test allergens in Europe: A call to action and proposal for recovery plan—An EAACI position paper. Allergy 2020, 75, 2161–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skypala, I.J.; Venter, C.; Meyer, R.; Dejong, N.W.; Fox, A.T.; Groetch, M.; Elberink, J.N.O.; Sprikkelman, A.B.; Diamandi, L.; Vlieg-Boerstra, B.J.; et al. The development of a standardised diet history tool to support the diagnosis of food allergy. Clin. Transl. Allergy 2015, 5, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Nadeau, K.C. Diagnosis of Food Allergy. Immunol. Allergy Clin. N. Am. 2018, 38, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kattan, J.D.; Sicherer, S.H. Optimizing the Diagnosis of Food Allergy. Immunol. Allergy Clin. N. Am. 2015, 35, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares-Weiser, K.; Takwoingi, Y.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Halken, S.; Poulsen, L.; Van Ree, R.; et al. The diagnosis of food allergy: A systematic review and meta-analysis. Allergy 2014, 69, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Valk, J.P.M.; Van Wijk, R.G.; Hoorn, E.; Groenendijk, L.; Groenendijk, I.M.; De Jong, N.W. Measurement and interpretation of skin prick test results. Clin. Transl. Allergy 2015, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, N.W.; Terlouw, S.; van Boven, F.E.; van Maaren, M.S.; Schreurs, M.W.J.; van den Berg-Somhorst, D.B.P.M.; Esser, D.; Bastiaan-Net, S. Birch Pollen Related Pear Allergy: A Single-Blind Oral Challenge TRIAL with 2 Pear Cultivars. Nutrients 2021, 13, 1355. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, N.W. Reproducubility and Stability of ‘in House Manufactured’ Extracts Used in the Diagnosis of IgE mediated allergy; 2004; pp. 45–53. ISBN 90-77595-73-2. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Conti, A.; Dubakiene, R.; Fernandez-Rivas, M.; Hoffmann-Sommergruber, K.; Lidholm, J.; Mustakov, T.; Elberink, J.N.G.O.; et al. IgE-Mediated food allergy diagnosis: Current status and new perspectives. Mol. Nutr. Food Res. 2006, 51, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.; Santos, A.F. Biomarkers of diagnosis and resolution of food allergy. Pediatr. Allergy Immunol. 2021, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Arends, N.J.T.; de Groot, N.E.H.; Emons, J.A.M.; Brand, H.K.A.; Verhoeven, D.; van Veen, L.N.; de Jong, N.W. Almond allergy in a cohort of Dutch atopic children: Results from 189 oral food challenges with almond. Linical Transl. Allergy 2017, 7 (Suppl. 1), OP07. [Google Scholar]
- Mortz, C.G.; Andersen, K.E.; Bindslev-Jensen, C. The prevalence of peanut sensitization and the association to pollen sensitization in a cohort of unselected adolescents—The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Pediatr. Allergy Immunol. 2005, 16, 501–506. [Google Scholar] [CrossRef]
