Short-Term Intake of Yellowstripe Scad versus Salmon Did Not Induce Similar Effects on Lipid Profile and Inflammatory Markers among Healthy Overweight Adults despite Their Comparable EPA+DHA Content
Abstract
:1. Introduction
2. Materials and Methods
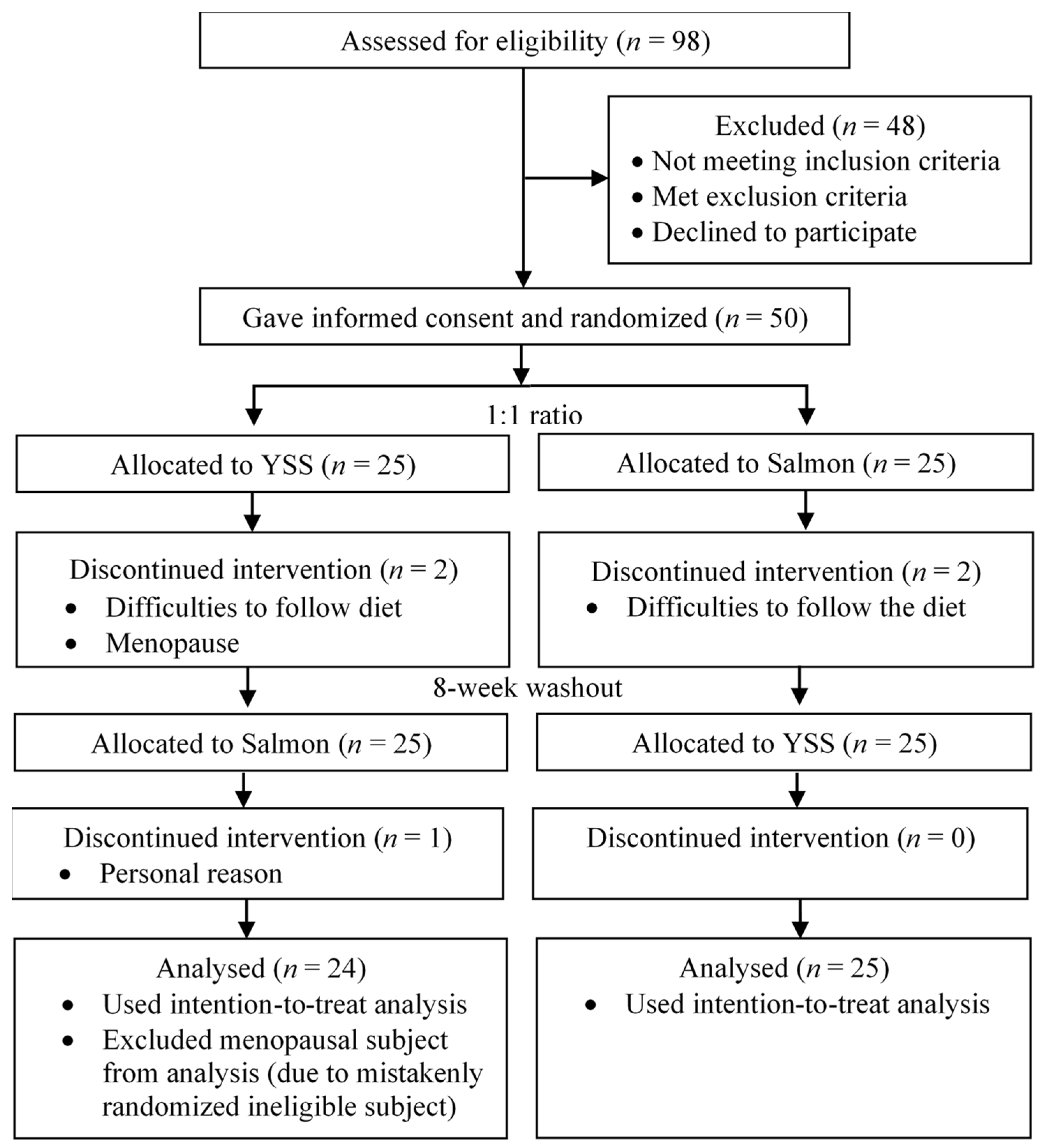
2.1. Study Design and Subjects
2.2. Diet
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Serum EPA+DHA Level
3.2. Blood Lipid Profile
3.3. Inflammatory Markers
3.4. EPA+DHA Content of Fish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaysians Eat More Fish than Japanese, Reveals Study. Available online: https://www.thestar.com.my/news/nation/2014/06/19/malaysians-eat-more-fish-than-japanese-reveals-study (accessed on 30 August 2019).
