Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes
Abstract
1. Introduction
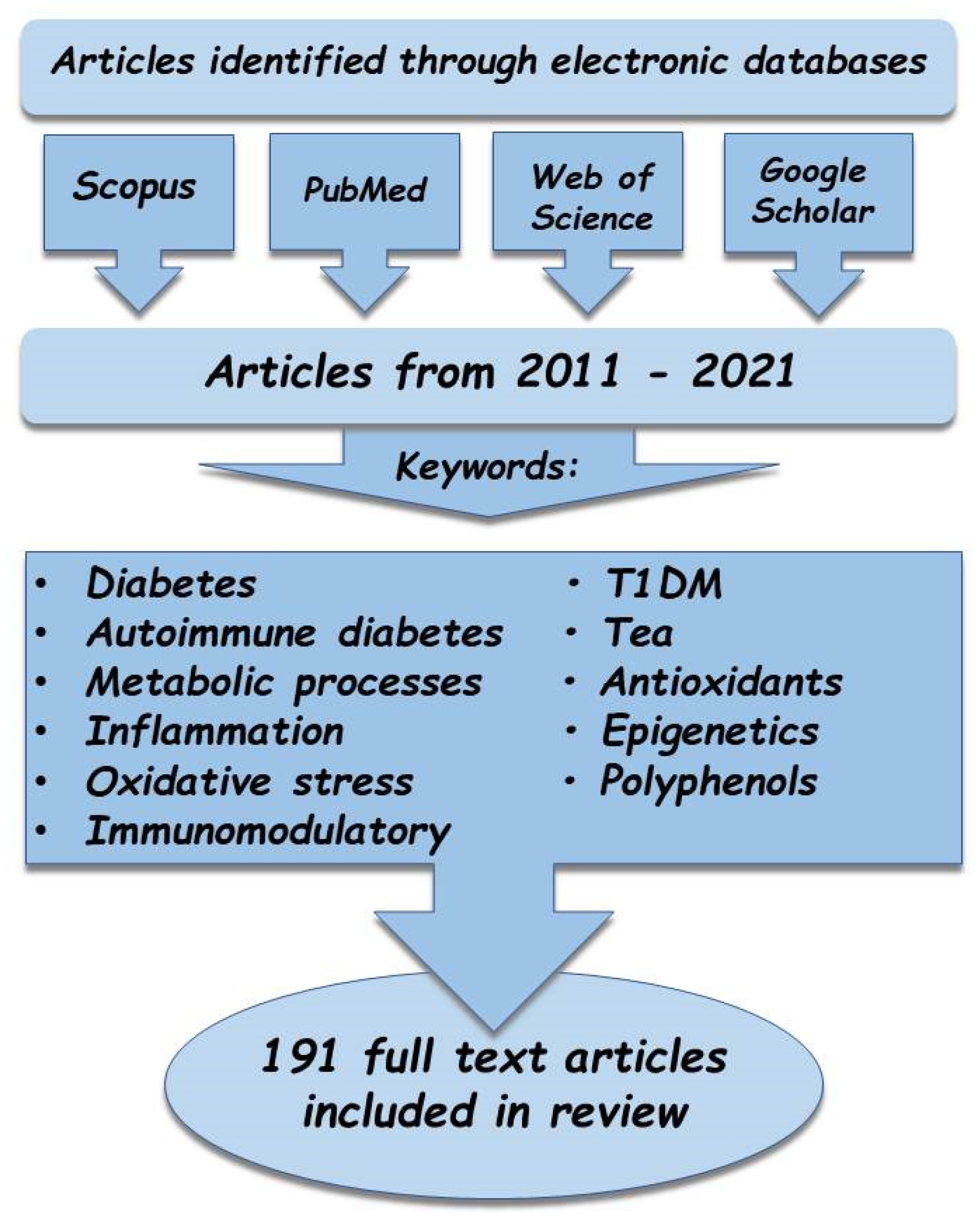
2. Materials and Methods
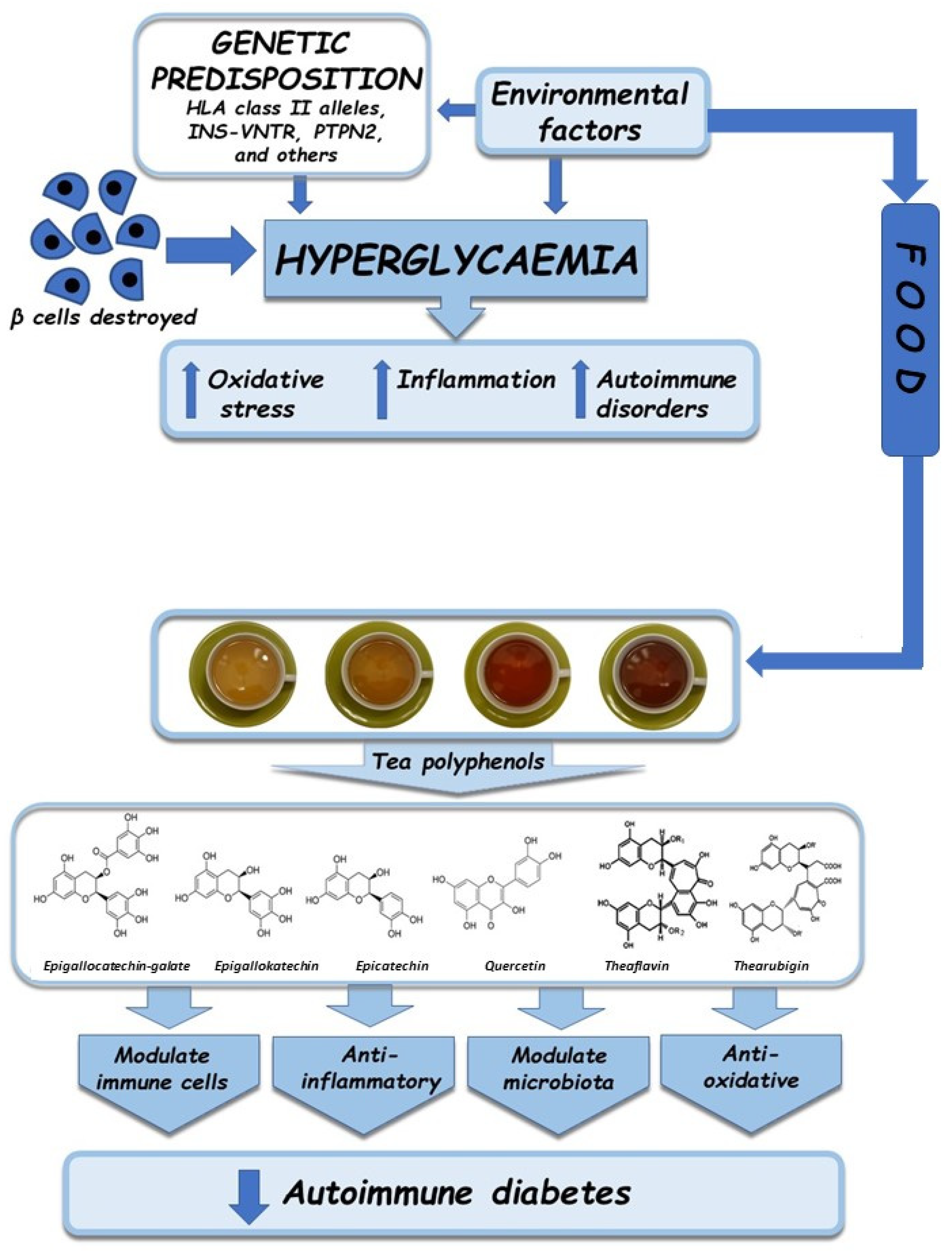
3. Pathogenesis of Autoimmune Diabetes
4. Metabolic Disorders in Diabetes
4.1. Oxidative Stress
4.2. Inflammation
4.3. Autoimmune Disorders
5. Antioxidative, Anti-Inflammatory, and Immunomodulatory Properties of Tea
5.1. Antioxidative Properties
5.2. Anti-Inflammatory and Immunomodulatory Properties
6. Impact of Tea on Organisms with Autoimmune Diabetes—A Review
6.1. Antioxidative Activity
6.2. Anti-Inflammatory Activity
6.3. Immunomodulatory Activity
7. Perspectives and Conclusions—Can Nutrigenomics Be the Future?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Egan, A.M.; Dinneen, S.F. What is diabetes? Medicine 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Thant, T.M.; Aminah, N.S.; Kristanti, A.N.; Ramadhan, R.; Aung, H.T.; Takaya, Y. Antidiabetes and Antioxidant agents from Clausena excavata root as medicinal plant of Myanmar. Open Chem. 2019, 17, 1339–1344. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Khan, A. Mini Review Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, 267–271. [Google Scholar]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef]
- Simó, R.; Bañeras, J.; Hernández, C.; Rodríguez-Palomares, J.; Valente, F.; Gutierrez, L.; González-Alujas, T.; Ferreira, I.; Aguadé-Bruix, S.; Montaner, J.; et al. Diabetic retinopathy as an independent predictor of subclinical cardiovascular disease: Baseline results of the PRECISED study. BMJ Open Diabetes Res. Care 2019, 7, e000845. [Google Scholar] [CrossRef]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T cell-mediated beta cell destruction: Autoimmunity and alloimmunity in the context of type 1 diabetes. Front. Endocrinol. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. The EPIC-InterAct Study: A Study of the Interplay between Genetic and Lifestyle Behavioral Factors on the Risk of Type 2 Diabetes in European Populations. Curr. Nutr. Rep. 2014, 3, 355–363. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Matsumura, K.; Kikuno, S.; Nagasawa, K.; Okubo, M.; Mori, Y.; Kobayashi, T. Slowly progressive type 1 diabetes mellitus: Current knowledge and future perspectives. Diabetes Metab. Syndr. Obes. 2019, 12, 2461–2477. [Google Scholar] [CrossRef]
- Pieralice, S.; Pozzilli, P. Latent autoimmune diabetes in adults: A review on clinical implications and management. Diabetes Metab. J. 2018, 42, 451. [Google Scholar] [CrossRef]
- Maddaloni, E.; Lessan, N.; Al Tikriti, A.; Buzzetti, R.; Pozzilli, P.; Barakat, M.T. Latent autoimmune diabetes in adults in the United Arab Emirates: Clinical features and factors related to insulin-requirement. PLoS ONE 2015, 10, e0131837. [Google Scholar] [CrossRef] [PubMed]
- Fadiga, L.; Saraiva, J.; Catarino, D.; Frade, J.; Melo, M.; Paiva, I. Adult-onset autoimmune diabetes: Comparative analysis of classical and latent presentation. Diabetol. Metab. Syndr. 2020, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, A.T.; Tajuddin, N.B.; Hussain, M.I.M.; Mathew, S.; Govindaraju, A.; Qadri, I. Antioxidant and hypoglycemic activities of Clausena anisata (Wild.) Hook F. Ex Benth. Root Mediated Synthesized Silver Nanoparticles. Pharmacogn. J. 2016, 8, 579–586. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef]
- Hicks, A. Current status and future development of global tea production and tea products. AU J. Technol. 2009, 12, 251–264. [Google Scholar]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Bhattacharjee, C. Extraction of polyphenols from dried tea leaves. J. Sci. Eng. Res. 2012, 3, 1–5. [Google Scholar]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharmacol. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Sharma, R.B.; Alonso, L.C. Lipotoxicity in the pancreatic beta cell: Not just survival and function, but proliferation as well? Curr. Diabetes Rep. 2014, 14, 492. [Google Scholar] [CrossRef]
- Bugya, Z.; Prechl, J.; Szénási, T.; Nemes, É.; Bácsi, A.; Koncz, G. Multiple Levels of Immunological Memory and Their Association with Vaccination. Vaccines 2021, 9, 174. [Google Scholar] [CrossRef]
- Homsak, E. Diabetes as autoimmune disease—Diabetes type I. Biochem. Med. 2014, 24, 1–7. [Google Scholar]
- Skrypnik, D.; Skrypnik, K.; Suliburska, J.; Bogdański, P.; Pupek-Musialik, D. Dietotherapy of selected metabolic diseases. Forum Zaburzeń Metab. 2013, 4, 80–89. [Google Scholar]
- Xie, Z.; Chang, C.; Zhou, Z. Molecular Mechanisms in Autoimmune Type 1 Diabetes: A Critical Review. Clin. Rev. Allergy Immunol. 2014, 47, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.A.; Ozdemir, D.; Bestepe, N.; Dellal, F.D.; Bilginer, M.C.; Faki, S.; Bicer, C.; Ersoy, R.; Cakir, B. Low rate of latent autoimmune diabetes in adults (LADA) in patients followed for type 2 diabetes: A single center’s experience in Turkey. Arch. Endocrinol. Metab. 2021, 64, 584–590. [Google Scholar]
