Lifestyle, WCRF/AICR Recommendations, and Esophageal Adenocarcinoma Risk: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Evaluation
3. Results
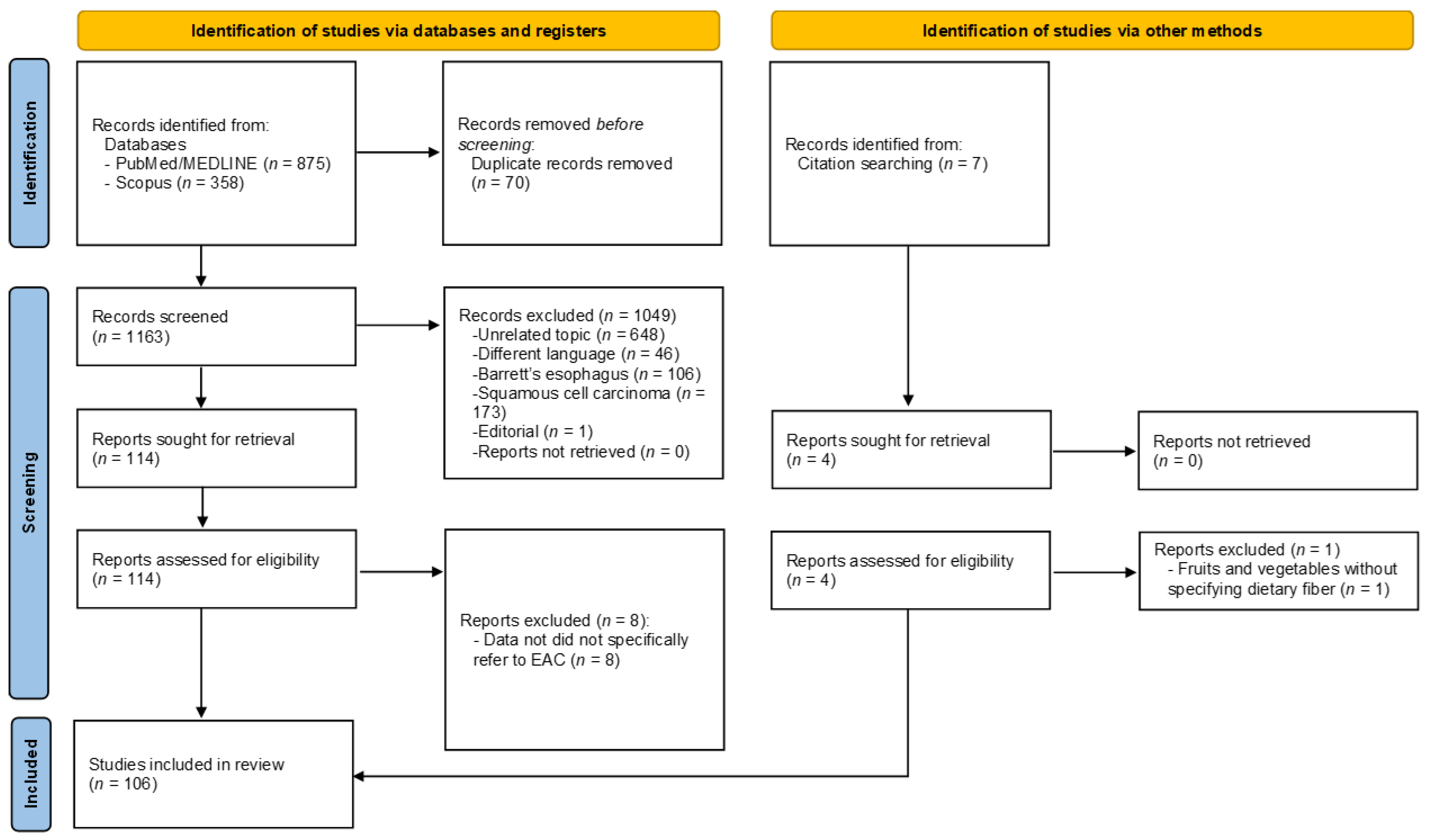
3.1. Literature Search and Quality Evaluation
3.2. Anthropometric Measures
3.3. Physical Activity
3.4. Dietary Patterns, Food Groups, and Beverages
3.4.1. Foods of Plant Origin and Dietary Fiber
3.4.2. Animal Products
3.4.3. Consumption of Nonalcoholic Beverages
3.5. Vitamins, Minerals, and Other Nutrients
3.5.1. Vitamin C
3.5.2. Vitamin E
3.5.3. Vitamin A and Carotenoids
3.5.4. B Vitamins
3.5.5. Vitamin D and Calcium
3.5.6. Iron
3.5.7. Other Compounds
3.5.8. Dietary Supplements
3.6. Cooking Process and Chemical Modification during Cooking
3.7. Alcohol
3.8. Smoking
3.9. Interaction between Smoking and Alcohol
3.10. Socioeconomic Factors and EAC Risk
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer Press: Lyon, France, 2020. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/tomorrow (accessed on 12 June 2021).