- Ahmad, N.I.; Wan Mahiyuddin, W.R.; Tengku Mohamad, T.R.; Ling, C.Y.; Daud, S.F.; Hussein, N.C.; Abdullah, N.A.; Shaharudin, R.; Sulaiman, L.H. Fish consumption pattern among adults of different ethnics in Peninsular Malaysia. Food Nutr. Res. 2016, 60, 32697. [Google Scholar] [CrossRef] [Green Version]
- Gammelmark, A.; Nielsen, M.S.; Bork, C.S.; Lundbye-Christensen, S.; Tjønneland, A.; Overvad, K.; Schmidt, E.B. Association of fish consumption and dietary intake of marine n-3 PUFA with myocardial infarction in a prospective Danish cohort study. Br. J. Nutr. 2016, 116, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Grung, B.; Hansen, A.L.; Berg, M.; Møen-Knudseth, M.P.; Olson, G.; Thornton, D.; Dahl, L.; Thayer, J.F. Exploratory multivariate analysis of the effect of fatty fish consumption and medicinal use on heart rate and heart rate variability data. Front. Psychol. 2015, 6, 135. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Park, S.; Yang, H.; Choi, Y.J.; Huh, K.B.; Chang, N. Association between fish and shellfish, and omega-3 PUFAs intake and CVD risk factors in middle-aged female patients with type 2 diabetes. Nutr. Res. Pract. 2015, 9, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Rhee, J.J.; Kim, E.; Buring, J.E.; Kurth, T. Fish consumption, omega-3 fatty acids, and risk of cardiovascular disease. Am. J. Prev. Med. 2017, 52, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Kastelein, J.J.P.; Maki, K.C.; Susekov, A.; Ezhov, M.; Nordestgaard, B.G.; Machielse, B.N.; Kling, D.; Davidson, M.H. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: The EpanoVa for lowering very high triglyceridEs (EVOLVE) trial. J. Clin. Lipidol. 2014, 8, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, C.; Li, L.; Man, Q.; Meng, L.; Song, P.; Frøyland, L.; Du, Z.Y. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br. J. Nutr. 2012, 108, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Daggy, B.P. The role of fish oil in inflammatory eye diseases. Biomed. Hub 2017, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Alhassan, A.; Young, J.; Lean, M.E.; Lara, J. Consumption of fish and vascular risk factors: A systematic review and meta-analysis of intervention studies. Atherosclerosis 2017, 266, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.D. Polyunsaturated fatty acid regulation of gene transcription: A molecular mechanism to improve the metabolic syndrome. J. Nutr. 2001, 131, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Faeh, D.; Minehira, K.; Schwarz, J.M.; Periasamy, R.; Park, S.; Tappy, L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005, 54, 1907–1913. [Google Scholar] [CrossRef] [Green Version]
- Jump, D.B. Fatty acid regulation of hepatic lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 115. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism, 7th ed.; Cengage Learning: Boston, MA, USA, 2017; pp. 126–171. [Google Scholar]
- Nettleton, J.A. Omega-3 Fatty Acids and Health; Springer Science and Business Media: Berlin, Germany, 2012; pp. 1–45. [Google Scholar]
- United States Department of Agriculture (USDA). National Nutrient Database for Standard Reference. Available online: https://fdc.nal.usda.gov/index.html (accessed on 25 May 2017).