- Carlsson, S. Etiology and Pathogenesis of Latent Autoimmune Diabetes in Adults (LADA) Compared to Type 2 Diabetes. Front. Physiol. 2019, 10, 320. [Google Scholar] [CrossRef]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA Region in the Prediction of Type 1 Diabetes. Curr. Diabetes 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A. Immunogenetics of type 1 diabetes: A comprehensive review. J. Autoimmun. 2015, 64, 101–112. [Google Scholar] [CrossRef]
- Sparks, A.E.; Chen, C.; Breslin, M.B.; Lan, M.S. Functional Domains of Autoimmune Regulator (AIRE) Modulate INS-VNTR Transcription in Human Thymic Epithelial Cells. J. Biol. Chem. 2016, 291, 11313–11322. [Google Scholar] [CrossRef]
- Mourad, D.; Azar, N.S.; Eid, A.; Azar, S.T. Immune Checkpoint Inhibitor-Induced Diabetes Mellitus: Potential Role of T Cells in the Underlying Mechanism. Int. J. Mol. Sci. 2021, 22, 2093. [Google Scholar] [CrossRef] [PubMed]
- Okruszko, A.; Szepietowska, B.; Wawrusiewicz-Kurylonek, N.; Górska, M.; Krętowski, A.; Szelachowska, M. HLA-DR, HLA-DQB1 and PTPN22 gene polymorphism: Association with age at onset for autoimmune diabetes. Arch. Med. Sci. 2012, 8, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Howson, J.M.; Rosinger, S.; Smyth, D.J.; Boehm, B.O.; Todd, J.A.; ADBW-END Study Group. Genetic analysis of adult-onset autoimmune diabetes. Diabetes 2011, 60, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, J.; Xu, Z.; Keller, S.J.L.; Xu, W. Relationship of CTLA-4 gene to latent autoimmune diabetes in adults and Type 2 diabetes: A population-based case-control study. Diabetes Manag. 2014, 4, 131–139. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Kullolli, O.; Parronchi, P.; Romagnani, S. How pregnancy can affect autoimmune diseases progression? Clin. Mol. Allergy 2016, 14, 11. [Google Scholar] [CrossRef]
- Ciortan, L.; Macarie, R.D.; Cecoltan, S.; Vadana, M.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Chronic High Glucose Concentration Induces Inflammatory and Remodeling Changes in Valvular Endothelial Cells and Valvular Interstitial Cells in a Gelatin Methacrylate 3D Model of the Human Aortic Valve. Polymers 2020, 12, 2786. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Andrisse, S.; Koehler, R.M.; Chen, J.E.; Patel, G.D.; Vallurupalli, V.R.; Ratliff, B.A.; Warren, D.E.; Fisher, J.S. Role of GLUT1 in regulation of reactive oxygen species. Redox Biol. 2014, 2, 764–771. [Google Scholar] [CrossRef]
- Clyne, A.M. Endothelial response to glucose: Dysfunction, metabolism, and transport. Biochem. Soc. Trans. 2021, 49, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 145–153. [Google Scholar]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef]
- Vinogradov, V.; Grivennikova, G. Oxidation of NADH and ROS production by respiratory complex I. Biochim. Biophys. Acta Bioenergy 2016, 1857, 863–871. [Google Scholar] [CrossRef]
- Kumar Rajendran, N.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The Influence of Light on Reactive Oxygen Species and NF-κB in Disease Progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Michalska, M.; Gorąca, A. Influence of NADPH oxidase inhibition on oxidative stress parameters in rat hearts. Pharm. Rep. 2013, 65, 898–905. [Google Scholar] [CrossRef]
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Khan, A.; Khan, I. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar]
- Pieme, C.A.; Tatangmo, J.A.; Simo, G.; Biapa Nya, P.C.; Ama Moor, V.J.; Moukette Moukette, B.; Tankeu Nzufo, F.; Njinkio Nono, B.L.; Sobngwi, E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes 2017, 10, 141. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effect of caffeine on biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. 2019, 15, 1073–1780. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The role of oxidative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- LaRocca, T.-J.; Sosunov, S.-A.; Shakerley, N.-L.; Ten, V.-S.; Ratner, A.-J. Hyper-glycemic conditions prime cells for RIP1-dependent necroptosis. J. Biol. Chem. 2016, 291, 13753–13761. [Google Scholar] [CrossRef]
- Rosa, M.-D.; Distefano, G.; Gagliano, C.; Rusciano, D.; Malaguarnera, L. Autophagy in diabetic retinopathy. Curr. Neuropharmacol. 2016, 14, 810–825. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Lee, H.; Hong, S.H.; Park, C.; Park, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Delierneux, C.; Kouba, S.; Shanmughapriya, S.; Potier-Cartereau, M.; Trebak, M.; Hempel, N. Mitochondrial Calcium Regulation of Redox Signaling. Cancer Cells 2020, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Jakob, U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019, 140, 14–27. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Cieluch, A.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Can We Prevent Mitochondrial Dysfunction and Diabetic Cardiomyopathy in Type 1 Diabetes Mellitus? Pathophysiology and Treatment Options. Int. J. Mol. Sci. 2020, 21, 2852. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Mieczan, T.; Wójcik, G. Importance of redox equilibrium in the pathogenesis of psoriasis—Impact of antioxidant-rich diet. Nutrients 2020, 12, 1841. [Google Scholar] [CrossRef]