- Pera, M.; Manterola, C.; Vidal, O.; Grande, L. Epidemiology of esophageal adenocarcinoma. J. Surg. Oncol. 2005, 92, 151–159. [Google Scholar] [CrossRef]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef]
- Dikken, J.L.; Lemmens, V.E.; Wouters, M.W.; Wijnhoven, B.P.; Siersema, P.D.; Nieuwenhuijzen, G.A.; Van Sandick, J.W.; Cats, A.; Verheij, M.; Coebergh, J.W.; et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur. J. Cancer 2012, 48, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Simard, E.P.; Ward, E.M.; Siegel, R.; Jemal, A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012, 62, 118–128. [Google Scholar] [CrossRef]
- Kubo, A.; Corley, D.A.; Jensen, C.D.; Kaur, R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett’s oesophagus. Nutr. Res. Rev. 2010, 23, 230–246. [Google Scholar] [CrossRef]
- World Cancer Research Fund & American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- World Cancer Research Fund & American Institute for Cancer Research. Continuous Update Project Report Diet, Nutrition, Physical Activity and Oesophageal Cancer. Available online: Dietandcancerreport.org (accessed on 12 June 2021).
- Singh, S.; Devanna, S.; Varayil, J.E.; Murad, M.H.; Iyer, P.G. Physical activity is associated with reduced risk of esophageal cancer, particularly esophageal adenocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2014, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, B.; Hao, C. Coffee consumption and risk of esophageal cancer incidence: A meta-analysis of epidemiologic studies. Medicine 2018, 97, e0514. [Google Scholar] [CrossRef] [PubMed]
- Asombang, A.W.; Chishinga, N.; Nkhoma, A.; Chipaila, J.; Nsokolo, B.; Manda-Mapalo, M.; Montiero, J.F.G.; Banda, L.; Dua, K.S. Systematic review and meta-analysis of esophageal cancer in Africa: Epidemiology, risk factors, management and outcomes. World J. Gastroenterol. 2019, 25, 4512–4533. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Zhou, M.; Whitlock, G.; Yang, G.; Offer, A.; Hui, G.; Peto, R.; Huang, Z.; Chen, Z. Esophageal cancer and body mass index: Results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int. J. Cancer 2007, 122, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Gianfredi, V.; Fatigoni, C. Lifestyle and Risk of Esophageal Adenocarcinoma, a Systematic Review. 2021. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD (accessed on 5 February 2021).
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Fatigoni, C.; Amerio, A.; Odone, A.; Gianfredi, V. Red and processed meat consumption and risk of depression: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6686. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Paterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; The Ottawa Hospital: Ottawa, ON, Canada, 2014; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 14 January 2021).
- Herzog, R.; Alvarez-Pasquin, M.J.; Diaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Nucci, D.; Fatigoni, C.; Salvatori, T.; Nardi, M.; Realdon, S.; Gianfredi, V. Association between dietary fibre intake and colorectal adenoma: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 4168. [Google Scholar] [CrossRef]
- Den Hoed, C.M.; Van Blankenstein, M.; Dees, J.; Kuipers, E.J. The minimal incubation period from the onset of Barrett’s oesophagus to symptomatic adenocarcinoma. Br. J. Cancer 2011, 105, 200–205. [Google Scholar] [CrossRef]
- Adair, T.; Hoy, D.; Dettrick, Z.; Lopez, A.D. Trends in oral, pharyngeal and oesophageal cancer mortality in Australia: The comparative importance of tobacco, alcohol and other risk factors. Aust. N. Z. J. Public Health 2011, 35, 212–219. [Google Scholar] [CrossRef]
- Ali, A.; Ersumo, T.; Johnson, O. Oesophageal carcinoma in Tikur Anbessa Hospital, Addis Ababa. East Afr. Med. J. 1998, 75, 590–593. [Google Scholar]
- Chyou, P.H.; Nomura, A.M.; Stemmermann, G.N. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: A prospective study among Hawaii Japanese men. Int. J. Cancer 1995, 60, 616–621. [Google Scholar] [CrossRef]
- Kinjo, Y.; Cui, Y.; Akiba, S.; Watanabe, S.; Yamaguchi, N.; Sobue, T.; Mizuno, S.; Beral, V. Mortality risks of oesophageal cancer associated with hot tea, alcohol, tobacco and diet in Japan. J. Epidemiol. 1998, 8, 235–243. [Google Scholar] [CrossRef]
- Kjaerheim, K.; Gaard, M.; Andersen, A. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: A prospective study of 10,900 Norwegian men. Cancer Causes Control 1998, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Su, M.; Tian, D.P.; Zhang, G.H.; Yang, H.L.; Gao, Y.X. Heredity, diet and lifestyle as determining risk factors for the esophageal cancer on Nanao Island in Southern China. Fam. Cancer 2009, 9, 229–238. [Google Scholar] [CrossRef]
