Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Gene Expression Analysis of the Immune System Using DNA Microarray Data
2.3. The Levels of Secretory Immunoglobulin A, IL-6, and IL-10 Determined by Enzyme-Linked Immunosorbent Assays
2.4. Analysis of Short-Chain Fatty Acids in the Cecum and Feces
2.5. Analysis of Counts of Predominant Bacterial Groups in the Cecum Digesta
2.6. Expression Analysis of mRNA Related to Immunity in the Ileum
2.7. Statistical Analysis
3. Results
3.1. KEGG Enrichment Analyses of DEGs by Using DNA Microarray Data
3.2. Food Intake, Body Weight, and Organ Weight
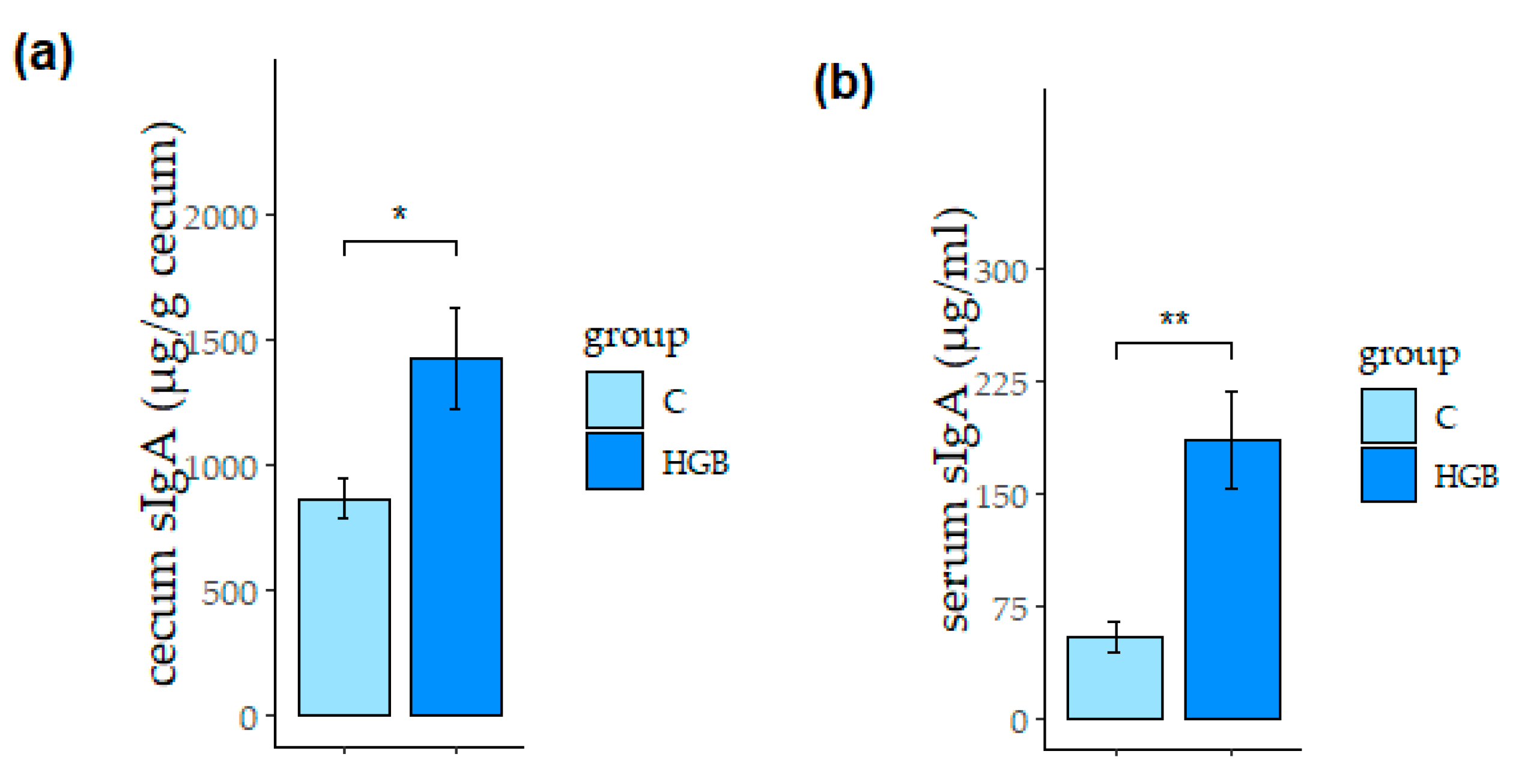
3.3. Secretory Immunoglobulin A (sIgA) Concentration in the Cecum and Serum
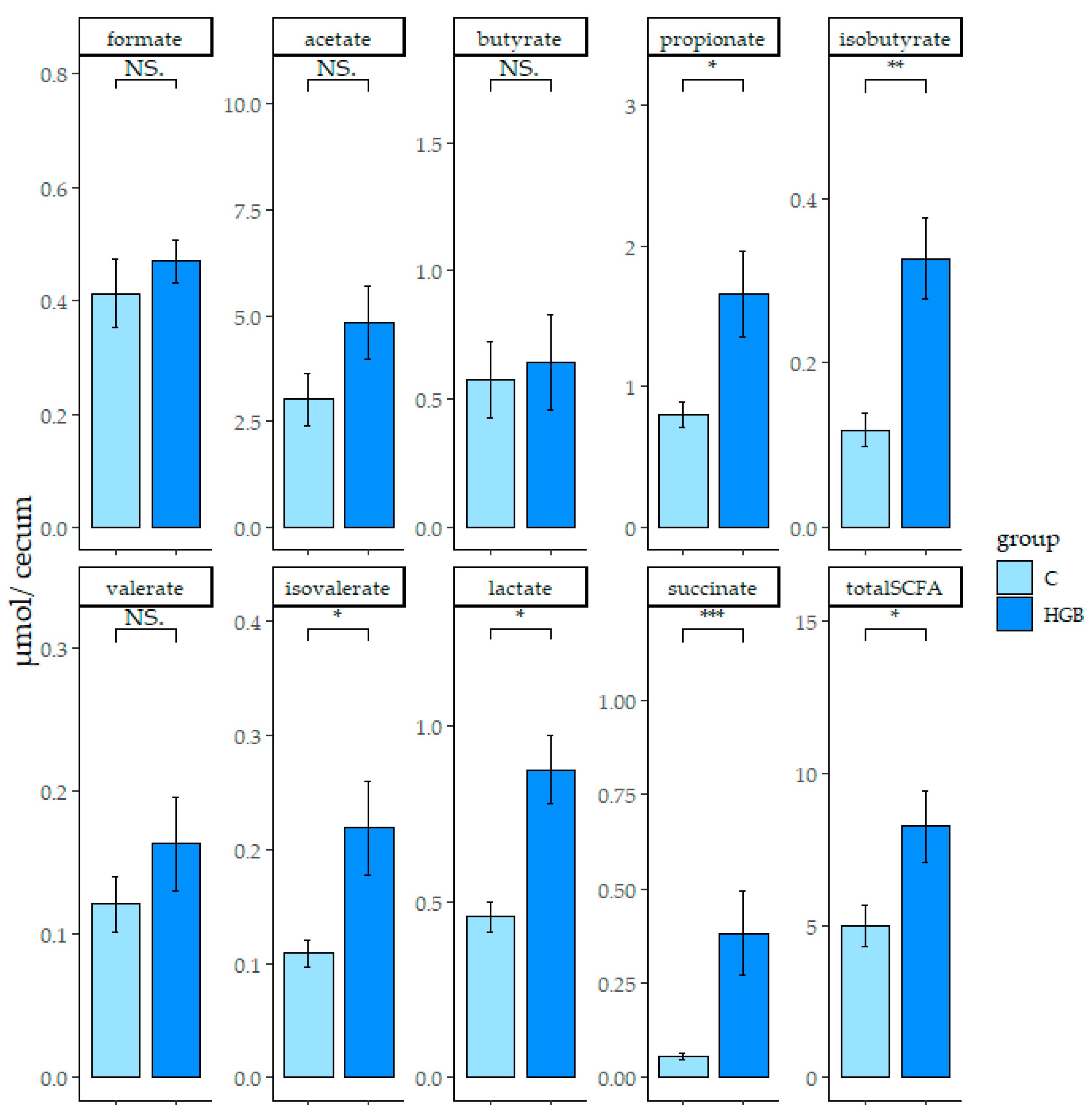
3.4. SCFA and Organic Acid Concentration in the Cecum Digesta and Feces
3.5. Counts of Predominant Bacterial Groups in the Cecum Digesta
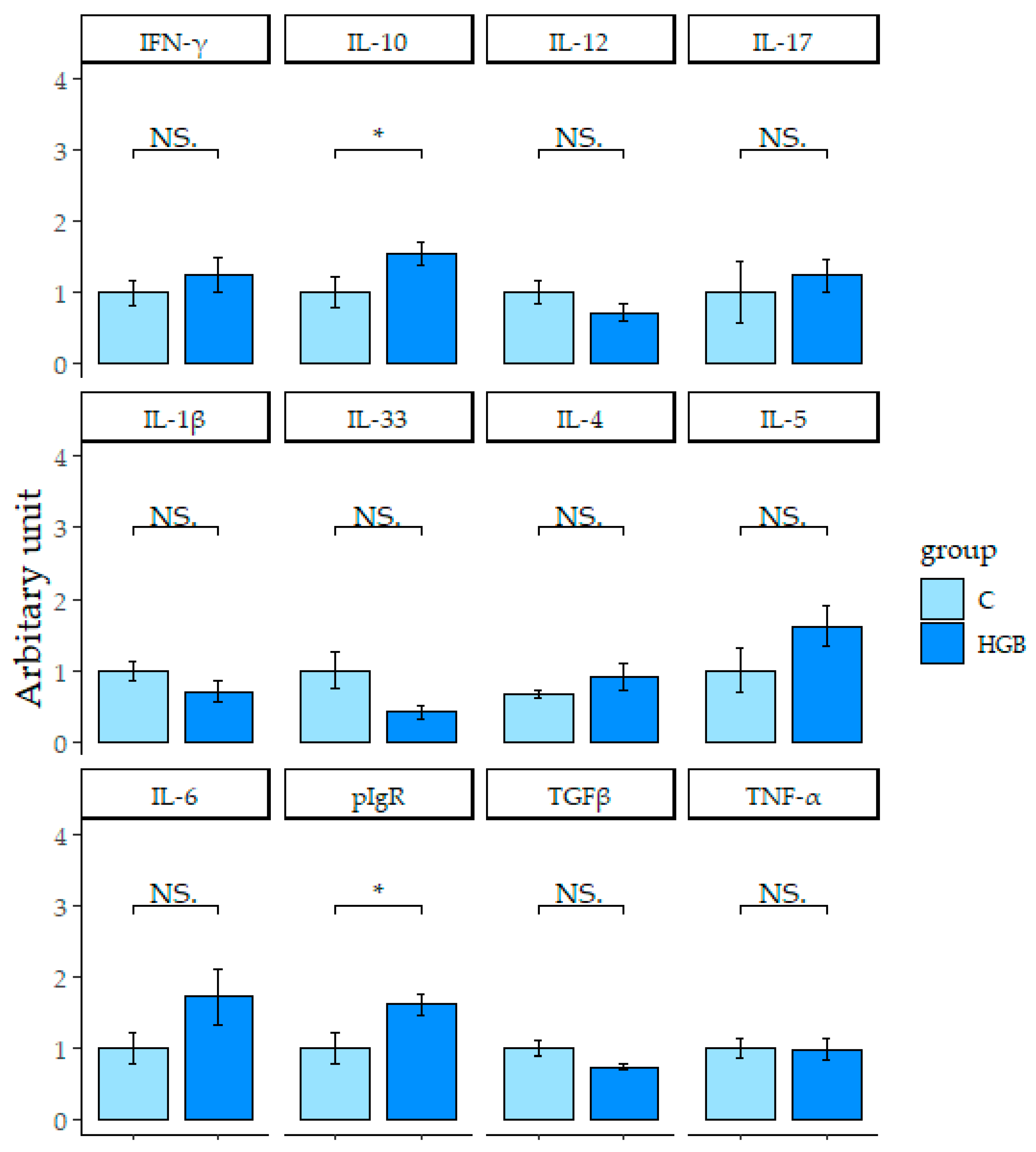
3.6. Expression of mRNA Related to the Immune System in the Ileum
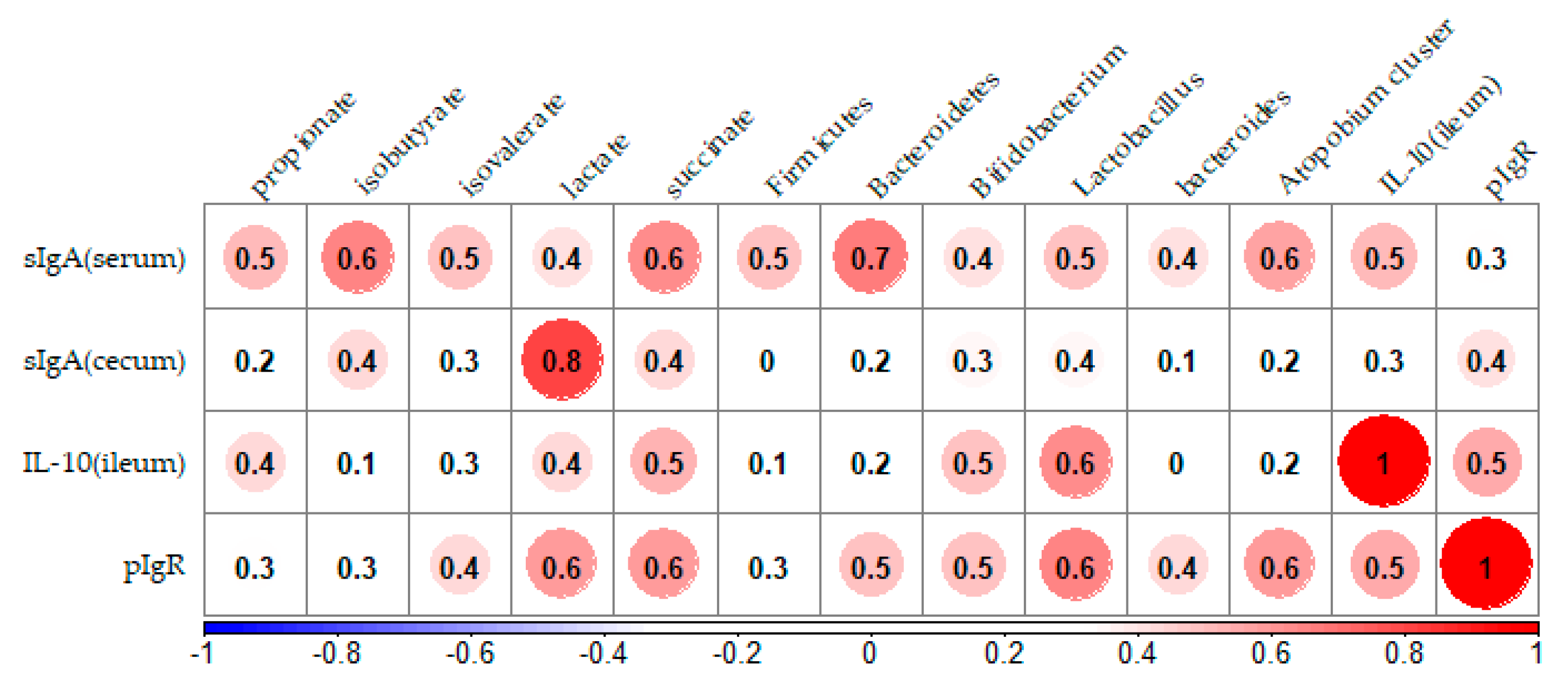
3.7. Correlation Analysis between sIgA Concentration and Parameters Related to the Immune System in the Ileum and Cecum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; MacIver, N.J. Role of T Cells in Malnutrition and Obesity. Front. Immunol. 2014, 5, 379. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R. Effects of dietary retinoids and carotenoids on immune development. Proc. Nutr. Soc. 2007, 66, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sherman, P.M.; Ossa, J.C.; Johnson-Henry, K. Unraveling Mechanisms of Action of Probiotics. Nutr. Clin. Pract. 2009, 24, 10–14. [Google Scholar] [CrossRef]