- Emmett, S.; Angus, F.; Fry, J.; Lee, P. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy 1999, 54, 380–385. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Novembre, E.; Cozza, G.; De Marco, A.; Bonazza, P.; Vierucci, A. Sensitivity to tomato and peanut allergens in children monosensitized to grass pollen. Allergy 1988, 43, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.M.; Stevens, M.; Matthews, S.; Ridout, S.; Twiselton, R.; Hide, D.W. Cohort study of peanut and tree nut sensitisation by age of 4 years. BMJ 1996, 313, 514–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, N.A.; Penumarti, A.; Burks, A.W.; Slater, J.E. Food allergen extracts to diagnose food-induced allergic diseases: How they are made. Ann. Allergy Asthma Immunol. 2017, 119, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristics | ||
|---|---|---|
| Numbers included | 54 | |
| Female/male | 42 | 12 |
| Mean age/range | 36 | 19–69 |
| n | % | |
| Inhalant allergy | 54 | 100 |
| Grass pollen | 40 | 74 |
| Birch pollen | 51 | 94 |
| House dust mite | 31 | 57 |
| Pets | 33 | 61 |
| Medication used | 46 | 85 |
| Anti-histamines | 45 | 83 |
| Corticosteroid nose spray | 17 | 31 |
| Eye drops | 4 | 7 |
| Lung medication | 13 | 24 |
| Adrenaline | 6 | 11 |
| Food allergy symptoms | ||
| GI + OAS | 50 | 93 |
| Skin | 14 | 26 |
| Lung | 18 | 33 |
| Symptoms Per Food Allergen | |||||||
|---|---|---|---|---|---|---|---|
| Almond | Apple | Hazelnut | Peach | Peanut | Walnut | Total | |
| n (%) | 27 (16) | 21 (12) | 44 (25) | 23 (13) | 28 (16) | 31 (18) | 174 (100) |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Consuming/ | 8 (30) | 3 (14) | 7 (16) | 6 (26) | 13 (46) | 3 (10) | 40 (23) |
| no symptoms | |||||||
| NA (strict diet) | 3 (11) | 1 (5) | 1 (2) | 1 (4) | 4 (14) | 6 (19) | 16 (9) |
| Symptoms: | |||||||
| GI/OAS | 12 (44) | 12 (57) | 23 (52) | 12 (52) | 5 (18) | 14 (45) | 78 (45) |
| Skin | 0 | 0 | 1 (2) | 0 | 1 (4) | 0 | 2 (1) |
| Lung | 1 (4) | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| GI/OAS + Skin | 1 (4) | 1 (5) | 2 (5) | 1 (4) | 0 | 1 (3) | 6 (3) |
| GI/OAS + Lung | 2 (7) | 3 (14) | 8 (18) | 3 (13) | 2 (7) | 5 (16) | 23 (13) |
| GI/OAS + Skin + Lung | 0 | 1 (5) | 2 (5) | 0 | 2 (7) | 2 (6) | 7 (4) |
| Skin + Lung | 0 | 0 | 0 | 0 | 1 (4) | 0 | 1 (1) |
| SPT Results Per Food Allergen | ||||||||
|---|---|---|---|---|---|---|---|---|
| Almond | Apple | Hazelnut | Peach | Peanut | Walnut | Total | ||
| ALK | Positive ≥3 mm | 24 | 19 | 41 | 4 | 21 | 14 | 123 |
| % | 89 | 90 | 93 | 17 | 75 | 45 | 71 | |
| Mean HEP index | 0.96 | 0.47 | 1.40 | 0.11 | 0.86 | 0.42 | ||
| Range HEP index | 0–4.05 | 0–1.14 | 0–18.85 | 0–1.42 | 0–5.17 | 0–2.57 | ||
| HM | Positive ≥3 mm | 22 | 17 | 42 | 20 | 23 | 14 | 138 |
| % | 81 | 81 | 95 | 87 | 82 | 45 | 79 | |
| Mean HEP index | 0.51 | 0.38 | 1.61 | 0.83 | 1.13 | 0.39 | ||
| Range HEP index | 0–1.37 | 0–1.44 | 0–11.44 | 0–1.91 | 0–6.07 | 0–2.31 | ||
| ICC | 0.36 | 0.74 | 0.79 | 0.17 | 0.66 | 0.78 | ||
| 95% CI for ICC | 0 to 0.65 | 0.47 to 0.89 | 0.65 to 0.88 | 0 to 0.49 | 0.39 to 0.82 | 0.59 to 0.89 | ||
| p-value HEP | 0.03 | ˂0.0001 | ˂0.0001 | 0.015 | ˂0.0001 | ˂0.0001 | ||
| Strength of ICC | weak | moderate | strong | weak | moderate | strong | ||
| Serum Specific IgE Measurements | |||||
|---|---|---|---|---|---|
| n = | Positive n = | Mean ISU | Range ISU | ||
| Almond * | 27 | 10 | 0.60 * | 0.0–2.98 * | |
| Apple | Mal d1 | 20 | 18 | 15.38 | 0–64.1 |
| Hazelnut | Cor a1 | 42 | 40 | 7.42 | 0–31.2 |
| Cor a8 | 42 | 2 | 1.34 | 0–54.4 | |
| Cor a9 | 42 | 2 | 0.30 | 0–9.83 | |
| Cor a14 | 42 | 3 | 3.03 | 0–105.6 | |
| Peach | Pru p1 | 22 | 20 | 7.50 | 0–60.9 |