- Siewiera, K.; Łabieniec-Watała, M. The role of plant polyphenols in alleviating the adverse effects of diabetes on mitochondrial homeostasis. Post Fitoter. 2013, 1, 36–41. [Google Scholar]
- Hua, Y.Y.; Zhang, Y.; Gong, W.W.; Ding, Y.; Shen, J.R.; Li, H.; Chen, Y.; Meng, G.L. Dihydromyricetin improves endothelial dysfunction in diabetic mice via oxidative stress inhibition in a SIRT3-dependent manner. Int. J. Mol. Sci. 2020, 21, 6699. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; El-Senousy, W.M.; Abdel-Latif, M.; Khalil, R.G. Association between antioxidant enzyme activities and Enterovirus-infected type 1 diabetic children. Med. Princ. Pract. 2018, 27, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Varvarovská, J.; Racek, J.; Stozický, F.; Soucek, J.; Trefil, L.; Pomahacová, R. Parameters of oxidative stress in children with Type 1 diabetes mellitus and their relatives. J. Diabetes Complicat. 2003, 17, 7–10. [Google Scholar] [CrossRef]
- Varvarovská, J.; Racek, J.; Stetina, R.; Sýkora, J.; Pomahacová, R.; Rusavý, Z.; Lacigová, S.; Trefil, L.; Siala, K.; Stozický, F. Aspects of oxidative stress in children with type 1 diabetes mellitus. Biomed. Pharmacother. 2004, 58, 539–545. [Google Scholar] [CrossRef]
- Jain, S.K.; McVie, R.; Bocchini, J.A., Jr. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology 2006, 13, 163–170. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Stentz, F.B.; Umpierrez, G.E. Diabetic ketoacidosis induces in vivo activation of human T-lymphocytes. Biochem. Biophys. Res. Commun. 2004, 315, 404–407. [Google Scholar] [CrossRef]
- Hodgkinson, A.D.; Bartlett, T.; Oates, P.J.; Millward, B.A.; Demaine, A.G. The response of antioxidant genes to hyperglycemia is abnormal in patients with type 1 diabetes and diabetic nephropathy. Diabetes 2003, 52, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Jorns, A.; Arndt, T.; Zu Vilsendorf, A.M. Islet infiltration, cytokine expression and beta cell Heath in the NOD Mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia 2014, 57, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Nocoń-Bohusz, J.; Noczyńska, A. Evaluation the concentration of selected markers of the atherosclerosis process in children with diabetes type 1. Pediatr. Endocrinol. 2016, 15, 17–27. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Francés, D.E.; Ingaramo, P.I.; Ronco, M.T.; Carnovale, C.E. Diabetes, an inflammatory process: Oxidative Stress and TNF-alpha involved in hepatic complication. J. Biomed. Sci. Eng. 2013, 6, 645–653. [Google Scholar] [CrossRef]
- Watters, O.; O’Connor, J. A role for Tumor Necrosis Factor in Ischemia and Ischemic Preconditioning. J. Neuroinflamm. 2011, 8, 87. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1 β, IL-2, and IL-6 in insulin-dependent diabetic children. Mediat. Inflamm. 2006, 2006, 1–6. [Google Scholar] [CrossRef]
- Popovic, D.; Lalic, K.; Jotic, A.; Milicic, T.; Bogdanovic, J.; Dordevic, M.; Stankovic, S.; Jeremic, V.; Lalic, N.M. The Inflammatory and Hemostatic Cardiovascular Risk Markers During Acute Hyperglycemic Crisis in Type 1 and Type 2 Diabetes. J. Med. Biochem. 2019, 38, 126–133. [Google Scholar] [CrossRef]
- Cardoso, J.F.; Domingueti, C.P.B.; Gomes, K.; Fernandes, A.P. Evaluation of cytokines in type 1 diabetes patients with and without retinopathy. J. Bras. Patol. Med. Lab. 2017, 53, 31–37. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Song, G.; Pang, S.; Peng, Z.; Li, Y.; Wang, P. High-Fructose Diet Increases Inflammatory Cytokines and Alters Gut Microbiota Composition in Rats. Mediat. Inflamm. 2020, 2020, 6672636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gui, S.; Wang, J.; Chen, Q.; Zeng, J.; Liu, A.; Chen, Z.; Lu, X. Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J. Funct. Foods 2019, 64, 103654. [Google Scholar] [CrossRef]
- Othman, Z.A.; Zakaria, Z.; Suleiman, J.B.; Ghazali, W.; Mohamed, M. Anti-Atherogenic Effects of Orlistat on Obesity-Induced Vascular Oxidative Stress Rat Model. Antioxidants 2021, 10, 251. [Google Scholar] [CrossRef]
- Mahdavifard, S.; Nakhjavani, M. Effect of Glutamine on Oxidative Stress, Inflammatory, and Glycation Markers, and the Activity of Glyoxalase System in Diabetic Rats with Atherosclerosis. J. Maz. Univ. Med. Sci. 2019, 28, 33–42. [Google Scholar]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef]