- Antoine, J.M. Probiotics: Beneficial factors of the defence system. Proc. Nutr. Soc. 2010, 69, 429–433. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Nagai, M.; Noguchi, R.; Takahashi, D.; Morikawa, T.; Koshida, K.; Komiyama, S.; Ishihara, N.; Yamada, T.; Kawamura, Y.I.; Muroi, K.; et al. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell 2019, 178, 1072–1087.e14. [Google Scholar] [CrossRef]
- Kawamoto, S.; Maruya, M.; Kato, L.M.; Suda, W.; Atarashi, K.; Doi, Y.; Tsutsui, Y.; Qin, H.; Honda, K.; Okada, T.; et al. Foxp3+ T Cells Regulate Immunoglobulin A Selection and Facilitate Diversification of Bacterial Species Responsible for Immune Homeostasis. Immunity 2014, 41, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, J.; El Kafsi, H.; Sterlin, D.; Juste, C.; Parizot, C.; Dorgham, K.; Autaa, G.; Gouas, D.; Almeida, M.; Lepage, P.; et al. Microbial ecology perturbation in human IgA deficiency. Sci. Transl. Med. 2018, 10, eaan1217. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Jang, E.Y.; Hong, K.-B.; Chang, Y.B.; Shin, J.; Jung, E.Y.; Jo, K.; Suh, H.J. In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules 2020, 25, 5201. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-Glucans-Containing Food Enhances Probiotic Performances of Beneficial Bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; López, P.; Capozzi, V.; de Palencia, P.F.; Dueñas, M.T.; Spano, G.; Fiocco, D. Beta-Glucans Improve Growth, Viability and Colonization of Probiotic Microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Capozzi, V.; Rascón, A.; Felis, G.E.; Spano, G.; Fiocco, D. Combinations of cereal β-glucans and probiotics can enhance the anti-inflammatory activity on host cells by a synergistic effect. J. Funct. Foods 2016, 23, 12–23. [Google Scholar] [CrossRef]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef]