- Yi, S.W.; Hong, J.S.; Yi, J.J.; Ohrr, H. Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: Prospective cohort study. Medicine 2016, 95, e4876. [Google Scholar] [CrossRef]
- Yi, S.W.; Sull, J.W.; Linton, J.A.; Nam, C.M.; Ohrr, H. Alcohol consumption and digestive cancer mortality in Koreans: The Kangwha Cohort Study. J. Epidemiol. 2010, 20, 204–211. [Google Scholar] [CrossRef]
- Anderson, L.A.; Cantwell, M.M.; Watson, R.G.P.; Johnston, B.T.; Murphy, S.J.; Ferguson, H.R.; McGuigan, J.; Comber, H.; Reynolds, J.V.; Murray, L.J. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology 2009, 136, 799–805. [Google Scholar] [CrossRef]
- Anderson, L.A.; Watson, R.G.; Murphy, S.J.; Johnston, B.T.; Comber, H.; Mc Guigan, J.; Reynolds, J.V.; Murray, L.J. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: Results from the FINBAR study. World J. Gastroenterol. 2007, 13, 1585–1594. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Ye, W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: A population-based case-control study in Sweden. Nutr. Cancer 2006, 54, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bollschweiler, E.; Wolfgarten, E.; Nowroth, T.; Rosendahl, U.; Mönig, S.P.; Hölscher, A.H. Vitamin intake and risk of subtypes of esophageal cancer in Germany. J. Cancer Res. Clin. Oncol. 2002, 128, 575–580. [Google Scholar] [CrossRef]
- Chen, H.; Tucker, K.L.; Graubard, B.I.; Heineman, E.F.; Markin, R.S.; Potischman, N.A.; Russell, R.M.; Weisenburger, D.D.; Ward, M.H. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer 2002, 42, 33–40. [Google Scholar] [CrossRef]
- Chen, H.; Ward, M.H.; Graubard, B.I.; Heineman, E.F.; Markin, R.M.; Potischman, N.A.; Russell, R.M.; Weisenburger, D.D.; Tucker, K. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am. J. Clin. Nutr. 2002, 75, 137–144. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, N.; Ling, Y.; Wakai, T.; He, Y.; Wei, L.; Wang, S.; Akazawa, K. Alcohol consumption as a risk factor for esophageal adenocarcinoma in North China. Tohoku J. Exp. Med. 2011, 224, 21–27. [Google Scholar] [CrossRef]
- Cheng, K.K.; Sharp, L.; McKinney, P.A.; Logan, R.F.A.; Chilvers, C.E.D.; Cook-Mozaffari, P.; Ahmed, A.; Day, N.E. A case-control study of oesophageal adenocarcinoma in women: A preventable disease. Br. J. Cancer 2000, 83, 127–132. [Google Scholar] [CrossRef]
- Chow, W.-H.; Blot, W.J.; Vaughan, T.L.; Risch, H.A.; Gammon, M.D.; Stanford, J.L.; Dubrow, R.; Schoenberg, J.B.; Mayne, S.T.; Farrow, D.C.; et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J. Natl. Cancer Inst. 1998, 90, 150–155. [Google Scholar] [CrossRef]
- Corley, D.A.; Kubo, A.; Zhao, W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol. Biomark. Prev. 2008, 17, 352–358. [Google Scholar] [CrossRef]
- Dai, Q.; Cantwell, M.M.; Murray, L.J.; Zheng, W.; Anderson, L.A.; Coleman, H.G. Dietary magnesium, calcium: Magnesium ratio and risk of reflux oesophagitis, Barrett’s oesophagus and oesophageal adenocarcinoma: A population-based case-control study. Br. J. Nutr. 2016, 115, 342–350. [Google Scholar] [CrossRef]
- De Jonge, P.J.F.; Steyerberg, E.; Kuipers, E.J.; Honkoop, P.; Wolters, L.M.M.; Kerkhof, M.; Van Dekken, H.; Siersema, P.D. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am. J. Gastroenterol. 2006, 101, 1421–1429. [Google Scholar] [CrossRef]
- Drahos, J.; Li, L.; Jick, S.S.; Cook, M.B. Metabolic syndrome in relation to Barrett’s esophagus and esophageal adenocarcinoma: Results from a large population-based case-control study in the Clinical Practice Research Datalink. Cancer Epidemiol. 2016, 42, 9–14. [Google Scholar] [CrossRef]
- Duan, L.; Wu, A.H.; Sullivan-Halley, J.; Bernstein, L. Passive smoking and risk of oesophageal and gastric adenocarcinomas. Br. J. Cancer 2009, 100, 1483–1485. [Google Scholar] [CrossRef]
- Engel, L.S.; Chow, W.; Vaughan, T.L.; Gammon, M.D.; Risch, H.A.; Stanford, J.L.; Schoenberg, J.B.; Mayne, S.T.; Dubrow, R.; Rotterdam, H.; et al. Population attributable risks of esophageal and gastric cancers. J. Natl. Cancer Inst. 2003, 95, 1404–1413. [Google Scholar] [CrossRef]
- Gammon, M.D.; Schoenberg, J.B.; Ahsan, H.; Risch, H.A.; Vaughan, T.L.; Chow, W.-H.; Rotterdam, H.; West, A.B.; Dubrow, R.; Stanford, J.L.; et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J. Natl. Cancer Inst. 1997, 89, 1277–1284. [Google Scholar] [CrossRef]
- Gao, Y.-T.; McLaughlin, J.K.; Blot, W.J.; Ji, B.-T.; Bénichou, J.; Dai, Q.; Fraumeni, J.F. Risk factors for esophageal cancer in Shanghai, China. I. Role of cigarette smoking and alcohol drinking. Int. J. Cancer 1994, 58, 192–196. [Google Scholar] [CrossRef]
- Garidou, A.; Tzonou, A.; Lipworth, L.; Signorello, L.B.; Kalapothaki, V.; Trichopoulos, D. Life-style factors and medical, conditions in relation to esophageal cancer by histologic type in a low-risk population. Int. J. Cancer 1996, 68, 295–299. [Google Scholar] [CrossRef]