- Trout Proud. Available online: https://www.pressreader.com/malaysia/the-star-malaysia-star2/20160605/282127815738258 (accessed on 5 June 2016).
- Blanchet, C.; Lucas, M.; Julien, P.; Morin, R.; Gingras, S.; Dewailly, E. Fatty acid composition of wild and farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Lipids 2005, 40, 529–531. [Google Scholar] [CrossRef]
- Abd Aziz, N.; Azlan, A.; Ismail, A.; Mohd Alinafiah, S.; Razman, M.R. Quantitative determination of fatty acids in marine fish and shellfish from warm water of Straits of Malacca for nutraceutical purposes. BioMed Res. Int. 2013, 2013, 284329. [Google Scholar] [CrossRef]
- Abdulrahman, Y.; Azlan, A.; Peng, L.S.; Ismail, I.Z.; Noor, S.M. Effects of EPA + DHA from yellow-stripe scad and salmon on platelet and endothelial cell-related cytokines of healthy overweight Malaysians. Life Sci. Med. Biomed. 2019, 3, 28. [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Patterson, A.C.; Chalil, A.; Henao, J.J.A.; Streit, I.T.; Stark, K.D. Omega-3 polyunsaturated fatty acid blood biomarkers increase linearly in men and women after tightly controlled intakes of 0.25, 0.5, and 1 g/d of EPA + DHA. Nutr. Res. 2015, 35, 1040–1051. [Google Scholar] [CrossRef]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.; et al. ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar]
- Koubaa, A.; Mihoubi, N.B.; Abdelmouleh, A.; Bouain, A. Comparison of the effects of four cooking methods on fatty acid profiles and nutritional composition of red mullet (Mullus barbatus) muscle. Food Sci. Biotechnol. 2012, 21, 1243–1250. [Google Scholar] [CrossRef]
- Choo, P.Y. Retention of EPA and DHA In Selected Fish Using Different Cooking Methods Compared to Salmon. Bachelor′s Thesis, Universiti Putra Malaysia, Seri Kembangan, Malaysia, 2015. [Google Scholar]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Blinding: Who, what, when, why, how? Can. J. Surg. 2010, 53, 345. [Google Scholar]
- Ministry of Health Malaysia. Malaysian Dietary Guidelines; National Coordinating Committee on Food and Nutrition: Putrajaya, Malaysia, 2010; pp. 1–16.
- Cao, J.; Schwichtenberg, K.A.; Hanson, N.Q.; Tsai, M.Y. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin. Chem. 2006, 52, 2265–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singal, A.G.; Higgins, P.D.R.; Waljee, A.K. A primer on effectiveness and efficacy trials. Clin. Transl. Gastroenterol. 2014, 5, e45. [Google Scholar] [CrossRef] [PubMed]
- Dahl Lassen, A.; Poulsen, S.; Ernst, L.; Kaae Andersen, K.; Biltoft-Jensen, A.; Tetens, I. Evaluation of a digital method to assess evening meal intake in a free-living adult population. Food Nutr. Res. 2010, 54, 5311. [Google Scholar] [CrossRef] [PubMed]
- Wandless, I.; Mucklow, J.C.; Smith, A.; Prudham, D. Compliance with prescribed medicines: A study of elderly patients in the community. J. R. Coll. Gen. Pract. 1979, 29, 391–396. [Google Scholar]
- Beer-Borst, S.; Amado, R. Validation of a self-administered 24-hour recall questionnaire used in a large-scale dietary survey. Eur. J. Nutr. 1995, 34, 183–189. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S.; Pottala, J.V.; Vasan, R.S.; Larson, M.G.; Robins, S.J. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J. Nutr. 2012, 142, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- David, F.; Sandra, P.; Vickers, A.K. Column selection for the analysis of fatty acid methyl esters. Food Anal. Appl. 2005, 19, 19. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Dobiášová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Machin, D.; Campbell, M.J.; Tan, S.B.; Tan, S.H. Sample Size Tables for Clinical Studies, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 58–66. [Google Scholar]