- Martínez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived β-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am. J. Physiol. Liver Physiol. 2012, 302, G1405–G1415. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, J.D.; Volman, J.J.; Biörklund, M.; Önning, G.; Mensink, R.P.; Plat, J. Fecal water from ileostomic patients consuming oat β-glucan enhances immune responses in enterocytes. Mol. Nutr. Food Res. 2007, 51, 211–220. [Google Scholar] [CrossRef]
- Volman, J.J.; Mensink, R.P.; Ramakers, J.D.; de Winther, M.P.; Carlsen, H.; Blomhoff, R.; Buurman, W.A.; Plat, J. Dietary (1→3), (1→4)-β-d-glucans from oat activate nuclear factor-κB in intestinal leukocytes and enterocytes from mice. Nutr. Res. 2010, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- MuhomahH, T.A.; Nishino, N.; Katsumata, E.; Haoming, W.; Tsuruta, T. High-fat diet reduces the level of secretory immunoglobulin A coating of commensal gut microbiota. Biosci. Microbiota Food Health 2019, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Yamanaka, C.; Matsuoka, T.; Kobayashi, T.; Aoe, S. Effects of β-glucan Rich Barley Flour on Glucose and Lipid Metabolism in the Ileum, Liver, and Adipose Tissues of High-Fat Diet Induced-Obesity Model Male Mice Analyzed by DNA Microarray. Nutrients 2020, 12, 3546. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Tsuji, H.; Asahara, T.; Kado, Y.; Nomoto, K. Sensitive Quantitative Detection of Commensal Bacteria by rRNA-Targeted Reverse Transcription-PCR. Appl. Environ. Microbiol. 2007, 73, 6695. [Google Scholar] [CrossRef]