- Hashibe, M.; Boffetta, P.; Janout, V.; Zaridze, D.; Shangina, O.; Mates, D.; Szeszenia-Dabrowska, N.; Bencko, V.; Brennan, P. Esophageal cancer in Central and Eastern Europe: Tobacco and alcohol. Int. J. Cancer 2007, 120, 1518–1522. [Google Scholar] [CrossRef]
- Ibiebele, T.I.; Hughes, M.C.; Whiteman, D.C.; Webb, P.M. Dietary patterns and risk of oesophageal cancers: A population-based case-control study. Br. J. Nutr. 2012, 107, 1207–1216. [Google Scholar] [CrossRef]
- Ibiebele, T.I.; Hughes, M.C.; Nagle, C.M.; Bain, C.J.; Whiteman, D.C.; Webb, P.M. Dietary antioxidants and risk of Barrett’s esophagus and adenocarcinoma of the esophagus in an Australian population. Int. J. Cancer 2013, 133, 214–224. [Google Scholar] [CrossRef]
- Jansson, C.; Johansson, A.L.; Nyren, O.; Lagergren, J. Socioeconomic factors and risk of esophageal adenocarcinoma: A nationwide Swedish case-control study. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1754–1761. [Google Scholar] [CrossRef]
- Lagergren, J.; Bergström, R.; Nyrén, O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann. Intern. Med. 1999, 130, 883–890. [Google Scholar] [CrossRef]
- Lagergren, J.; Bergström, R.; Lindgren, A.; Nyrén, O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int. J. Cancer 2000, 85, 340–346. [Google Scholar] [CrossRef]
- Lagergren, J.; Viklund, P.; Jansson, C. Carbonated soft drinks and risk of esophageal adenocarcinoma: A population-based case-control study. J. Natl. Cancer Inst. 2006, 98, 1158–1161. [Google Scholar] [CrossRef]
- Lagergren, K.; Lindam, A.; Lagergren, J. Dietary proportions of carbohydrates, fat, and protein and risk of oesophageal cancer by histological type. PLoS ONE 2013, 8, e54913. [Google Scholar] [CrossRef]
- Lagergren, J.; Mattsson, F.; Nyrén, O. Gastroesophageal reflux does not alter effects of body mass index on risk of esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2014, 12, 45–51. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Ibiebele, T.I.; Webb, P.M.; Nagle, C.M.; Whiteman, D.C. A case-control study of glycemic index, glycemic load and dietary fiber intake and risk of adenocarcinomas and squamous cell carcinomas of the esophagus: The Australian Cancer Study. BMC Cancer 2014, 14, 877. [Google Scholar] [CrossRef]
- Li, N.; Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; McClain, K.M.; Niehoff, N.M.; Engel, L.S.; Shaheen, N.J.; Risch, H.A.; Vaughan, T.L.; et al. A pooled analysis of dietary sugar/carbohydrate intake and esophageal and gastric cardia adenocarcinoma incidence and survival in the USA. Int. J. Epidemiol. 2017, 46, 1836–1846. [Google Scholar] [CrossRef]
- Lin, Y.; Lagergren, J.; Lu, Y. Dietary acrylamide intake and risk of esophageal cancer in a population-based case-control study in Sweden. Int. J. Cancer 2011, 128, 676–681. [Google Scholar] [CrossRef]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. A dietary pattern rich in lignans, quercetin and resveratrol decreases the risk of oesophageal cancer. Br. J. Nutr. 2014, 112, 2002–2009. [Google Scholar] [CrossRef]
- Lin, Y.; Yngve, A.; Lagergren, J.; Lu, Y. Dietary intake of lignans and risk of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer Causes Control 2012, 23, 837–844. [Google Scholar] [CrossRef]
- Lindblad, M.; Rodríguez, L.A.; Lagergren, J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control 2005, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shivappa, N.; Lin, Y.; Lagergren, J.; Hébert, J.R. Diet-related inflammation and oesophageal cancer by histological type: A nationwide case-control study in Sweden. Eur. J. Nutr. 2015, 55, 1683–1694. [Google Scholar] [CrossRef]
- Massl, R.; van Blankenstein, M.; Jeurnink, S.; Hermans, J.J.; de Haan, M.C.; Stoker, J.; Koek, M.; Niessen, W.J.; Steyerberg, E.W.; Looman, C.W.; et al. Visceral adipose tissue: The link with esophageal adenocarcinoma. Scand. J. Gastroenterol. 2014, 49, 449–457. [Google Scholar] [CrossRef]
- Mayne, S.T.; Risch, H.A.; Dubrow, R.; Chow, W.H.; Gammon, M.D.; Vaughan, T.L.; Farrow, D.C.; Schoenberg, J.B.; Stanford, J.L.; Ahsan, H.; et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1055–1062. [Google Scholar]
- Mayne, S.T.; Risch, H.A.; Dubrow, R.; Chow, W.-H.; Gammon, M.D.; Vaughan, T.L.; Borchardt, L.; Schoenberg, J.B.; Stanford, J.L.; West, A.B.; et al. Carbonated soft drink consumption and risk of esophageal adenocarcinoma. J. Natl. Cancer Inst. 2006, 98, 72–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulholland, H.G.; Murray, L.J.; Anderson, L.A.; Cantwell, M.M. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br. J. Nutr. 2011, 106, 732–741. [Google Scholar] [CrossRef]