- Phang, M.; Lincz, L.F.; Garg, M.L. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J. Nutr. 2013, 143, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J. Analysis of variance is easily misapplied in the analysis of randomized trials: A critique and discussion of alternative statistical approaches. Psychosom. Med. 2005, 67, 652–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fergusson, D.; Aaron, S.D.; Guyatt, G.; Hébert, P. Post-randomisation exclusions: The intention to treat principle and excluding patients from analysis. Br. Med. J. 2002, 325, 652–654. [Google Scholar] [CrossRef] [Green Version]
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef]
- Cleophas, T.J.M.; De Vogel, E.M. Crossover studies are a better format for comparing equivalent treatments than parallel-group studies. Pharm. World Sci. 1998, 20, 113–117. [Google Scholar] [CrossRef]
- Richens, A. Proof of efficacy trials: Cross-over versus parallel-group. Epilepsy Res. 2001, 45, 43–47. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of fish and krill oil on gene expression in peripheral blood mononuclear cells and circulating markers of inflammation: A randomised controlled trial. J. Nutr. Sci. 2018, 7, e10. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, H.M.; Langkilde, A.M.; Undeland, I.; Sandberg, A.S. Herring (Clupea harengus) intake influences lipoproteins but not inflammatory and oxidation markers in overweight men. Br. J. Nutr. 2009, 101, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Sami Khaza, M. Atherogenic Index of Plasma (AIP) as a parameter in predicting cardiovascular risk in males compared to the conventional dyslipidemic indices (cholesterol ratios). Kerbala J. Med. 2013, 6, 1506–1513. [Google Scholar]
- Derosa, G.; Cicero, A.F.; D′Angelo, A.; Borghi, C.; Maffioli, P. Effects of n-3 PUFAs on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors 2016, 42, 316–322. [Google Scholar]
- Zibaeenezhad, M.J.; Ghavipisheh, M.; Attar, A.; Aslani, A. Comparison of the effect of omega-3 supplements and fresh fish on lipid profile: A randomized, open-labeled trial. Nutr. Diabetes 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devadawson, C.; Jayasinghe, C.; Sivakanesan, R.; Arulnithy, K. Assessment of lipid profile and atherogenic indices for cardiovascular disease risk based on different fish consumption habits. Assessment 2016, 9, 156–160. [Google Scholar]
- Gordon, D.J.; Knoke, J.; Probstfield, J.L.; Superko, R.; Tyroler, H.A. High-density lipoprotein cholesterol and coronary heart disease in hypercholesterolemic men: The lipid research clinics coronary primary prevention trial. Circulation 1986, 74, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, C.; Botella-Carretero, J.I.; Corella, D.; Fiol, M.; Lage, M.; Lurbe, E.; Richart, C.; Fernández-Real, J.M.; Fuentes, F.; Ordóñez, A.; et al. White fish reduces cardiovascular risk factors in patients with metabolic syndrome: The WISH-CARE study, a multicenter randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- 5th Edition of Clinical Practice Guidelines: Management of Dyslipidaemia 2017. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/CPG/CARDIOVASCULAR/4.pdf (accessed on 11 September 2018).
