Effect of Individual Nutrition Therapy and Exercise Regime on Gait Speed, Physical Function, Strength and Balance, Body Composition, Energy and Protein, in Injured, Vulnerable Elderly: A Multisite Randomized Controlled Trial (INTERACTIVE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Recruitment
2.3. Randomization and Blinding
2.4. Intervention
2.5. Attention Control
2.6. Primary and Secondary Outcome Measures
2.7. Ethics Approval and Consent to Participate
2.8. Sample Size
2.9. Statistical Analysis
3. Results
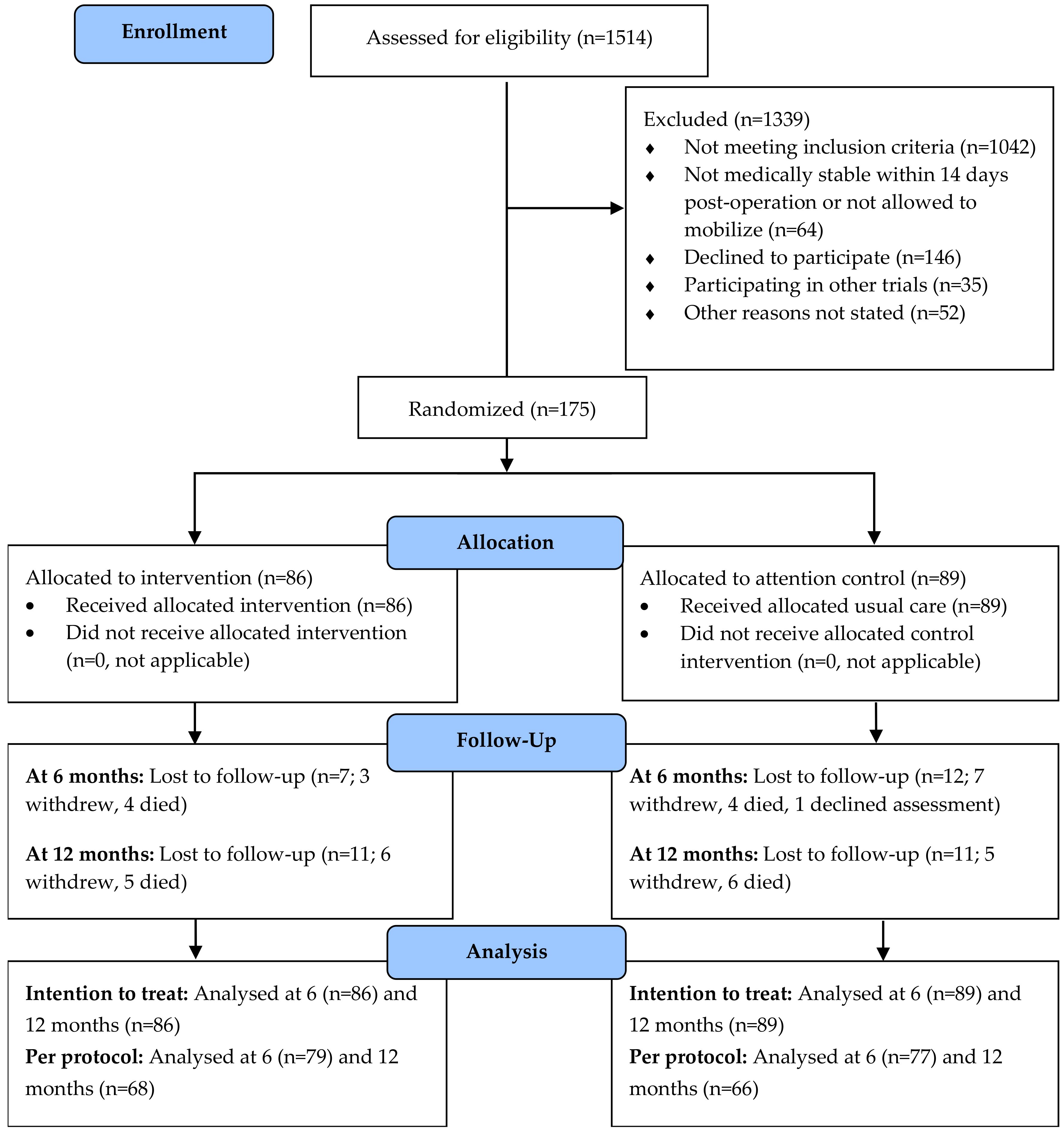
3.1. Recruitment
3.2. Characteristics of the Study Population
3.3. Primary Outcome
3.4. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van de Ree, C.L.; Landers, M.J.; Kruithof, N.; de Munter, L.; Slaets, J.P.; Gosens, T.; Jongh, M.A. Effect of frailty on quality of life in elderly patients after hip fracture: A longitudinal study. BMJ Open 2019, 9, e025941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-S.; Watts, J.; Peel, N.; Hubbard, R. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Jiang, C.; Shen, J.; Tang, P.; Wang, Y. Preoperative predictors for mortality following hip fracture surgery: A systematic review and meta-analysis. Injury 2012, 43, 676–685. [Google Scholar] [CrossRef]
- Panula, J.; Pihlajamäki, H.; Mattila, V.M.; Jaatinen, P.; Vahlberg, T.; Aarnio, P.; Kivelä, S.-L. Mortality and cause of death in hip fracture patients aged 65 or older-a population-based study. BMC Musculoskelet. Disord. 2011, 12, 105. [Google Scholar] [CrossRef] [Green Version]
- Leal, J.; Gray, A.; Prieto-Alhambra, D.; Arden, N.K.; Cooper, C.; Javaid, M.K.; Judge, A.; Group, R.S. Impact of hip fracture on hospital care costs: A population-based study. Osteoporos. Int. 2016, 27, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.; Landeiro, F.; McConnell, T.; Fulford-Smith, L.; Javaid, M.K.; Judge, A.; Leal, J. Costs of fragility hip fractures globally: A systematic review and meta-regression analysis. Osteoporos. Int. 2017, 28, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Ftouh, S.; Morga, A.; Swift, C. Management of hip fracture in adults: Summary of NICE guidance. BMJ 2011, 342, d3304. [Google Scholar] [CrossRef]
- Uda, K.; Matsui, H.; Fushimi, K.; Yasunaga, H. Intensive in-hospital rehabilitation after hip fracture surgery and activities of daily living in patients with dementia: Retrospective analysis of a nationwide inpatient database. Arch. Phys. Med. Rehabil. 2019, 100, 2301–2307. [Google Scholar] [CrossRef]
- Latham, N.K.; Harris, B.A.; Bean, J.F.; Heeren, T.; Goodyear, C.; Zawacki, S.; Heislein, D.M.; Mustafa, J.; Pardasaney, P.; Giorgetti, M. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: A randomized clinical trial. JAMA 2014, 311, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Handoll, H.H.; Cameron, I.D.; Mak, J.C.; Finnegan, T.P. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst. Rev. 2009, 4, CD007125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, J.C.; Cameron, I.D.; March, L.M. Evidence-based guidelines for the management of hip fractures in older persons: An update. Med. J. Aust. 2010, 192, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Riemen, A.H.; Hutchison, J.D. The multidisciplinary management of hip fractures in older patients. Orthop. Trauma 2016, 30, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevet, S.; Bioteau, C.; Maziere, S.; Couturier, P.; Merloz, P.; Tonetti, J.; Gavazzi, G. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop. Traumatol. Surg. Res. 2014, 100, 669–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Misu, S.; Tanaka, T.; Sakamoto, H.; Iwata, K.; Chuman, Y.; Ono, R. Pre-fracture nutritional status is predictive of functional status at discharge during the acute phase with hip fracture patients: A multicenter prospective cohort study. Clin. Nutr. 2017, 36, 1320–1325. [Google Scholar] [CrossRef]
- Brox, W.T.; Roberts, K.C.; Taksali, S.; Wright, D.G.; Wixted, J.J.; Tubb, C.C.; Patt, J.C.; Templeton, K.J.; Dickman, E.; Adler, R.A. The American Academy of Orthopaedic Surgeons evidence-based guideline on management of hip fractures in the elderly. J. Bone Jt. Surg. Am. Vol. 2015, 97, 1196. [Google Scholar] [CrossRef]
- Avenell, A.; Smith, T.O.; Curtain, J.P.; Mak, J.C.; Myint, P.K. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst. Rev. 2016, 11, CD001880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemm, H.J.; Bailey, J.K.; Desneves, K.J.; Crowe, T.C. Can early dietetic intervention improve outcomes in patients with hip fracture? Nutr. Diet. 2016, 73, 336–341. [Google Scholar] [CrossRef]
- Wyers, C.E.; Reijven, P.L.; Breedveld-Peters, J.J.; Denissen, K.F.; Schotanus, M.G.; van Dongen, M.C.; Eussen, S.J.; Heyligers, I.C.; van den Brandt, P.A.; Willems, P.C. Efficacy of nutritional intervention in elderly after hip fracture: A multicenter randomized controlled trial. J. Gerontol. Ser. A 2018, 73, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, M.; de Sire, A.; D’Andrea, F.; Carrera, D.; Renò, F.; Migliaccio, S.; Iolascon, G.; Cisari, C. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: A pilot randomized controlled trial. Aging Clin. Exp. Res. 2019, 31, 1517–1524. [Google Scholar] [CrossRef]
- Magaziner, J.; Mangione, K.K.; Orwig, D.; Baumgarten, M.; Magder, L.; Terrin, M.; Fortinsky, R.H.; Gruber-Baldini, A.L.; Beamer, B.A.; Tosteson, A.N. Effect of a multicomponent home-based physical therapy intervention on ambulation after hip fracture in older adults: The CAP randomized clinical trial. JAMA 2019, 322, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.K.; Humphreys, K.J.; Miller, M.D.; Cameron, I.D.; Whitehead, C.; Kurrle, S.; Mackintosh, S.; Crotty, M. Individual nutrition therapy and exercise regime: A controlled trial of injured, vulnerable elderly (INTERACTIVE trial). BMC Geriatr. 2008, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, H.A.; Chan, L.N.; Li, F. Indirect calorimetry: A practical guide for clinicians. Nutr. Clin. Pract. 2007, 22, 377–388. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C.; Gardner, M.M.; Norton, R.N.; Buchner, D.M. Falls prevention over 2 years: A randomized controlled trial in women 80 years and older. Age Ageing 1999, 28, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, A.J.; Robertson, M.C.; Gardner, M.M.; Norton, R.N.; Tilyard, M.W.; Buchner, D.M. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997, 315, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Gardner, M.M.; Robertson, M.C.; McGee, R.; Campbell, A.J. Application of a falls prevention program for older people to primary health care practice. Prev. Med. 2002, 34, 546–553. [Google Scholar] [CrossRef]
- Worsfold, C.; Simpson, J.M. Standardisation of a three-metre walking test for elderly people. Physiotherapy 2001, 87, 125–132. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Smyer, M.A. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J. Gerontol. 1981, 36, 428–434. [Google Scholar] [CrossRef]
- Bohannon, R.W. Alternatives for measuring knee extension strength of the elderly at home. Clin. Rehabil. 1998, 12, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Schaubert, K.L.; Bohannon, R.W. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J. Strength Cond. Res. 2005, 19, 717. [Google Scholar] [PubMed]
- Langley, F. The Reliability and Validity of the Modified Berg Balance Scale; University of South Australia: Adelaide, Australia, 2007. [Google Scholar]
- McLennan, W.; McLennan, W.; Podger, A.S. National Nutrition Survey Users’ Guide, 1995; Australian Bureau of Statistics [and] Commonwealth Department of Health and Welfare: Canberra, Australia, 1998. [Google Scholar]
- Hawthorne, G.; Richardson, J.; Osborne, R. The Assessment of Quality of Life (AQoL) instrument: A psychometric measure of health-related quality of life. Qual. Life Res. 1999, 8, 209–224. [Google Scholar] [CrossRef]
- Milte, R.; Miller, M.D.; Crotty, M.; Mackintosh, S.; Thomas, S.; Cameron, I.D.; Whitehead, C.; Kurrle, S.; Ratcliffe, J. Cost-effectiveness of individualized nutrition and exercise therapy for rehabilitation following hip fracture. J. Rehabil. Med. 2016, 48, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.D.; Crotty, M.; Whitehead, C.; Bannerman, E.; Daniels, L.A. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture: A randomized controlled trial. Clin. Rehabil. 2006, 20, 311–323. [Google Scholar] [CrossRef]
- Lane, P. Handling drop-out in longitudinal clinical trials: A comparison of the LOCF and MMRM approaches. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2008, 7, 93–106. [Google Scholar] [CrossRef]
- Morris, S.B. Estimating effect sizes from pretest-posttest-control group designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Mangione, K.K.; Craik, R.L.; Palombaro, K.M.; Tomlinson, S.S.; Hofmann, M.T. Home-based leg-strengthening exercise improves function 1 year after hip fracture: A randomized controlled study. J. Am. Geriatr. Soc. 2010, 58, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Mangione, K.K.; Craik, R.L.; Lopopolo, R.; Tomlinson, J.D.; Brenneman, S.K. Predictors of gait speed in patients after hip fracture. Physiother. Can. 2008, 60, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mantel, A.; Trapuzzano, A.; Chizmar, S.; Haffke, L.; Dawson, N. An investigation of the predictors of comfortable and fast gait speed in community-dwelling older adults. J. Geriatr. Phys. Ther. 2019, 42, E62–E68. [Google Scholar] [CrossRef]