- Matsuki, T. Development of quantitative PCR detection method with 16S rRNA gene-targeted genus- and species-specific primers for the analysis of human intestinal microflora and its application. Nihon Saikingaku Zasshi 2007, 62, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N.Y.; Bemark, M. The regulation of gut mucosal IgA B-cell responses: Recent developments. Mucosal Immunol. 2017, 10, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Dollé, L.; Tran, H.Q.; Etienne-Mesmin, L.; Chassaing, B. Policing of gut microbiota by the adaptive immune system. BMC Med. 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sparks, J.B.; Karyala, S.V.; Settlage, R.; Luo, X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015, 9, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Tao Chan, Y.; Nøhr, M.K.; et al. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance. Nat. Commun. 2019, 10, 3650. [Google Scholar] [CrossRef] [PubMed]
- Yuri, C.; Estrada, A.; Van Kessel, A.; Gajadhar, A.; Redmond, M.; Laarveld, B. Immunomodulatory Effects of Oat β-Glucan Administered Intragastrically or Parenterally on Mice Infected with Eimeria vermiformis. Microbiol. Immunol. 1998, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Iwai, K.; Yoshikawa, Y.; Shimamura, Y.; Miyoshi, N.; Hiramoto, S.; Asada, K.; Fukutomi, R.; Su, H.; Ohashi, N. Wheat Bran Intake Enhances the Secretion of Bacteria-Binding IgA in a Lumen of the Intestinal Tract by Incrementing Short Chain Fatty Acid Production Through Modulation of Gut Microbiota. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science (80-) 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Komatsu, N.; Kawamoto, S.; Suzuki, K.; Kanagawa, O.; Honjo, T.; Hori, S.; Fagarasan, S. Preferential Generation of Follicular B Helper T Cells from Foxp3+ T Cells in Gut Peyer’s Patches. Science (80-) 2009, 323, 1488–1492. [Google Scholar] [CrossRef]