- Pinart, M.; Jeran, S.; Boeing, H.; Stelmach-Mardas, M.; Standl, M.; Schulz, H.; Harris, C.; von Berg, A.; Herberth, G.; Koletzko, S.; et al. Dietary macronutrient composition in relation to circulating HDL and non-HDL cholesterol: A federated individual-level analysis of cross-sectional data from adolescents and adults in 8 European studies. J. Nutr. 2021, 151, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- El Bilbeisi, A.H.; Hosseini, S.; Djafarian, K. The association between physical activity and the metabolic syndrome among type 2 diabetes patients in Gaza strip, Palestine. Ethiop. J. Health Sci. 2017, 27, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieger, J.A.; Miller, M.D.; Cobiac, L. Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr. Res. 2014, 58, 20369. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.K.; Calder, P.C. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: A systematic review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Masui, Y.; Katsuyama, H.; Adachi, H.; Kawaguchi, A.; Hakoshima, M.; Waragai, Y.; Harigae, T.; Sako, A. An improvement of cardiovascular risk factors by omega-3 polyunsaturated fatty acids. J. Clin. Med. Res. 2018, 10, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Giorgi, G.; Cesari, F.; Gori, A.M.; Mannini, L.; Parisi, G.; Casini, A.; Abbate, R.; Gensini, G.F.; Poli, B.M. The atherosclerotic risk profile is affected differently by fish flesh with a similar EPA and DHA content but different n-6/n-3 ratio. Asia Pac. J. Clin. Nutr. 2013, 22, 32–40. [Google Scholar]
- Chang, W.L.; Azrina, A.; Sabariah, M.N.; Ismail, I.Z.; Loh, S.P. Effects of consuming yellowstripe scad versus salmon on lipid profile, fasting glucose, body weight status and blood pressure among healthy overweight Malaysian adults. Malays. J. Nutr. 2017, 23, 343–352. [Google Scholar]
- Fish, Fish Oils, n-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Heart Foundation Position Statement 2008. Available online: https://www.heartfoundation.org.au/getmedia/5195fde5-87f6-4c2a-b7c3-0745a47e5ab7/Summary_Evidence_FISH_FISH-OILS_FINAL.pdf (accessed on 8 July 2019).
- Harris, W.S.; Pottala, J.V.; Sands, S.A.; Jones, P.G. Comparison of the effects of fish and fish-oil capsules on the n–3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 2007, 86, 1621–1625. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Gambell, J.; Madden, J.; Calder, P.C.; Jebb, S.A. Compared with daily, weekly n–3 PUFA intake affects the incorporation of eicosapentaenoic acid and docosahexaenoic acid into platelets and mononuclear cells in humans. J. Nutr. 2014, 144, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Ghasemifard, S.; Sinclair, A.J.; Kaur, G.; Lewandowski, P.; Turchini, G.M. What is the most effective way of increasing the bioavailability of dietary long chain omega-3 fatty acids—Daily vs. weekly administration of fish oil? Nutrients 2015, 7, 5241. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, D.V. A recommended analysis for 2 × 2 crossover trials with baseline measurements. Pharm. Stat. 2014, 13, 376–387. [Google Scholar] [CrossRef]