- Ekström, H.; Elmståhl, S. Pain and fractures are independently related to lower walking speed and grip strength: Results from the population study “Good Ageing in Skåne”. Acta Orthop. 2006, 77, 902–911. [Google Scholar] [CrossRef] [Green Version]
- Kirk, B.; Mooney, K.; Cousins, R.; Angell, P.; Jackson, M.; Pugh, J.N.; Coyles, G.; Amirabdollahian, F.; Khaiyat, O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: A secondary analysis of the Liverpool Hope University—Sarcopenia Ageing Trial (LHU-SAT). Eur. J. Appl. Physiol. 2020, 120, 493–503. [Google Scholar] [CrossRef]
- Whitehurst, M.A.; Johnson, B.L.; Parker, C.M.; Brown, L.E.; Ford, A.M. The benefits of a functional exercise circuit for older adults. J. Strength Cond. Res. 2005, 19, 647. [Google Scholar] [PubMed] [Green Version]
- Liu, C.-j.; Shiroy, D.M.; Jones, L.Y.; Clark, D.O. Systematic review of functional training on muscle strength, physical functioning, and activities of daily living in older adults. Eur. Rev. Aging Phys. Act. 2014, 11, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Shubert, T.E.; Smith, M.L.; Goto, L.; Jiang, L.; Ory, M.G. Otago exercise program in the United States: Comparison of 2 implementation models. Phys. Ther. 2017, 97, 187–197. [Google Scholar] [CrossRef]
- Resnick, B.; Spellbring, A.M. Understanding what motivates older adults to exercise. J. Gerontol. Nurs. 2000, 26, 34–42. [Google Scholar] [CrossRef]
- Volkert, D.; Kreuel, K.; Heseker, H.; Stehle, P. Energy and nutrient intake of young-old, old-old and very-old elderly in Germany. Eur. J. Clin. Nutr. 2004, 58, 1190–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, B.L.; Coyle, D.H.; Santos, J.A.; Burrows, T.; Rosewarne, E.; Peters, S.A.; Carcel, C.; Jaacks, L.M.; Norton, R.; Collins, C.E. Investigating sex differences in the accuracy of dietary assessment methods to measure energy intake in adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 1241–1255. [Google Scholar] [CrossRef]
- Bell, J.J.; Bauer, J.D.; Capra, S.; Pulle, R.C. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients–results of a pragmatic intervention. Clin. Nutr. 2014, 33, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Anbar, R.; Beloosesky, Y.; Cohen, J.; Madar, Z.; Weiss, A.; Theilla, M.; Hakim, T.K.; Frishman, S.; Singer, P. Tight calorie control in geriatric patients following hip fracture decreases complications: A randomized, controlled study. Clin. Nutr. 2014, 33, 23–28. [Google Scholar] [CrossRef]