- Belhiba, O.; Aadam, Z.; Jeddane, L.; Saile, R.; Salih, A.L.J.H.; Bousfiha, A.A.; Jennane, F. Research of anti-GAD and anti-IA2 autoantibodies by ELISA test in a series of Moroccan pediatric patients with diabetes type 1. Afr. Health Sci. 2020, 20, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Kikkas, I.; Mallone, R.; Larger, E.; Volland, H.; Morel, N. A Rapid Lateral Flow Immunoassay for the Detection of Tyrosine Phosphatase-Like Protein IA-2 Autoanti-bodies in Human Serum. PLoS ONE 2014, 9, e103088. [Google Scholar] [CrossRef]
- Zamanfar, D.; Mohsen, A.; Monireh, A.; Monajati, M. Prevalence of autoantibodies in type 1 diabetes mellitus pediatrics in Mazandaran, North of Iran. J. Pediatr. Endocrinol. Metab. 2020, 33, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Delitala, A.P. Autoimmunity in latent autoimmune diabetes in adults. AIMS Med. Sci. 2019, 6, 132–139. [Google Scholar] [CrossRef]
- Pipi, E.; Marketou, M.; Tsirogianni, A. Distinct clinical and laboratory characteristics of latent autoimmune diabetes in adults in relation to type 1 and type 2 diabetes mellitus. World J. Diabetes 2014, 5, 505–510. [Google Scholar] [CrossRef]
- Szepietowska, B.; Wawrusiewicz-Kurylonek, N.; Krętowski, A.; Górska, M.; Szelachowska, M. Endocrine autoimmunity in patients with Latent Autoimmune Diabetes in Adults (LADA)—Association with HLA genotype. Endokrynol. Pol. 2016, 67, 197–201. [Google Scholar] [CrossRef][Green Version]
- Unanue, E.R.; Ferris, S.T.; Carrero, J.A. The role of islet antigen presenting cells and the presentation of insulin in the initiation of autoimmune diabetes in the NOD mouse. Immunol. Rev. 2016, 27, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, F.; Yue, T.T.; Wang, F.X.; Yang, C.L.; Luo, J.H.; Rong, S.J.; Xiong, F.; Zhang, S.; Wang, C.Y. Revisiting the Antigen-Presenting Function of β Cells in T1D Pathogenesis. Front. Immunol. 2021, 12, 690783. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, A.; Harrison, L.C. T cels as mediators of mucosal tolerance: The autoimmune diabetes model. Immunol. Rev. 2000, 173, 109–119. [Google Scholar] [CrossRef]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The antioxidant activity of quercetin in water solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef]
- Shannon, E.; Jaiswal, A.K.; Abu-Ghannam, N. Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas: A kinetic study. Food Res. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Wołosiak, R.; Mazurkiewicz, M.; Drużyńska, B.; Worobiej, E. Antioxidant activity of the selected green teas. Żywn Nauka Technol. Jakość 2008, 4, 290–297. [Google Scholar]
- Korir, M.W.; Wachira, F.N.; Wanyoko, J.K.; Ngure, R.M.; Khalid, R. The fortification of tea with sweeteners and milk and its effect on in vitro antioxidant potential of tea product and glutathione levels in an animal model. Food Chem. 2014, 145, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ. Toxicol. Pharmacol. 2013, 36, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K. Possible protective effect of kombucha tea ferment on cadmium chloride induced liver and kidney damage in irradiated rats. Int. J. Biol. Life Sci. 2013, 9, 7–12. [Google Scholar]
- El-Beltagy, M.A.; Saleh, S.Y.; El-Ghannam, A.E.R.; Ibrahim, I.A. Protective effect of green tea extract on heavy metals-induced oxidative testicular damage in rats. Indian J. Appl. Res. 2015, 5, 577–583. [Google Scholar]
- Nna, V.U.; Usman, U.Z.; Ofulet, E.O.; Owu, D.U. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride-induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem. Toxicol. 2017, 102, 143–155. [Google Scholar] [CrossRef]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef]
- Yi, R.; Wang, R.; Sun, P.; Zhao, X. Antioxidant-mediated preventative effect of Dragon-pearl tea crude polyphenol extract on reserpine-induced gastric ulcers. Exp. Med. 2015, 10, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.; Radwan, N.M.; Aboul Ezz, H.S.; Salama, N.A. The antioxidant effect of green tea mega EGCG against electromagnetic radiation-induced oxidative stress in the hippocampus and striatum of rats. Electromagn. Biol. Med. 2017, 36, 63–73. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Miao, L.F.; Qian, L.L.; Wang, N.; Qi, M.M.; Zhang, Y.M.; Dang, S.P.; Wu, Y.; Wang, R.X. Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Front. Endocrinol. 2019, 10, 640. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, C.; Rao, J.; Xue, Q.; Wu, H.; Wu, D.; Zhang, A.; Chen, L.; Shen, Z.; Lei, L. Systematic identification of the druggable interactions between human protein kinases and naturally occurring compounds in endometriosis. Comput. Biol. Chem. 2017, 71, 136–143. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 8, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Kesharwani, R.K.; Misra, K.; Rizvi, S.I. Protective effect of theaflavin on erythrocytes subjected to in vitro oxidative stress. Biochem. Res. Int. 2013, 2013, 649759. [Google Scholar] [CrossRef]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Litterio, M.C.; Vazquez Prieto, M.A.; Adamo, A.M.; Elesgaray, R.; Oteiza, P.I.; Galleano, M.; Fraga, C.G. (-)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: Effects on the determinants of nitric oxide bioavailability. J. Nutr. Biochem. 2015, 26, 745–751. [Google Scholar] [CrossRef]