| Pru p3 | 22 | 1 | 0.23 | 0–4.67 | |
| Peanut | Ara h2 | 28 | 5 | 1.56 | 0–20.3 |
| Ara h6 | 28 | 5 | 1.15 | 0–14.4 | |
| Ara h8 | 28 | 19 | 2.88 | 0–14.0 | |
| Ara h9 | 28 | 3 | 3.39 | 0–8.29 | |
| Walnut | Jug r1 | 30 | 3 | 2.41 | 0–65 |
| Jug r3 | 30 | 0 | 0 | 0 | |
| Accuracy of Sensitization Measurements in Relation to Reported Symptoms | |||||||
|---|---|---|---|---|---|---|---|
| Extract | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | |
| Almond | ALK | 1.00 | 0.25 | 0.73 | 1.00 | 1.33 | 0.00 |
| HM | 0.88 | 0.25 | 0.70 | 0.50 | 1.17 | 0.50 | |
| sIgE Almond | 0.44 | 0.20 | 0.25 | 0.38 | 0.56 | 2.78 | |
| Apple | ALK | 0.88 | 0.00 | 0.83 | 0.00 | 0.88 | NA |
| HM | 0.82 | 0.33 | 0.88 | 0.25 | 1.24 | 0.53 | |
| Mal d1 | 0.88 | 0.00 | 0.88 | 0.00 | 0.88 | NA | |
| Hazelnut | ALK | 0.97 | 0.25 | 0.85 | 0.67 | 1.30 | 0.11 |
| HM | 0.97 | 0.13 | 0.83 | 0.50 | 1.11 | 0.23 | |
| Cor a1 | 0.82 | 0.50 | 0.97 | 0.13 | 1.64 | 0.36 | |
| Peach | ALK | 0.19 | 0.83 | 0.75 | 0.28 | 1.13 | 0.98 |
| HM | 0.94 | 0.33 | 0.79 | 0.67 | 1.41 | 0.19 | |
| Pru p1 | 0.75 | 0.50 | 0.94 | 0.17 | 1.50 | 0.50 | |
| Peanut | ALK | 0.82 | 0.23 | 0.47 | 0.60 | 1.06 | 0.79 |
| HM | 0.91 | 0.23 | 0.50 | 0.75 | 1.18 | 0.39 | |
| Ara h2 | 1.00 | 0.65 | 0.36 | 1.00 | 2.86 | 0.00 | |
| Ara h8 | 0.53 | 0.71 | 0.82 | 0.38 | 1.85 | 0.66 | |
| Walnut | ALK | 0.50 | 0.67 | 0.92 | 0.15 | 1.50 | 0.75 |
| HM | 0.50 | 1.00 | 1.00 | 0.21 | NA | 0.50 | |
| Jug r1 | 1.00 | 0.14 | 0.10 | 1.00 | 1.16 | 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlouw, S.; van Boven, F.E.; Borsboom-van Zonneveld, M.; de Graaf-in ‘t Veld, C.; van Splunter, M.E.; van Daele, P.L.A.; van Maaren, M.S.; Schreurs, M.W.J.; de Jong, N.W. Homemade Food Allergen Extracts for Use in Skin Prick Tests in the Diagnosis of IgE-Mediated Food Allergy: A Good Alternative in the Absence of Commercially Available Extracts? Nutrients 2022, 14, 475. https://doi.org/10.3390/nu14030475
Terlouw S, van Boven FE, Borsboom-van Zonneveld M, de Graaf-in ‘t Veld C, van Splunter ME, van Daele PLA, van Maaren MS, Schreurs MWJ, de Jong NW. Homemade Food Allergen Extracts for Use in Skin Prick Tests in the Diagnosis of IgE-Mediated Food Allergy: A Good Alternative in the Absence of Commercially Available Extracts? Nutrients. 2022; 14(3):475. https://doi.org/10.3390/nu14030475
Chicago/Turabian StyleTerlouw, Severina, Frank E. van Boven, Monika Borsboom-van Zonneveld, Catharina de Graaf-in ‘t Veld, Marloes E. van Splunter, Paul L. A. van Daele, Maurits S. van Maaren, Marco W. J. Schreurs, and Nicolette W. de Jong. 2022. "Homemade Food Allergen Extracts for Use in Skin Prick Tests in the Diagnosis of IgE-Mediated Food Allergy: A Good Alternative in the Absence of Commercially Available Extracts?" Nutrients 14, no. 3: 475. https://doi.org/10.3390/nu14030475
APA StyleTerlouw, S., van Boven, F. E., Borsboom-van Zonneveld, M., de Graaf-in ‘t Veld, C., van Splunter, M. E., van Daele, P. L. A., van Maaren, M. S., Schreurs, M. W. J., & de Jong, N. W. (2022). Homemade Food Allergen Extracts for Use in Skin Prick Tests in the Diagnosis of IgE-Mediated Food Allergy: A Good Alternative in the Absence of Commercially Available Extracts? Nutrients, 14(3), 475. https://doi.org/10.3390/nu14030475