- Nordström, P.; Thorngren, K.-G.; Hommel, A.; Ziden, L.; Anttila, S. Effects of geriatric team rehabilitation after hip fracture: Meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 2018, 19, 840–845. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool Hope University–Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Kashef, M.A.; Giugliano, G. Legacy effect of statins: 20-year follow up of the West of Scotland Coronary Prevention Study (WOSCOPS). Glob. Cardiol. Sci. Pract. 2016, 2016, e201635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, M.; Cosentino, F.; Tocci, G.; Palano, F.; Paneni, F. Antihypertensive therapy in diabetes: The legacy effect and RAAS blockade. Curr. Hypertens. Rep. 2011, 13, 318–324. [Google Scholar] [CrossRef]
- Bernocchi, P.; Giordano, A.; Pintavalle, G.; Galli, T.; Spoglia, E.B.; Baratti, D.; Scalvini, S. Feasibility and clinical efficacy of a multidisciplinary home-telehealth program to prevent falls in older adults: A randomized controlled trial. J. Am. Med Dir. Assoc. 2019, 20, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; Miller, W.C.; Ashe, M.C. The Effect of Telehealth Interventions on Function and Quality of Life for Older Adults with Pre-Frailty or Frailty: A Systematic Review and Meta-Analysis. J. Appl. Gerontol. 2021. [Google Scholar] [CrossRef]
- Visser, M.; Fuerst, T.; Lang, T.; Salamone, L.; Harris, T.B.; For The Health, Aging, Body Composition Study Dual-Energy X-Ray Absorptiometry; Body Composition Working Group. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. J. Appl. Physiol. 1999, 87, 1513–1520. [Google Scholar] [CrossRef] [Green Version]
- Han, C.Y.; Miller, M.; Yaxley, A.; Baldwin, C.; Woodman, R.; Sharma, Y. Effectiveness of combined exercise and nutrition interventions in prefrail or frail older hospitalised patients: A systematic review and meta-analysis. BMJ Open 2020, 10, e040146. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.A.F. Exercise, nutrition and managing hip fracture in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 12–24. [Google Scholar]
| Intervention (n = 86) | Control (n = 89) | p-Value 1 | |
|---|---|---|---|
| Characteristic | |||
| Age, years, n, mean ± SD | 86, 82.4 ± 5.7 | 89, 83.0 ± 6.2 | 0.51 |
| Female, n, (%) | 86, 58 (67.4) | 89, 77 (86.5) | 0.002 |
| BMI 2, kg/m 2, n, mean ± SD | 86, 25.1 ± 3.5 | 89, 24.8 ± 4.2 | 0.67 |
| MMSE 3 score, n, mean ± SD | 59, 23.5 ± 3.2 | 62, 23.9 ± 3.4 | 0.53 |
| Baseline resting energy expenditure (kcal/d), n, mean ± SD | 36, 1334 ± 431 | 32, 1321±441 | 0.47 |
| Estimated energy requirement (kcal/d), n, mean ± SD | 86, 1734 ± 388 | 88, 1652±330 | 0.14 |
| Estimated protein requirement (g/d), n, mean ± SD | 86, 78.2 ± 16.9 | 88, 77.0 ± 15.6 | 0.61 |
| Physical function, strength and balance | |||
| Gait speed (m/s), n, mean ± SD | 86, 0.33 ± 0.28 | 89, 0.28 ± 0.28 | 0.25 |
| Knee strength (injured) (kg), n, mean ± SD | 74, 6.1 ± 3.08 | 73, 5.2 ± 3.08 | 0.06 |
| Grip strength (kg), n, mean ± SD | 85, 18.1 ± 6.6 | 88, 15.7 ± 5.9 | 0.01 |
| MoBERG 4 score, n, mean ± SD | 85, 16.3 ± 7.8 | 87, 15.1 ± 8.3 | 0.32 |
| Physical Activities of Daily living 5, n, mean ± SD | 86, 9.63 ± 1.72 | 89, 9.43 ± 1.97 | 0.47 |