- Calabró, V.; Piotrkowski, B.; Fischerman, L.; Prieto, M.A.V.; Galleano, M.; Fraga, C.G. Modifications in nitric oxide and superoxide anion metabolism induced by fructose overload in rat heart are prevented by (−)-epicatechin. Food Funct. 2016, 7, 1876–1883. [Google Scholar] [CrossRef]
- Prince, P.D.; Lanzi, C.R.; Fraga, C.G.; Galleano, M. Dietary (−)-epicatechin affects NF-κB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food Funct. 2019, 10, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Thichanpiang, P.; Wongprasert, K. Green tea polyphenol epigallocatechin-3-gallate attenuates TNF-α-induced intercellular adhesion molecule-1 expression and monocyte adhesion to retinal pigment epithelial cells. Am. J. Chin. Med. 2015, 43, 103–119. [Google Scholar] [CrossRef]
- Gothandam, K.; Ganesan, V.S.; Ayyasamy, T.; Ramalingam, S. Antioxidant potential of theaflavin ameliorates the activities of key enzymes of glucose metabolism in high fat diet and streptozotocin—Induced diabetic rats. Redox Rep. 2019, 24, 41–50. [Google Scholar] [CrossRef]
- Bulboaca, A.E.; Boarescu, P.M.; Porfire, A.S.; Dogaru, G.; Barbalata, C.; Valeanu, M.; Munteanu, C.; Râjnoveanu, R.M.; Nicula, C.A.; Stanescu, I.C. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants 2020, 9, 172. [Google Scholar] [CrossRef]
- Leu, J.G.; Lin, C.Y.; Jian, J.H.; Shih, C.Y.; Liang, Y.J. Epigallocatechin-3-gallate combined with alpha lipoic acid attenuates high glucose-induced receptor for advanced glycation end products (RAGE) expression in human embryonic kidney cells. An. Acad. Bras. Cienc. 2013, 85, 745–752. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin Treatment Ameliorates Diabetes and Its Complications in Streptozotocin-Induced Diabetic Rats. Dose Response 2017, 15, 1559325817691158. [Google Scholar] [CrossRef]
- Mota, M.A.; Landim, J.S.; Targino, T.S.; Silva, S.F.; Silva, S.L.; Pereira, M.R. Evaluation of the anti-inflammatory and analgesic effects of green tea (Camellia sinensis) in mice. Acta Cir. Bras. 2015, 30, 242–246. [Google Scholar] [CrossRef]
- Szulińska, M.; Stępień, M.; Kręgielska-Narożna, M.; Suliburska, J.; Skrypnik, D.; Bąk-Sosnowska, M.; Kujawska-Łuczak, M.; Grzymisławska, M.; Bogdański, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61, 1295525. [Google Scholar] [CrossRef]
- Abolfathi, A.A.; Mohajeri, D.; Rezaie, A.; Nazeri, M. Protective Effects of Green Tea Extract against Hepatic Tissue Injury in Streptozotocin-Induced Diabetic Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 740671. [Google Scholar] [CrossRef]
- Al-Shiekh, A.A.M.; Al-Shati, A.A.; Sarhan, M.A.A. Effect of White Tea Extract on Antioxidant Enzyme Activities of Streptozotocin—Induced Diabetic Rats. Egypt. Acad. J. Biolog. Sci. 2014, 6, 17–30. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Ranjbar, A.; Hosseini, A.; Khanavi, M. The Effect of Green Tea Extract on Oxidative Stress and Spatial Learning in Streptozotocin-diabetic Rats. Iran. J. Pharm. Sci. 2017, 16, 201–209. [Google Scholar]
- Rohman, M.S.; Lukitasari, M.; Nugroho, D.A.; Ramadhiani, R.; Widodo, N.; Kusumastuty, I.; Nugrahini, N.I.P. Decaffeinated light-roasted green coffee and green tea extract combination improved metabolic parameters and modulated inflammatory genes in metabolic syndrome rats. F1000Research 2021, 10, 467. [Google Scholar] [CrossRef]
- Gennaro, G.; Claudino, M.; Cestari, T.M.; Ceolin, D.; Germino, P.; Garlet, G.P.; De Assis, G.F. Green tea modulates cytokine expression in the periodontium and attenuates alveolar bone resorption in type 1 diabetic rats. PLoS ONE 2015, 10, e0134784. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Mohamad Shahi, M. Green Tea (Camellia sinensis) Supplementation to Diabetic Rats Improves Serum and Hepatic Oxidative Stress Markers. Iran. J. Pharm. Sci. 2013, 12, 109–114. [Google Scholar]
- Carito, V.; Ciafrè, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, G.N.; Ceccanti, M.; Boccardo, C.; et al. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super. Sanita 2015, 51, 382–386. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, K.; Fichna, J.; Lewandowska, U. Anti-inflammatory activity of polyphenolic compounds. Post Fitoter. 2017, 18, 17–23. [Google Scholar]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, M.; Liang, Z. (−)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yip, Y.W.Y.; Ren, J.; Hui, W.K.; He, J.N.; Yu, Q.X.; Chu, K.O.; Ng, T.K.; Chan, S.O.; Pang, C.P.; et al. Green tea catechins alleviate autoimmune symptoms and visual impairment in a murine model for human chronic intraocular inflammation by inhibiting Th17-associated pro-inflammatory gene expression. Sci. Rep. 2019, 9, 2301. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, D.; Wang, X.; Zhang, J.; Shan, Z.; Teng, W. (-)-Epigallocatechin gallate inhibits TNF-α-induced PAI-1 production in vascular endothelial cells. J. Cardiovasc. Pharmacol. 2013, 62, 452–456. [Google Scholar] [CrossRef]