- Jin, J.; Sklar, G.E.; Oh, V.M.S.; Chuen Li, S. Factors affecting therapeutic compliance: A review from the patient′s perspective. Ther. Clin. Risk Manag. 2008, 4, 269. [Google Scholar]
- Mohd Suan, M.A.; Asli, S.E.; Abdullah, W.M.; Shafie, Z.; Johari, N.H. Patient perspective on factors contributing to nonadherence to dietary therapy: A qualitative study in multicultural population of Kedah, Malaysia. Int. Q. Community Health Educ. 2019, 39, 217–223. [Google Scholar] [CrossRef]
| Diet | Amount Given | Frequency | Corresponding EPA+DHA Content * |
|---|---|---|---|
| Steamed YSS | ≈385 g whole fish/day ** | 3 times (days)/week | 2329 mg/day (≈7000 mg/week) |
| Steamed salmon | ≈246 g fillet/day | 3 times (days)/week | 2330 mg/day (≈7000 mg/week) |
| Food Group | Food |
|---|---|
| Fish and seafood | Salmon |
| Yellowstripe scad | |
| Mackerel | |
| Sardine | |
| Tuna | |
| Trout | |
| Herring | |
| Threadfin bream | |
| Anchovies | |
| Meat, eggs and poultry | Omega-3 enriched eggs |
| Dairy products | Omega-3 enriched milk |
| Nuts and seeds | Flaxseeds/linseeds |
| Chia seeds | |
| Walnuts | |
| Fats and oils | Cod liver oil |
| Flaxseed oil | |
| Omega-3 enriched margarine |
| Socio-Demographic Characteristics | n | % |
|---|---|---|
| Age (years) | ||
| Mean | 29 | |
| SD | 7 | |
| Range | 21–46 | |
| Sex | ||
| Male | 17 | 34.7 |
| Female | 32 | 65.3 |
| Ethnicity | ||
| Malay | 35 | 71.4 |
| Chinese | 12 | 24.5 |
| Indian | 2 | 4.1 |
| Fatty Acid Composition | YSS | Salmon |
|---|---|---|
| Total fat (g/100g) | 3.33 | 15.17 |
| Saturated fat (mg/100 g sample) | 1751.64 | 2557.48 |
| Caprylic acid 8:0 | 1.61 | 0.66 |
| Capric acid 10:0 | 0.00 | 2.38 |
| Undecanoic acid 11:0 | 0.76 | 0.24 |
| Lauric acid 12:0 | 26.29 | 169.65 |
| Tridecanoic acid 13:0 | 2.46 | 1.96 |
| Myristic acid 14:0 | 246.37 | 334.74 |
| Pentadecanoic acid 15:0 | 58.94 | 30.36 |
| Palmitic acid 16:0 | 895.24 | 1462.68 |
| Heptadecanoic acid 17:0 | 25.97 | 35.65 |
| Stearic acid 18:0 | 375.17 | 405.72 |
| Arachidic acid 20:0 | 24.45 | 53.39 |
| Henicosanoic acid 21:0 | 7.32 | 4.44 |
| Behenic acid 22:0 | 21.26 | 27.80 |
| Tricosanoic acid 23:0 | 55.26 | 19.84 |
| Lignoceric acid 24:0 | 10.58 | 7.98 |
| Monounsaturated fat (mg/100 g sample) | 561.46 | 8017.96 |
| Myristoleic acid 14:1 | 1.42 | 6.05 |
| Cis-10-pentadecenoic acid 15:1 | 0.13 | 3.42 |
| Palmitoleic acid 16:1 | 254.24 | 374.85 |
| Cis-10-heptadecanoic acid 17:1 | 63.41 | 32.69 |
| Elaidic acid 18:1n9t | 3.60 | 0.00 |
| Oleic acid 18:1n9c | 210.96 | 6870.41 |