- Murphy, S.J.; Ferguson, H.R.; Johnston, B.T.; Watson, P.R.; Murray, L.J.; Cantwell, M.M.; Anderson, L.A.; McGuigan, J.; Comber, H.; Reynolds, J.V. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett’s esophagus. J. Nutr. 2010, 140, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.A.N.; Mayne, S.T.; Risch, H.; Gammon, M.D.; Vaughan, T.L.; Chow, W.-H.; Dubrow, R.; Schoenberg, J.B.; Stanford, J.L.; West, A.B.; et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int. J. Cancer 2008, 123, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.A.N.; Mayne, S.T.; Risch, H.A.; Gammon, M.D.; Vaughan, T.; Chow, W.-H.; Dubin, J.A.; Dubrow, R.; Schoenberg, J.; Stanford, J.L.; et al. Principal component analysis of dietary and lifestyle patterns in relation to risk of subtypes of esophageal and gastric cancer. Ann. Epidemiol. 2011, 21, 543–550. [Google Scholar] [CrossRef]
- Silvera, S.A.N.; Mayne, S.T.; Gammon, M.D.; Vaughan, T.L.; Chow, W.-H.; Dubin, J.A.; Dubrow, R.; Stanford, J.L.; West, A.B.; Rotterdam, H.; et al. Diet and lifestyle factors and risk of subtypes of esophageal and gastric cancers: Classification tree analysis. Ann. Epidemiol. 2014, 24, 50–57. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Abnet, C.; Murray, L.J.; Woodside, J.; Anderson, L.; Brockman, J.; Cantwell, M.M. Iron intake and markers of iron status and risk of Barrett’s esophagus and esophageal adenocarcinoma. Cancer Causes Control 2010, 21, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, M.G.; Cantwell, M.M.; Murray, L.J.; Anderson, L.A.; Abnet, C.C. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Cancer 2011, 129, 1493–1502. [Google Scholar] [CrossRef]
- Olsen, C.M.; Pandeya, N.; Green, A.C.; Webb, P.; Whiteman, D.C. Population attributable fractions of adenocarcinoma of the esophagus and gastroesophageal junction. Am. J. Epidemiol. 2011, 174, 582–590. [Google Scholar] [CrossRef]
- Pandeya, N.; Williams, G.; Sadhegi, S.; Green, A.C.; Webb, P.; Whiteman, D.C. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am. J. Epidemiol. 2011, 168, 105–114. [Google Scholar] [CrossRef]
- Pandeya, N.; Williams, G.; Green, A.C.; Webb, P.M.; Whiteman, D.C. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009, 136, 1215–1224.e2. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, N.; Webb, P.M.; Sadeghi, S.; Green, A.C.; Whiteman, D.C. Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: Are the effects modified by smoking, NSAIDs or acid suppressants? Gut 2009, 59, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Steck, S.E.; Bradshaw, P.T.; Trivers, K.F.; Abrahamson, P.E.; Engel, L.; He, K.; Chow, W.-H.; Mayne, S.T.; Risch, H.A.; et al. Dietary intake of flavonoids and oesophageal and gastric cancer: Incidence and survival in the United States of America (USA). Br. J. Cancer 2015, 112, 1291–1300. [Google Scholar] [CrossRef]
- Pohl, H.; Wrobel, K.; Bojarski, C.; Voderholzer, W.; Sonnenberg, A.; Rösch, T.; Baumgart, D.C. Risk factors in the development of esophageal adenocarcinoma. Am. J. Gastroenterol. 2013, 108, 200–207. [Google Scholar] [CrossRef]
- Ryan, A.M.; Rowley, S.P.; Fitzgerald, A.P.; Ravi, N.; Reynolds, J.V. Adenocarcinoma of the oesophagus and gastric cardia: Male preponderance in association with obesity. Eur. J. Cancer 2006, 42, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Sharp, L.; Carsin, A.E.; Cantwell, M.M.; Anderson, L.A.; Murray, L.J. Intakes of dietary folate and other B vitamins are associated with risks of esophageal adenocarcinoma, Barrett’s esophagus, and reflux esophagitis. J. Nutr. 2013, 143, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Lagergren, J.; Ye, W.; Nyrén, O.; Wolk, A. Antioxidants and cancers of the esophagus and gastric cardia. Int. J. Cancer 2000, 87, 750–754. [Google Scholar] [CrossRef]
- Terry, P.; Lagergren, J.; Hansen, H.; Wolk, A.; Nyrén, O. Fruit and vegetable consumption in the prevention of oesophageal and cardia cancers. Eur. J. Cancer Prev. 2001, 10, 365–369. [Google Scholar] [CrossRef]
- Terry, P.D.; Lagergren, J.; Wolk, A.; Steineck, G.; Nyrén, O. Dietary intake of heterocyclic amines and cancers of the esophagus and gastric cardia. Cancer Epidemiol. Biomark. Prev. 2003, 12, 940–944. [Google Scholar]
- Thrift, A.P.; Shaheen, N.J.; Gammon, M.D.; Bernstein, L.; Reid, B.J.; Onstad, L.; Risch, H.A.; Liu, G.; Bird, N.C.; Wu, A.H.; et al. Obesity and risk of esophageal adenocarcinoma and barrett’s esophagus: A mendelian randomization study. J. Natl. Cancer Inst. 2014, 106, 111–dju252. [Google Scholar] [CrossRef]
- Tzonou, A.; Lipworth, L.; Garidou, A.; Signorello, L.B.; Lagiou, P.; Hsieh, C.C.; Trichopoulos, D. Diet and risk of esophageal cancer by histologic type in a low-risk population. Int. J. Cancer 1996, 68, 300–304. [Google Scholar] [CrossRef]
- Veugelers, P.J.; Porter, G.A.; Guernsey, D.L.; Casson, A.G. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis. Esophagus 2006, 19, 321–328. [Google Scholar] [CrossRef]