- Bogdański, P.; Szulińska, M.; Dytfeld, J.; Pupek-Musialik, D. Evaluation of plasminogen activator inhibitor 1 concentration in patients with simple obesity. Endocrinol. Obes. Metab. Disord. 2012, 8, 1–7. [Google Scholar]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef]
- Wang, Z.M.; Gao, W.; Wang, H.; Zhao, D.; Nie, Z.L.; Shi, J.Q.; Zhao, S.; Lu, X.; Wang, L.S.; Yang, Z.J. Green tea polyphenol epigallocatechin-3-gallate inhibits TNF-α-induced production of monocyte chemoattractant protein-1 in human umbilical vein endothelial cells. Cell Physiol. Biochem. 2014, 33, 1349–1358. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.T.; Pang, X.M.; Han, C.J.; Mao, J.J. Epigallocatechin-3-gallate inhibits angiotensin II and interleukin-6-induced C-reactive protein production in macrophages. Pharm. Rep. 2012, 64, 912–918. [Google Scholar] [CrossRef]
- Bagheri, R.; Rashidlamir, A.; Ashtary-Larky, D.; Wong, A.; Alipour, M.; Motevalli, M.S.; Chebbi, A.; Laher, I.; Zouhal, H. Does green tea extract enhance the anti-inflammatory effects of exercise on fat loss? Br. J. Clin. Pharmacol. 2020, 86, 753–762. [Google Scholar] [CrossRef]
- Yilmaz, R.; Akoglu, H.; Altun, B.; Yildirim, T.; Arici, M.; Erdem, Y. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur. J. Clin. Nutr. 2012, 66, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Li, W.X.; He, R.R.; Li, Y.F.; Tsoi, B.; Zhai, Y.J.; Kurihara, H. Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxid. Med. Cell Longev. 2012, 2012, 537923. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.R.; Vanarsa, K.; Kim, H.Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, J. The ability of green tea to alleviate autoimmune diseases: Fact or fiction? Expert Rev. Clin. Immunol. 2011, 7, 711–713. [Google Scholar] [CrossRef][Green Version]
- Huang, S.C.; Kao, Y.H.; Shih, S.F.; Tsai, M.C.; Lin, C.S.; Chen, L.W.; Chuang, Y.P.; Tsui, P.F.; Ho, L.J.; Lai, J.H.; et al. Epigallocatechin-3-gallate exhibits immunomodulatory effects in human primary T cells. Biochem. Biophys. Res. Commun. 2021, 550, 70–76. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.; Yu, B.; He, J.; Zhang, K.; Ding, X.; Chen, D. Effect of dietary tea polyphenols on growth performance and cell mediated immune response of post-weaning piglets under oxidative stress. Arch. Anim. Nutr. 2010, 64, 12–21. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, B.; Zhu, F. Epigallocatechin-3-gallate protects Kuruma shrimp Marsupeneaus japonicus from white spot syndrome virus and Vibrio alginolyticus. Fish. Shellfish Immun. 2018, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic Effects of Tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef]
- Sharma, V.; Gupta, A.K.; Walia, A. Effect of Green Tea on Diabetes Mellitus. ASNH 2019, 3, 27–31. [Google Scholar]
- Li, B.; Fu, L.; Kojima, R.; Yamamoto, A.; Uemo, T.; Matsui, T. Theaflavins prevent the onset of diabetes through ameliorating glucose tolerance mediated by promoted incretin secretion in spontaneous diabetic Torii rats. J. Funct. Foods 2021, 86, 104702. [Google Scholar] [CrossRef]
- Yang, K.; Hashemi, Z.; Han, W.; Jin, A.; Yang, H.; Ozga, J.; Li, L.; Chan, C.B. Hydrolysis enhances bioavailability of proanthocyanidin-derived metabolites and improves β-cell function in glucose intolerant rats. J. Nutr. Biochem. 2015, 26, 850–859. [Google Scholar] [CrossRef]
- Suzuki, T.; Pervin, M.; Goto, S.; Isemura, M.; Nakamura, Y. Beneficial effects of tea and the green tea catechin epigallocatechin-3-gallate on obesity. Molecules 2016, 21, 1305. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C. Epicatechin potentiation of glucose-stimulated insulin secretion in INS-1 cells is not dependent on its antioxidant activity. Acta Pharm. Sin. 2018, 39, 893–902. [Google Scholar] [CrossRef]
- Komorita, Y.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Jodai-Kitamura, T.; Yoshinari, M.; Oku, Y.; Higashi, T.; Nakamura, U.; et al. Additive effects of green tea and coffee on all-cause mortality in patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. BMJ Open Diabetes Res. Care 2020, 8, e001252. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Kanzaki, N.; Hirakawa, Y.; Yoshinari, M.; Higashioka, M.; Honda, T.; Shibata, M.; Sakata, S.; Yoshida, D.; Teramoto, T.; et al. Serum Ethylamine Levels as an Indicator of l-Theanine Consumption and the Risk of Type 2 Diabetes in a General Japanese Population: The Hisayama Study. Diabetes Care 2019, 42, 1234–1240. [Google Scholar] [CrossRef]
- Adi, P.J.; Burra, S.P.; Vataparti, A.R.; Matcha, B. Calcium, zinc and vitamin E ameliorate cadmium-induced renal oxidative damage in albino Wistar rats. Toxicol. Rep. 2016, 3, 591–597. [Google Scholar] [CrossRef]