- Linterman, M.A.; Pierson, W.; Lee, S.K.; Kallies, A.; Kawamoto, S.; Rayner, T.F.; Srivastava, M.; Divekar, D.P.; Beaton, L.; Hogan, J.J.; et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011, 17, 975–982. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Lin, D.; Zheng, P.; Zhang, P.; Hu, M.; Wang, Q.; Pan, W.; Yang, X.; Hu, T.; et al. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, M.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science (80-) 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.M.; Wildeboer-Veloo, A.C.M.; Grijpstra, J.; Knol, J.; Degener, J.E.; Welling, G.W. Development of 16S rRNA-Based Probes for theCoriobacterium Group and the Atopobium Cluster and Their Application for Enumeration of Coriobacteriaceaein Human Feces from Volunteers of Different Age Groups. Appl. Environ. Microbiol. 2000, 66, 4523–4527. [Google Scholar] [CrossRef] [PubMed]
- Perdigón, G.; Maldonado Galdeano, C.; Valdez, J.; Medici, M. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 2002, 56, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Sashihara, T.; Sueki, N.; Ikegami, S. An Analysis of the Effectiveness of Heat-Killed Lactic Acid Bacteria in Alleviating Allergic Diseases. J. Dairy Sci. 2006, 89, 2846–2855. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; van der Kleij, D.; de Jong, E.C.; Schipper, K.; van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; van Kooyk, Y. Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Yamanaka, C.; Ichinose, Y.; Kohyama, N.; Yanagisawa, T.; Aoe, S. Effects of barley β-glucan with various molecular weights partially hydrolyzed by endogenous β-glucanase on glucose tolerance and lipid metabolism in mice. Cereal Chem. 2020, 97, 1056–1065. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Ikeda, F.; Aoki, K.; Yoshikawa, M.; Kato, Y.; Adachi, Y.; Tanioka, A.; Ishibashi, K.; Tsubaki, K.; Ohno, N. Barley-derived β-d-glucan induces immunostimulation via a dectin-1-mediated pathway. Immunol. Lett. 2009, 123, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Adachi, Y.; Ishibashi, K.; Tsubaki, K.; Ohno, N. Binding Capacity of a Barley β- D-Glucan to the β-Glucan Recognition Molecule Dectin-1. J. Agric. Food Chem. 2008, 56, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
| LogCFU/g (Cecum Digesta) | Control | HGB |
|---|---|---|
| Phylum | ||
| Total bacteria | 13.3 ± 0.3 | 14.9 ± 0.4 * |
| Bacteroidetes | 10.0 ± 0.2 | 11.2 ± 0.2 * |
| Firmicutes | 12.7 ± 0.1 | 12.9 ± 0.1 * |
| Actinobacteria | 9.6 ± 0.4 | 10.1 ± 0.1 |
| Genus | ||
| Bacteroides fragilis group | 9.7 ± 0.1 | 10.3 ± 0.2 * |
| Bifidobacterium | 10.3 ± 0.3 | 11.1 ± 0.1 * |
| Lactobacillus | 10.2 ± 0.2 | 11.3 ± 0.1 * |
| Prevotella | 7.4 ± 0.1 | 7.6 ± 0.1 |
| Clostridium coccoides group | 9.8 ± 0.2 | 10.3 ± 0.1 |
| Clostridium leptum subgroup | 11.2 ± 0.2 | 11.7 ± 0.1 |
| Atopobium cluster | 7.8 ± 0.2 | 9.1 ± 0.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mio, K.; Otake, N.; Nakashima, S.; Matsuoka, T.; Aoe, S. Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects. Nutrients 2021, 13, 907. https://doi.org/10.3390/nu13030907
Mio K, Otake N, Nakashima S, Matsuoka T, Aoe S. Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects. Nutrients. 2021; 13(3):907. https://doi.org/10.3390/nu13030907
Chicago/Turabian StyleMio, Kento, Nami Otake, Satoko Nakashima, Tsubasa Matsuoka, and Seiichiro Aoe. 2021. "Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects" Nutrients 13, no. 3: 907. https://doi.org/10.3390/nu13030907
APA StyleMio, K., Otake, N., Nakashima, S., Matsuoka, T., & Aoe, S. (2021). Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects. Nutrients, 13(3), 907. https://doi.org/10.3390/nu13030907