- Vigen, C.; Bernstein, L.; Wu, A.H. Occupational physical activity and risk of adenocarcinomas of the esophagus and stomach. Int. J. Cancer. 2006, 118, 1004–1009. [Google Scholar] [CrossRef]
- Ward, M.H.; Sinha, R.; Heineman, E.F.; Rothman, N.; Markin, R.; Weisenburger, D.D.; Correa, P.; Zahm, S.H. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int. J. Cancer 1997, 71, 14–19. [Google Scholar] [CrossRef]
- Ward, M.H.; Heineman, E.F.; Markin, R.S.; Weisenburger, D.D. Adenocarcinoma of the stomach and esophagus and drinking water and dietary sources of nitrate and nitrite. Int. J. Occup. Environ. Health 2008, 14, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Cross, A.J.; Abnet, C.C.; Sinha, R.; Markin, R.S.; Weisenburger, D.D. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur. J. Cancer Prev. 2012, 21, 134–138. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Sadeghi, S.; Pandeya, N.; Smithers, B.M.; Gotley, D.; Bain, C.; Webb, P.; Green, A.C. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut 2007, 57, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wolfgarten, E.; Rosendahl, U.; Nowroth, T.; Leers, J.; Metzger, R.; Hölscher, A.; Bollschweiler, E. Coincidence of nutritional habits and esophageal cancer in Germany. Onkologie 2001, 24, 546–551. [Google Scholar] [CrossRef]
- Wu, A.H.; Wan, P.; Bernstein, L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001, 12, 721–732. [Google Scholar] [CrossRef]
- Wu, A.H.; Tseng, C.C.; Hankin, J.; Bernstein, L. Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control 2007, 18, 713–722. [Google Scholar] [CrossRef]
- Abnet, C.C.; Freedman, N.D.; Hollenbeck, A.R.; Fraumeni, J.F.; Leitzmann, M., Jr.; Schatzkin, A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur. J. Cancer 2008, 44, 465–471. [Google Scholar] [CrossRef]
- Allen, N.E.; Beral, V.; Casabonne, D.; Kan, S.W.; Reeves, G.K.; Brown, A.; Green, J. Moderate alcohol intake and cancer incidence in women. J. Natl. Cancer Inst. 2009, 101, 296–305. [Google Scholar] [CrossRef]
- Carman, S.; Kamangar, F.; Freedman, N.D.; Wright, M.E.; Dawsey, S.M.; Dixon, L.B.; Subar, A.; Schatzkin, A.; Abnet, C. Vitamin E intake and risk of esophageal and gastric cancers in the NIH-AARP Diet and Health Study. Int. J. Cancer 2009, 125, 165–170. [Google Scholar] [CrossRef]
- Cook, M.B.; Matthews, C.E.; Gunja, M.Z.; Abid, Z.; Freedman, N.D.; Abnet, C.C. Physical activity and sedentary behavior in relation to esophageal and gastric cancers in the NIH-AARP cohort. PLoS ONE 2013, 8, e84805. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Freedman, N.D.; Ren, J.; Ward, M.H.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R.; Abnet, C. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am. J. Gastroenterol. 2011, 106, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Dawsey, S.P.; Hollenbeck, A.; Schatzkin, A.; Abnet, C.C. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS ONE 2014, 9, e88774. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, P.J.F.; Wolters, L.M.M.; Steyerberg, E.W.; Van Dekken, H.; Kusters, J.G.; Kuipers, E.J.; Siersema, P.D. Environmental risk factors in the development of adenocarcinoma of the oesophagus or gastric cardia: A cross-sectional study in a Dutch cohort. Aliment. Pharmacol. Ther. 2007, 26, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Engeland, A.; Tretli, S.; Bjørge, T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control 2004, 15, 837–843. [Google Scholar] [CrossRef]
- Freedman, N.D.; Abnet, C.; Leitzmann, M.F.; Mouw, T.; Subar, A.F.; Hollenbeck, A.R.; Schatzkin, A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am. J. Epidemiol. 2007, 165, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int. J. Cancer 2007, 121, 2753–2760. [Google Scholar] [CrossRef]
- Gatenby, P.A.C.; Caygill, C.P.J.; Ramus, J.R.; Charlett, A.; Watson, A. Barrett’s columnar-lined oesophagus: Demographic and lifestyle associations and adenocarcinoma risk. Dig. Dis. Sci. 2008, 53, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Pera, G.; Agudo, A.; Bueno-De-Mesquita, H.B.; Ceroti, M.; Boeing, H.; Schulz, M.; Del Giudice, G.; Plebani, M.; Carneiro, F.; et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int. J. Cancer 2006, 118, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Jakszyn, P.; Pera, G.; Agudo, A.; Bingham, S.; Palli, D.; Ferrari, P.; Boeing, H.; Del Giudice, G.; Plebani, M.; et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European prospective investigation into cancer and nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hardikar, S.; Onstad, L.; Blount, P.L.; Odze, R.D.; Reid, B.J.; Vaughan, T.L. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: A prospective study of Barrett’s esophagus. PLoS ONE 2013, 8, e52192. [Google Scholar] [CrossRef] [PubMed]