- Sarkar, D.; Bose, S.K.; Chakraborty, T.; Roy, S. Theaflavin Enriched Black Tea Extract Alleviates Diabetic Nephropathy by Suppressing Hyperglycaemia-Mediated Oxidative Stress and Inflammation in Streptozotocin-Induced Rats. Nat. Prod. J. 2021, 11, 512–521. [Google Scholar]
- Rusak, G.; Šola, I.; Vujčić Bok, V. Matcha and Sencha green tea extracts with regard to their phenolics pattern and antioxidant and antidiabetic activity during in vitro digestion. J. Food Sci. Technol. 2021, 58, 3568–3578. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.; Rodríguez, A.; Moreno-Ulloa, A.; Ceballos, G.; Villarreal, F. (-)-Epicatechin-induced recovery of mitochondria from simulated diabetes: Potential role of endothelial nitric oxide synthase. Diabetes Vasc. Dis. Res. 2016, 13, 201–210. [Google Scholar] [CrossRef]
- Joo, S.Y.; Song, Y.A.; Park, Y.L.; Myung, E.; Chung, C.Y.; Park, K.J.; Cho, S.B.; Lee, W.S.; Kim, H.S.; Rew, J.S.; et al. Epigallocatechin-3-gallate Inhibits LPS-Induced NF-κB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver 2012, 6, 188–196. [Google Scholar] [CrossRef]
- Deng, X.; Hou, Y.; Zhou, H.; Li, Y.; Xue, Z.; Xue, X.; Huang, G.; Huang, K.; He, X.; Xu, W. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutr. 2021, 9, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green Tea and Its Relation to Human Gut Microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Samriz, O.; Mizrahi, H.; Werbner, M.; Shoenfeld, Y.; Avni, O.; Koren, O. Microbiota at the crossroads of autoimmunity. Autoimmun Rev. 2016, 15, 859–869. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Dias, R.; Bergamo, P.; Maurano, F.; Aufiero, V.R.; Luongo, D.; Mazzarella, G.; Bessa-Pereira, C.; Pérez-Gregorio, M.; Rossi, M.; Freitas, V. First morphological-level insights into the efficiency of green tea catechins and grape seed procyanidins on a transgenic mouse model of celiac disease enteropathy. Food Funct. 2021, 12, 5903–5912. [Google Scholar] [CrossRef] [PubMed]
- Westerlind, H.; Palmqvist, I.; Saevarsdottir, S.; Alfredsson Lars Klareskog, L.; Di Giuseppe, D. Is tea consumption associated with reduction of risk of rheumatoid arthritis? A Swedish case-control study. Arthritis Res. Ther. 2021, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Brás, N.; Fernandes, I.; Pérez-Gregorio, M.; Mateus, N.; Freitas, V. Molecular insights on the interaction and preventive potential of epigallocatechin-3-gallate in Celiac Disease. Int. J. Biol. Macromol. 2018, 112, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.B. The Immunological Benefits of Green Tea (Camellia sinensis). Int. J. Biol. 2017, 9, 10–17. [Google Scholar] [CrossRef][Green Version]
- Berná, G.; Oliveras-López, M.J.; Jurado-Ruíz, E.; Tejedo, J.; Bedoya, F.; Soria, B.; Martín, F. Nutrigenetics and nutrigenomics insights into diabetes etiopathogenesis. Nutrients 2014, 6, 5338–5369. [Google Scholar] [CrossRef]
- Akil, A.A.; Jerman, L.F.; Yassin, E.; Padmajeya, S.S.; Al-Kurbi, A.; Fakhro, K.A. Reading between the (Genetic) Lines: How Epigenetics is Unlocking Novel Therapies for Type 1 Diabetes. Cells 2020, 11, 2403. [Google Scholar] [CrossRef]
- Poczęta, M.; Nowak, E.; Bieg, D.; Bednarek, I. Epigenetic modifications and gene expression in cancerogenesis. Ann. Acad. Med. Silesiensis 2018, 72, 80–89. [Google Scholar] [CrossRef]
- Al Theyab, A.; Almutairi, T.; Al-Suwaidi, A.M.; Bendriss, G.; McVeigh, C.; Chaari, A. Epigenetic Effects of Gut Metabolites: Exploring the Path of Dietary Prevention of Type 1 Diabetes. Front. Nutr. 2020, 7, 563605. [Google Scholar] [CrossRef]
- Gallo, E.; Maggini, V.; Berardi, M.; Pugi, A.; Notaro, R.; Talini, G.; Vannozzi, G.; Bagnoli, S.; Forte, P.; Mugelli, A.; et al. Is green tea a potential trigger for autoimmune hepatitis? Phytomedicine 2013, 20, 1186–1189. [Google Scholar] [CrossRef]
- Yang, E.J.; Lee, J.; Lee, S.Y.; Kim, E.K.; Moon, Y.M.; Jung, Y.O.; Cho, M.L. EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF-1α with Th17/Treg control. PLoS ONE 2014, 9, e86062. [Google Scholar]
- Rana, S.; Kumar, S.; Rathore, N.; Padwad, Y.; Bhushana, S. Nutrigenomics and its Impact on Life Style Associated Metabolic Diseases. Curr. Genom. 2016, 17, 261–278. [Google Scholar] [CrossRef]
- Ortsäter, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjöholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 9, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, Y.; Dai, X.; Wang, J.; Li, Y. Epigallocatechin-3-gallate protects pro-inflammatory cytokine induced injuries in insulin-producing cells through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 670, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Palacio Sánchez, E.; Ribero Vargas, M.E.; Restrepo Gutiérrez, J.C. Hepatotoxicity due to green tea consumption (Camellia sinensis): A review. Rev. Col. Gastroenterol. 2013, 28, 46–52. [Google Scholar]