| Instrumental Activities of Daily living 6, n, mean ± SD | 86, 12.0 ± 2.0 | 89, 11.8 ± 2.6 | 0.50 |
| Body composition, energy, and protein intake | |||
| Reported weight loss (kg), n (%) | 24 (13.7) | 18 (10.3) | 0.20 |
| Reported amount of weight loss (kg), n (%) | |||
| 5 kg or less | 9 (37.5) | 7 (38.9) | 0.99 |
| >5 kg | 12 (50) | 9 (50) | |
| unknown | 3 (12.5) | 2 (4.8) | |
| % Fat-free mass, DEXA 7, n, mean ± SD | 43, 67.4 ± 9.4 | 36, 66.1 ± 10.7 | 0.59 |
| Estimated energy intake (kcal/d), n, mean ± SD | 86, 1143 ± 422 | 88, 1125 ± 417 | 0.77 |
| Estimated protein intake (g/d), n, mean ± SD | 86, 49.5 ± 19.0 | 88, 47.1 ± 20.1 | 0.42 |
| Intervention Group | Control Group | Mean Difference between Groups 1 | p-Value 2 | Cohen D | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Gait speed (m/s), | |||||
| 6-month, n, mean ± SD | 77, 0.83 ± 0.3 | 76, 0.83 ± 0.3 | −0.02 (−0.11 to 0.07) | 0.64 | 0.18 |
| 12-month, n, mean ± SD | 65, 0.92 ± 0.4 | 67, 0.84 ± 0.3 | 0.08 (−0.04 to 0.21) | 0.19 | 0.11 |
| Change from 0 to 6-month | 77, 0.49 ± 0.32 | 76, 0.53 ± 0.32 | |||
| Change from 0 to 12-month | 65, 0.60 ± 0.47 | 67 0.57 ± 0.44 | |||
| Secondary outcomes—Physical function, strength and balance | |||||
| Knee strength (injured) (kg) | |||||
| 6-month, n, mean ± SD | 74, 10.7 ± 3.7 | 73, 10.7 ± 4.7 | −0.15 (−1.59 to 1.29) | 0.84 | 0.39 |
| 12-month, n, mean ± SD | 63, 11.1 ± 5.1 | 63, 11.1 ± 5.1 | 0.12 (−1.99 to 1.76) | 0.9 | 0.29 |
| Change from 0 to 6-month | 58, 4.77 ± 3.90 | 65, 5.43 ± 4.54 | |||
| Change from 0 to 12-month | 52, 5.32 ± 5.21 | 55, 5.82 ± 5.15 | |||
| Grip strength (kg) | |||||
| 6-month, n, mean ± SD | 79, 18.7 ± 7.6 | 76, 17.7 ± 6.4 | −1.24 (−2.29 to −0.18) | 0.02 | 0.21 |
| 12-month, n, mean ± SD | 64, 19.4 ± 8.3 | 67, 17 ± 5.7 | 2.36 (−0.15 to 4.88) | 0.07 | 0 |
| Change from 0 to 6-month | 79, 0.57 ± 3.61 | 76, 1.85 ± 2.81 | |||
| Change from 0 to 12-month | 64, 0.82 ± 10.85 | 67, 0.67 ± 8.11 | |||
| MoBERG 3 score | |||||
| 6-month, n, mean ± SD | 78, 38.2 ± 11.9 | 74, 37.2 ± 10.9 | 0.77 (−2.46 to 4.01) | 0.64 | 0.03 |
| 12-month, n, mean ± SD | 63, 39.1 ± 12.6 | 66, 36.5 ± 11.8 | 3.04 (−1.30 to 7.38) | 0.17 | 0.17 |
| Change from 0 to 6-month | 78, 22.13 ± 10.86 | 73, 21.57 ± 9.81 | |||
| Change from 0 to 12-month | 62, 23.6 ± 15.4 | 64, 21.8 ± 14.6 | |||
| PADL 4 score | |||||
| 6-month, n, mean ± SD | 79, 12.6 ± 1.54 | 78, 12.6 ± 1.86 | −0.05 (−0.53 to 0.43) | 0.84 | 0.11 |
| 12-month, n, mean ± SD | 65, 10.98 ± 1.46 | 68, 10.85 ± 1.22 | 0.12 (−0.34 to 0.58) | 0.6 | 0.04 |
| Change from 0 to 6-month | 79, 3.06 ± 1.87 | 78, 3.18 ± 1.81 | |||
| Change from 0 to 12-month | 65, 1.51 ± 2.19 | 68, 1.51 ± 2.28 | |||
| IADL 5 score | |||||
| 6-month, n, mean ± SD | 79, 10.9 ± 2.8 | 78, 10.8 ± 3.4 | −0.09 (−0.83 to 0.66) | 0.82 | 0.04 |
| 12-month, n, mean ± SD | 65, 11.4 ± 2.9 | 68, 10.8 ± 3 | 0.51 (−0.52−1.54) | 0.33 | 0.17 |
| Change from 0 to 6-month | 79, −1.24 ± 2.2 | 78, −1.14 ± 2.48 | |||
| Change from 0 to 12-month | 65, −0.85 ± 3.55 | 68, −0.88 ± 3.83 | |||
| Secondary outcomes—Body composition, energy and protein intake | |||||