- Huerta, J.M.; Navarro, C.; Chirlaque, M.-D.; Tormo, M.-J.; Steindorf, K.; Buckland, G.; Carneiro, F.; Johnsen, N.F.; Overvad, K.; Stegger, J.; et al. Prospective study of physical activity and risk of primary adenocarcinomas of the oesophagus and stomach in the EPIC (European prospective investigation into cancer and nutrition) cohort. Cancer Causes Control 2010, 21, 657–669. [Google Scholar] [CrossRef]
- Jakszyn, P.; Lujan-Barroso, L.; Agudo, A.; Bueno-De-Mesquita, H.B.; Molina-Montes, E.; Sánchez, M.J.; Fonseca-Nunes, A.; Siersema, P.D.; Matiello, A.; Tumino, R.; et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2013, 133, 2744–2750. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, J.; Sundquist, K. Associations of alcohol use disorders with esophageal and gastric cancers: A population-based study in Sweden. Eur. J. Cancer Prev. 2017, 26, 119–124. [Google Scholar] [CrossRef]
- Keszei, A.P.; Schouten, L.J.; Goldbohm, R.A.; Van den Brandt, P.A. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Ann. Oncol. 2012, 23, 2319–2326. [Google Scholar] [CrossRef]
- Keszei, A.P.; Goldbohm, R.A.; Schouten, L.J.; Jakszyn, P.; Van den Brandt, P.A. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2012, 97, 135–146. [Google Scholar] [CrossRef]
- Levi, Z.; Kark, J.D.; Shamiss, A.; Derazne, E.; Tzur, D.; Keinan-Boker, L.; Liphshitz, I.; Niv, Y.; Furman, M.; Afek, A. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer 2013, 119, 4086–4093. [Google Scholar] [CrossRef]
- Li, W.; Park, Y.; Wu, J.W.; Ren, J.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin. Gastroenterol. Hepatol. 2013, 11, 1130–1136.e2. [Google Scholar] [CrossRef]
- Lin, Y.; Ness-Jensen, E.; Hveem, K.; Lagergren, J.; Lu, Y. Metabolic syndrome and esophageal and gastric cancer. Cancer Causes Control 2015, 26, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Merry, A.H.; Schouten, L.J.; Goldbohm, R.A.; Van den Brandt, P.A. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: A prospective cohort study. Gut 2007, 56, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, M.G.; Freedman, N.D.; Hollenbeck, A.R.; Schatzkin, A.; Murray, L.J.; Cantwell, M.M.; Abnet, C. Association of dietary fat intakes with risk of esophageal and gastric cancer in the NIH-AARP diet and health study. Int. J. Cancer 2012, 131, 1376–1387. [Google Scholar] [CrossRef]
- O’Doherty, M.G.; Freedman, N.D.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 2011, 61, 1261–1268. [Google Scholar] [CrossRef]
- Petrick, J.L.; Kelly, S.P.; Liao, L.M.; Freedman, N.D.; Graubard, B.I.; Cook, M.B. Body weight trajectories and risk of oesophageal and gastric cardia adenocarcinomas: A pooled analysis of NIH-AARP and PLCO Studies. Br. J. Cancer 2017, 116, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef]
- Ren, J.; Freedman, N.; Kamangar, F.; Dawsey, S.; Hollenbeck, A.; Schatzkin, A.; Abnet, C. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef]
- Samanic, C.; Chow, W.H.; Gridley, G.; Jarvholm, B.; Fraumeni, J.F., Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control 2006, 17, 901–909. [Google Scholar] [CrossRef]
- Sanikini, H.; Muller, D.; Sophiea, M.; Rinaldi, S.; Agudo, A.; Duell, E.J.; Weiderpass, E.; Overvad, K.; Tjønneland, A.; Halkjær, J.; et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: Results from the European prospective investigation into cancer and nutrition (EPIC) cohort. Int. J. Cancer 2019, 146, 929–942. [Google Scholar] [CrossRef]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; Van Den Brandt, P.A. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: A prospective cohort study. Gut 2009, 59, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; Van den Brandt, P.A.; Goldbohm, R.A.; Schouten, L.J. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology 2010, 138, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; Schouten, L.J.; Goldbohm, R.A.; Van den Brandt, P.A. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int. J. Cancer 2011, 129, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Schulze, M.B.; Pischon, T.; Dietrich, T.; Molina-Montes, E.; Chirlaque, M.-D.; Barricarte, A.; Amiano, P.; Quirós, J.R.; Tumino, R.; et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2079–2089. [Google Scholar] [CrossRef]
- Steffen, A.; Huerta, J.M.; Weiderpass, E.; Bueno-De-Mesquita, H.; May, A.M.; Siersema, P.D.; Kaaks, R.; Neamat-Allah, J.; Pala, V.; Panico, S.; et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 137, 646–657. [Google Scholar] [CrossRef]