- Reddy, M.A.; Kumar, B.K.; Boobalan, G.; Reddy, M.K.; Kumar, C.S.V.S.; Reddy, G.A.; Lakshman, M. Hepatoprotective potential of green tea extract against experimental hepatotoxicity in rats. Indian J. Pharm Sci. 2017, 79, 58–64. [Google Scholar] [CrossRef]
- Ali, A.H.A. Hepatoprotective effect of green tea extractagainst cyclophosphamide induced liver injury in albino rats. FMAR 2018, 6, 11–19. [Google Scholar] [CrossRef]
- Esposito, S.; Toni, G.; Tascini, G.; Santi, E.; Berioli, M.G.; Principi, N. Environmental Factors Associated with Type 1 Diabetes. Front. Endocrinol. 2019, 10, 592. [Google Scholar] [CrossRef]
| Polyphenols | Protective Effect | Design | Animals | References | |
|---|---|---|---|---|---|
| Antioxidant parameters | Inflammatory parameters | ||||
| (-)-epicatechin | ↓ TBARS; ↓ SOD; ↓ GPX | ↓ ratio nuclear/cytosolic p65; ↓TNF-α; ↓ iNOS | 10% (w/v) fructose in the drinking water for 8 weeks; (-)-epicatechin (20 mg/kg body weight/day) in diet for 8 weeks | Male Sprague Dawley rats | [114] |
| (-)-epicatechin | ↑ NOS; ↓ O2-; | 10% (w/v) fructose in the drinking water for 8 weeks; (-)-epicatechin (20 mg/kg body weight/day) in diet for 8 weeks | Male Sprague Dawley rats | [115] | |
| (-)-epicatechin | ↑ NOS; ↑ SOD; ↑ GPX; ↓ CAT; ↓ TBARS | 10% (w/v) fructose in the drinking water for 8 weeks; (-)-epicatechin (20 mg/kg body weight/day) in diet for 8 weeks | Male Sprague Dawley rats | [116] | |
| (-)-epicatechin | ↓ TBARS; ↓ SOD; ↑ NOS | ↓ TNFα; ↓ iNOS; ↓ IL-6 | 10% (w/v) fructose in the drinking water for 8 weeks; (-)-epicatechin (20 mg/kg body weight/day) in diet for 8 weeks | Male Sprague Dawley rats | [117] |
| EGCG | ↓ ROS; | ↓ ICAM-1; ↓ NF-κB | Cells were pretreated with or without 100 µM EGCG for 1 h prior to exposure without or with 20 ng/mL of TNF- for 24 h | Human retinal pigment epithelial ARPE-19 cells | [118] |
| Theaflavin | ↑ SOD; ↑ CAT; ↑ GSH; ↑ GST; ↓ TBARS; ↓ HP | 100 mg/kg bw /day theaflavin administered orally to diabetic rats for 30 days | Male Wistar diabetic rats | [119] | |
| EGCG | ↓ MDA; ↓ TOS; ↑ thiols; ↑ CAT; ↑ TAC; | 60 mg/100 g bw streptozotocin by intraperitoneal injection; 2.5 mg/100 g bw/day EGCG in saline solution or in liposomal form by intraperitoneal injection for 2 days | Male Wistar-Bratislava diabetic rats | [120] | |
| EGCG | ↑ SOD; ↓ ROS; ↓ RAGE mRNA; | ↓ TNF-α; ↓ IL-6 | 25 mM glucose; 2.2 mM EGCG | Human embryonic kidney 293 (HEK293) cells | [121] |
| Catechin | ↓ MDA; ↑ SOD; ↑ CAT; ↑ GST | Streptozocin by intraperitoneal injection; 40 or 80 mg/kg/day catechin by intraperitoneal injection for 4 weeks | Male diabetic Wistar rats | [122] |
| Polyphenols | Protective Effect | Design | Animals | References | |
|---|---|---|---|---|---|
| Antioxidant parameters | Inflammatory parameters | ||||
| Alcoholic extracts of green tea | ↓ inflammatory cell migration in the peritoneum | 0.07 or 0.14 g alcoholic extracts of green tea per kg by gavage or subcutaneously one hour before intraperitoneal injection of carrageenan (inflammation induction) | Male Swiss mice | [123] | |
| Green tea extract | ↑ TAS | ↓ TNF-α; ↓ CRP | 2 or 4 g extract of green tea per 1 kg of high-sodium-diet (35 g/kg) for 42 days | Male Wistar rats | [124] |
| Green tea extract | ↑ GSH; ↑ SOD; ↑ CAT; ↑ GSH-Px; ↓ MDA | Green tea extract (1.5%, w/v) as a sole drinking source | Male Wistar diabetic rats | [125] | |
| White tea extract | ↑ SOD; ↑ CAT; ↑ GPX; ↑ GSH-Px; ↓ MDA | White tea extract (2%, w/v) as a sole drinking source | Male diabetic rats | [126] | |
| Green tea extract | ↓ LPO; ↓ total thiol groups | Green tea extract (3 mg/L) as a sole drinking source | Male diabetic Wistar rats | [127] | |
| Green tea extract | ↓ TNF-α; ↓ CRP; ↓ IL-6; ↓ NF-κB | Streptozocin by intraperitoneal injection; 300 mg green tea extract for 9 weeks | Male Sprague-Dawley rats | [128] | |
| Green tea water extract | ↓ TNF-α; ↑ IL-10 | Streptozocin by intraperitoneal injection; green tea solution (7 g/L) ad libitum for 5, 30, 60 or 90 days | Male diabetic Wistar rats | [129] | |
| Green tea alcoholic extract | ↓ MDA; ↑ TAC | Streptozocin by intraperitoneal injection; 100 or 200 mg/kg green tea alcoholic extract by oral gavage for 4 weeks | Male diabetic Wistar rats | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Tomaszewska, E.; Jachimowicz, K. Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes. Nutrients 2021, 13, 3972. https://doi.org/10.3390/nu13113972
Winiarska-Mieczan A, Tomaszewska E, Jachimowicz K. Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes. Nutrients. 2021; 13(11):3972. https://doi.org/10.3390/nu13113972
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Ewa Tomaszewska, and Karolina Jachimowicz. 2021. "Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes" Nutrients 13, no. 11: 3972. https://doi.org/10.3390/nu13113972
APA StyleWiniarska-Mieczan, A., Tomaszewska, E., & Jachimowicz, K. (2021). Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes. Nutrients, 13(11), 3972. https://doi.org/10.3390/nu13113972