| Cis-11-eicosenoic acid 20:1n9 | 5.48 | 324.02 |
| Erucic acid 22:1n9 | 4.21 | 350.91 |
| Nervonic acid 24:1 | 18.02 | 55.61 |
| Polyunsaturated fat (mg/100 g sample) | 1020.83 | 4593.94 |
| Linolelaidic acid 18:2n6t | 0.00 | 17.04 |
| Linoleic acid 18:2n6c | 73.65 | 2274.80 |
| γ-Linolenic acid 18:3n6 | 13.19 | 40.29 |
| α-Linolenic acid 18:3n3 | 28.52 | 894.95 |
| Cis-11,14-eicosadienoic acid 20:2n6 | 14.54 | 173.17 |
| Cis-8,11,14-eicosatrienoic acid 20:3n6 | 7.57 | 48.79 |
| Cis-11,14,17-eicosatrienoic acid 20:3n3 | 99.92 | 40.15 |
| Arachidonic acid 20:4n6 | 3.49 | 74.86 |
| Cis-5,8,11,14,17-eicosapentaenoic acid 20:5n3 | 214.16 | 400.52 |
| Docosadienoic acid 22:2 | 10.16 | 18.75 |
| Cis-4,7,10,13,16,19-docosahexaenoic acid 22:6n3 | 555.66 | 610.63 |
| YSS | Salmon | Between−Group Difference 2 | 95% CI | p Value ** | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline 1 | Week 8 | p Value * | Baseline 1 | Week 8 | p Value * | ||||
| Dietary intake | |||||||||
| Energy (kcal/day) | 1359 (368) | 1448 (487) | 0.16 | 1356 (357) | 1413 (548) | 0.44 | 32.00 | −135; 206 | 0.06 |
| Carbohydrate (g/day) | 58.61 (23.82) | 68.29 (25.66) | 0.06 | 58.79 (20.63) | 60.76 (27.11) | 0.49 | 7.71 | −1.64; 16.71 | 0.18 |
| Protein (g/day) | 173.78 (52.23) | 181.01 (68.33) | 0.42 | 173.47 (45.44) | 180.08 (65.52) | 0.44 | 0.62 | −19.78; 21.63 | 0.10 |
| Total Fat (g/day) | 47.61 (18.75) | 50.28 (26.37) | 0.31 | 47.53 (17.73) | 49.93 (25.82) | 0.49 | 0.27 | −7.59; 8.30 | 0.10 |
| PUFA 20:5, EPA (g/day) 3 | 0.01 (0.04) | 0.00 (0.02) | 0.34 § | 0.01 (0.03) | 0.03 (0.15) | 0.002 § | −0.03 | 0.00; 0.05 | 0.01 † |
| PUFA 22:6, DHA (g/day) 3 | 0.04 (0.11) | 0.04 (0.11) | 0.74 § | 0.03 (0.06) | 0.06 (0.20) | 0.002 § | −0.03 | 0.01; 0.15 | 0.24 † |
| Saturated fat (g/day) | 13.66 (6.84) | 11.34 (6.51) | 0.66 | 14.76 (10.56) | 13.40 (10.87) | 0.37 | −0.96 | 1.55; 5.67 | 0.71 |
| Total PA (MET-min/wk) | 4134 (3571) | 3909 (4598) | 0.09 | 5326 (6298) | 5157 (5273) | 0.55 | −56 | −2826; 331 | 0.24 |
| Body mass index (kg/m2) | 25.20 (1.57) | 25.14 (1.69) | 0.25 | 25.29 (1.59) | 25.27 (1.66) | 0.78 | −0.040 | −0.28; 0.03 | 0.48 |
| Serum EPA+DHA (ug/mL) | 94.31 (150.56) | 100.46 (90.34) | 0.81 | 98.21 (78.99) | 123.97 (116.48) | 0.11 | −19.61 | −45.95; 14.04 | 0.29 |
| Serum EPA | 3.80 (16.02) | 2.05 (10.06) | 0.43 | 4.47 (13.75) | 13.75 (26.62) | 0.04 | −11.03 | −8.63; 1.10 | 0.13 |
| Serum DHA | 90.51 (150.07) | 98.41 (89.65) | 0.76 | 93.73 (78.91) | 110.22 (114.85) | 0.32 | −8.59 | −41.73; 17.34 | 0.41 |
| Blood lipid profile | |||||||||
| Total cholesterol (mmol/1) | 5.18 (0.83) | 5.19 (0.97) | 0.71 | 5.12 (0.15) | 5.24 (0.89) | 0.14 | −0.11 | −0.24; 0.12 | 0.92 |
| Triglycerides (mmol/1) | 1.11 (0.52) | 1.04 (0.47) | 0.38 | 1.06 (0.49) | 0.90 (0.38) | <0.001 | 0.09 | 0.06; 0.22 | 0.01 |