| % Fat-free mass 6 | |||||
| 6-month, n, mean ± SD | 33, 69 ± 9.7 | 36, 64 ± 10.3 | 1.28 (−1.53 to 4.10) | 0.37 | 0.37 |
| 12-month, n, mean ± SD | 41, 67.5 ± 10.4 | 34, 65.7 ± 10.6 | −0.53 (−3.51 to 2.45) | 0.72 | 0.05 |
| Change from 0 to 6-month | 29, 0.39 ± 5.47 | 37, −0.95 ± 5.96 | |||
| Change from 0 to 12-month | 25, −0.02 ± 5.87 | 30, 0.65 ± 5.51 | |||
| Estimated energy intake (kcal/d) | |||||
| 6-month, n, mean ± SD | 79, 1701 ± 466 | 75, 1444 ± 444 | 235 (95 to 375) | 0.01 | 0.57 |
| 12-month, n, mean ± SD | 64, 1634 ± 436 | 67, 1620 ± 544 | 22 (−153 to 196) | 0.81 | 0.01 |
| Change from 0 to 6-month | 76, 563 ± 578 | 72, 294 ± 509 | |||
| Change from 0 to 12-month | 61, 480 ± 597 | 64, 503 ± 643 | |||
| Estimated protein Intake (g/d) | |||||
| 6-month, n, mean ± SD | 79, 67.4 ± 25.8 | 75, 55.8 ± 25.2 | 9.1 (1.50 to 16.8) | 0.02 | 0.47 |
| 12-month, n, mean ± SD | 64, 65.1 ± 23.6 | 67, 59.5 ± 20.8 | 6.1 (−1.76 to 14.0) | 0.13 | 0.16 |
| Change from 0 to 6-month | 76, 16.95 ± 29.5 | 72, 7.93 ± 26.9 | |||
| Change from 0 to 12-month | 61, 14.8 ± 30.3 | 64, 11.6 ± 27.8 | |||
| Intervention Group (n = 86) | Control Group (n = 89) | Mean Difference between Groups 1 | p-Value 2 | Cohen D | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Gait speed (m/s) | |||||
| 6-month, mean ± SD | 0.82 ± 0.04 | 0.81 ± 0.04 | −0.02 (−0.11 to 0.08) | 0.72 | 0.14 |
| 12-month, mean ± SD | 0.69 ± 0.04 | 0.66 ± 0.04 | 0.07 (−0.06 to 0.19) | 0.36 | 0.07 |
| Change from 0 to 6-month | 0.49 ± 0.04 | 0.53 ± 0.04 | |||
| Change from 0 to 12-month | 0.58 ± 0.07 | 0.56 ± 0.06 | |||
| Secondary outcomes–Physical function, strength and balance | |||||
| Knee strength (injured) (kg) | |||||
| 6-month, mean ± SD | 10.4 ± 0.45 | 10.3 ± 0.54 | −0.12 (−1.34 to 1.10) | 0.85 | 0.26 |
| 12-month, mean ± SD | 10.9 ± 5.04 | 11.0 ± 5.65 | −0.34 (−1.96 to 1.27) | 0.7 | 0.26 |
| Change from 0 to 6-month | 4.77 ± 0.50 | 5.17 ± 0.50 | |||
| Change from 0 to 12-month | 4.93 ± 0.76 | 5.68 ± 0.66 | |||
| Grip strength (kg) | |||||
| 6-month, mean ± SD | 18.8 ± 0.83 | 17.3 ± 0.68 | −0.90 (−1.92 to 0.13) | 0.1 | 0.14 |
| 12-month, mean ± SD | 18.8 ± 0.99 | 17.3 ± 0.77 | 0.19 (−0.70 to 3.90) | 0.19 | 0.14 |
| Change from 0 to 6-month | 0.65 ± 0.44 | 1.62 ± 0.35 | |||
| Change from 0 to 12-month | 0.72 ± 1.24 | 1.68 ± 0.99 | |||
| MoBERG 3 score | |||||
| 6-month, mean ± SD | 38.0 ± 1.3 | 36.8 ± 1.3 | 0.40 (−2.81 to 3.62) | 0.75 | 0 |
| 12-month, mean ± SD | 39.3 ± 1.77 | 37.2 ± 1.46 | 2.23 (−1.56 to 6.02) | 0.32 | 0.11 |
| Change from 0 to 6-month | 21.71 ± 1.23 | 21.7 ± 1.25 | |||
| Change from 0 to 12-month | 22.99 ± 1.97 | 22.11 ± 1.76 | |||
| PADL 4 score | |||||
| 6-month, mean ± SD | 12.6 ± 0.18 | 12.6 ± 0.20 | −0.09 (−0.55 to 0.37) | 0.72 | 0.11 |
| 12-month, mean ± SD | 11.1 ± 0.19 | 10.9 ± 0.17 | 0.49 (−0.25 to 0.62) | 0.49 | 0 |
| Change from 0 to 6-month | 3.0 ± 0.22 | 3.2 ± 0.2 | |||
| Change from 0 to 12-month | 1.52 ± 0.26 | 1.52 ± 0.26 | |||
| IADL 5 score | |||||