| HDL-cholesterol (mmol/1) | 1.56 (0.27) | 1.55 (0.30) | 0.88 | 1.56 (0.37) | 1.62 (0.29) | 0.008 | −0.07 | −0.13; −0.01 | 0.41 |
| LDL-cholesterol (mmol/1) | 3.12 (0.77) | 3.11 (0.85) | 0.86 | 3.08 (0.88) | 3.19 (0.82) | 0.13 | -0.12 | −0.23; 0.08 | 0.20 |
| VLDL-cholesterol (mmol/1) | 0.51 (0.23) | 0.48 (0.22) | 0.55 | 0.48 (0.22) | 0.41 (0.17) | <0.001 | 0.04 | 0.03; 0.11 | 0.01 |
| Atherogenic coefficient | 2.43 (0.76) | 2.44 (0.88) | 0.79 | 2.39 (0.86) | 2.34 (0.83) | 0.43 | 0.06 | −0.04; 0.18 | 0.19 |
| AIP | −0.18 (0.22) | −0.21 (0.22) | 0.31 | −0.20 (0.24) | −0.28 (0.20) | <0.001 | 0.05 | 0.01; 0.08 | 0.006 |
| Inflammatory markers (pg/ml) 4 | |||||||||
| IL-1β | 1.17 (0.16) | 1.14 (0.16) | 0.77 | 1.13 (0.15) | 1.13 (0.16) | 0.19 | −0.03 | −0.02; 0.05 | 0.97 |
| IL-6 | 0.69 (0.21) | 0.66 (0.21) | 0.43 | 0.68 (0.18) | 0.64 (0.20) | 0.03 | 0.01 | −0.04; 0.07 | 0.03 |
| TNF-α | 1.38 (0.19) | 1.36 (0.21) | 0.19 | 1.37 (0.21) | 1.34 (0.21) | 0.007 | 0.01 | −0.01; 0.04 | 0.95 |
| IFN-γ | 2.15 (0.36) | 2.12 (0.36) | 0.91 | 2.10 (0.33) | 2.11 (0.35) | 0.43 | −0.04 | −0.03; 0.05 | 0.25 |
| Nutrient Content (mg/100 g Sample) | YSS | Salmon | Between-Group Difference § | 95% CI | p Value |
|---|---|---|---|---|---|
| Total EPA+DHA | 769.82 (80.48) | 1011.16 (94.40) | −241.35 | −627.95; 145.26 | 0.11 |
| EPA | 214.16 (22.77) | 400.53 (17.32) | −186.37 | −273.40; −99.33 | 0.01 |
| DHA | 555.66 (57.71) | 610.64 (77.08) | −54.98 | −347.93; 237.97 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.L.; Azlan, A.; Noor, S.M.; Ismail, I.Z.; Loh, S.P. Short-Term Intake of Yellowstripe Scad versus Salmon Did Not Induce Similar Effects on Lipid Profile and Inflammatory Markers among Healthy Overweight Adults despite Their Comparable EPA+DHA Content. Nutrients 2021, 13, 3524. https://doi.org/10.3390/nu13103524
Chang WL, Azlan A, Noor SM, Ismail IZ, Loh SP. Short-Term Intake of Yellowstripe Scad versus Salmon Did Not Induce Similar Effects on Lipid Profile and Inflammatory Markers among Healthy Overweight Adults despite Their Comparable EPA+DHA Content. Nutrients. 2021; 13(10):3524. https://doi.org/10.3390/nu13103524
Chicago/Turabian StyleChang, Wei Lin, Azrina Azlan, Sabariah Md Noor, Irmi Zarina Ismail, and Su Peng Loh. 2021. "Short-Term Intake of Yellowstripe Scad versus Salmon Did Not Induce Similar Effects on Lipid Profile and Inflammatory Markers among Healthy Overweight Adults despite Their Comparable EPA+DHA Content" Nutrients 13, no. 10: 3524. https://doi.org/10.3390/nu13103524
APA StyleChang, W. L., Azlan, A., Noor, S. M., Ismail, I. Z., & Loh, S. P. (2021). Short-Term Intake of Yellowstripe Scad versus Salmon Did Not Induce Similar Effects on Lipid Profile and Inflammatory Markers among Healthy Overweight Adults despite Their Comparable EPA+DHA Content. Nutrients, 13(10), 3524. https://doi.org/10.3390/nu13103524