| 6-month, mean ± SD | 10.9 ± 0.31 | 10.62 ± 0.37 | 0.78 (−0.63 to 0.79) | 0.76 | 0.03 |
| 12-month, mean ± SD | 11.4 ± 0.4 | 10.9 ± 0.38 | 0.48 (−0.49 to 1.44) | 0.38 | 0.03 |
| Change from 0 to 6-month | −1.12 ± 0.26 | −1.17 ± 0.28 | |||
| Change from 0 to 12-month | −0.64 ± 0.46 | −0.91 ± 0.44 | |||
| Secondary outcomes–Body composition, energy and protein intake | |||||
| % Fat-free mass 6 | |||||
| 6-month, mean ± SD | 68.1 ± 2.4 | 67.1 ± 2.1 | 1.07 (−1.71 to 3.86) | 0.44 | 0.03 |
| 12-month, mean ± SD | 67.9 ± 3.7 | 68.7 ± 3.7 | −0.71 (0.47 to 0.96) | 0.51 | 0.21 |
| Change from 0 to 6-month | 1.0 ± 1.5 | −0.13 ± 1.13 | |||
| Change from 0 to 12-month | 0.84 ± 2.01 | 1.49 ± 2.63 | |||
| Estimated energy intake (kcal/d) | |||||
| 6-month, mean ± SD | 1689 ± 293 | 1440 ± 58 | 240 (101 to379) | 0.01 | 0.55 |
| 12-month, mean ± SD | 1655 ± 63 | 1630 ± 81 | 20 (−136 to 177) | 0.68 | 0.02 |
| Change from 0 to 6-month | 526 ± 70 | 320 ± 67 | |||
| Change from 0 to 12-month | 493 ± 82 | 511±89 | |||
| Estimated protein intake (g/d) | |||||
| 6-month, mean ± SD | 66.7 ± 3.1 | 55.4 ± 3.0 | 10.54 (2.73 to 18.36) | 0.01 | 0.45 |
| 12-month, mean ± SD | 64.1 ± 3.4 | 59.9 ± 2.9 | 4.09 (−3.02 to 11.20) | 0.29 | 0.09 |
| Change from 0 to 6-month | 16.7 ± 3.62 | 8.31 ± 3.37 | |||
| Change from 0 to 12-month | 14.1 ± 4.09 | 12.8 ± 3.36 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.Y.; Crotty, M.; Thomas, S.; Cameron, I.D.; Whitehead, C.; Kurrle, S.; Mackintosh, S.; Miller, M. Effect of Individual Nutrition Therapy and Exercise Regime on Gait Speed, Physical Function, Strength and Balance, Body Composition, Energy and Protein, in Injured, Vulnerable Elderly: A Multisite Randomized Controlled Trial (INTERACTIVE). Nutrients 2021, 13, 3182. https://doi.org/10.3390/nu13093182
Han CY, Crotty M, Thomas S, Cameron ID, Whitehead C, Kurrle S, Mackintosh S, Miller M. Effect of Individual Nutrition Therapy and Exercise Regime on Gait Speed, Physical Function, Strength and Balance, Body Composition, Energy and Protein, in Injured, Vulnerable Elderly: A Multisite Randomized Controlled Trial (INTERACTIVE). Nutrients. 2021; 13(9):3182. https://doi.org/10.3390/nu13093182
Chicago/Turabian StyleHan, Chad Yixian, Maria Crotty, Susie Thomas, Ian D. Cameron, Craig Whitehead, Susan Kurrle, Shylie Mackintosh, and Michelle Miller. 2021. "Effect of Individual Nutrition Therapy and Exercise Regime on Gait Speed, Physical Function, Strength and Balance, Body Composition, Energy and Protein, in Injured, Vulnerable Elderly: A Multisite Randomized Controlled Trial (INTERACTIVE)" Nutrients 13, no. 9: 3182. https://doi.org/10.3390/nu13093182
APA StyleHan, C. Y., Crotty, M., Thomas, S., Cameron, I. D., Whitehead, C., Kurrle, S., Mackintosh, S., & Miller, M. (2021). Effect of Individual Nutrition Therapy and Exercise Regime on Gait Speed, Physical Function, Strength and Balance, Body Composition, Energy and Protein, in Injured, Vulnerable Elderly: A Multisite Randomized Controlled Trial (INTERACTIVE). Nutrients, 13(9), 3182. https://doi.org/10.3390/nu13093182