- Vermeulen, E.; Zamora-Ros, R.; Duell, E.J.; Luján-Barroso, L.; Boeing, H.; Aleksandrova, K.; Bueno-De-Mesquita, H.B.; Scalbert, A.; Romieu, I.; Fedirko, V.; et al. Dietary flavonoid intake and esophageal cancer risk in the European prospective investigation into cancer and nutrition cohort. Am. J. Epidemiol. 2013, 178, 570–581. [Google Scholar] [CrossRef]
- Yates, M.; Cheong, E.; Luben, R.; Igali, L.; Fitzgerald, R.; Khaw, K.-T.; Hart, A. Body mass index, smoking, and alcohol and risks of Barrett’s esophagus and esophageal adenocarcinoma: A UK prospective cohort study. Dig. Dis. Sci. 2014, 59, 1552–1559. [Google Scholar] [CrossRef]
- Xiao, Q.; Freedman, N.D.; Ren, J.; Hollenbeck, A.R.; Abnet, C.C.; Park, Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br. J. Cancer 2014, 110, 1328–1333. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Lujan-Barroso, L.; Bueno-De-Mesquita, H.B.; Dik, V.K.; Boeing, H.; Steffen, A.; Tjønneland, A.; Olsen, A.; Bech, B.H.; Overvad, K.; et al. Tea and coffee consumption and risk of esophageal cancer: The European prospective investigation into cancer and nutrition study. Int. J. Cancer 2014, 135, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, K.; Nyrén, O.; Luo, J.; Dickman, P.; Boffetta, P.; Englund, A.; Ye, W. Risk of gastroesophageal cancer among smokers and users of Scandinavian moist snuff. Int. J. Cancer 2007, 122, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar]
- Gevaart-Durkin, A.; de Peyster, A. High temperature cooked meats. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 912–915. [Google Scholar]
- Lauby-Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens-Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Turati, F.; Tramacere, I.; La Vecchia, C.; Negri, E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 609–617. [Google Scholar] [CrossRef]
- Kubo, A.; Corley, D.A. Body mass index and adenocarcinomas of the esophagus or gastric cardia: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 872–878. [Google Scholar] [CrossRef]
- Drahos, J.; Xiao, Q.; Risch, H.A.; Freedman, N.D.; Abnet, C.C.; Anderson, L.A.; Bernstein, L.; Brown, L.; Chow, W.-H.; Gammon, M.D.; et al. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int. J. Cancer 2015, 138, 55–64. [Google Scholar] [CrossRef]
- Salehi, M.; Moradi-Lakeh, M.; Salehi, M.H.; Nojomi, M.; Kolahdooz, F. Meat, fish, and esophageal cancer risk: A systematic review and dose-response meta-analysis. Nutr. Rev. 2013, 71, 257–267. [Google Scholar] [CrossRef]
- Zhu, H.-C.; Yang, X.; Xu, L.; Zhao, L.-J.; Tao, G.-Z.; Zhang, C.; Qin, Q.; Cai, J.; Ma, J.-X.; Mao, W.-D.; et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig. Dis. Sci. 2014, 59, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Song, S.; Song, Y.; Lee, J.E. Consumption of red and processed meat and esophageal cancer risk: Meta-analysis. World J. Gastroenterol. 2013, 19, 1020–1029. [Google Scholar] [CrossRef]
- Huang, W.; Han, Y.; Xu, J.; Zhu, W.; Li, Z. Red and processed meat intake and risk of esophageal adenocarcinoma: A meta-analysis of observational studies. Cancer Causes Control 2012, 24, 193–201. [Google Scholar] [CrossRef]
- Han, Y.J.; Li, J.; Huang, W.; Fang, Y.; Xiao, L.N.; Liao, Z.E. Fish consumption and risk of esophageal cancer and its subtypes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2013, 67, 147–154. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Salvatori, T.; Dallagiacoma, G.; Fatigoni, C.; Moretti, M.; Realdon, S. Rectal cancer: 20% risk reduction thanks to dietary fibre intake. Systematic review and meta-analysis. Nutrients 2019, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Salvatori, T.; Villarini, M.; Moretti, M.; Nucci, D.; Realdon, S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2018, 69, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Mechanisms for the impact of whole grain foods on cancer risk. J. Am. Coll. Nutr. 2000, 19 (Suppl. 3), 300S–307S. [Google Scholar] [CrossRef]
- Brown, L.M.; Swanson, C.A.; Gridley, G.; Swanson, G.M.; Schoenberg, J.B.; Greenberg, R.S.; Silverman, D.T.; Pottern, L.M.; Hayes, R.B.; Schwartz, A.G.; et al. Adenocarcinoma of the esophagus: Role of obesity and diet. J. Natl. Cancer Inst. 1995, 87, 104–109. [Google Scholar] [CrossRef]
- Tullio, V.; Gasperi, V.; Catani, M.V.; Savini, I. The impact of whole grain intake on gastrointestinal tumors: A focus on colorectal, gastric, and esophageal cancers. Nutrients 2020, 13, 81. [Google Scholar] [CrossRef]
- Nobel, Y.R.; Snider, E.J.; Compres, G.; Freedberg, D.E.; Khiabanian, H.; Lightdale, C.J.; Toussaint, N.; Abrams, J.A. Increasing dietary fiber intake is associated with a distinct esophageal microbiome. Clin. Transl. Gastroenterol. 2018, 9, e199. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.W.; Riggs, D.R.; Jackson, B.J.; Cunningham, C. Corn-derived carbohydrate inositol hexaphosphate inhibits Barrett’s adenocarcinoma growth by pro-apoptotic mechanisms. Oncol. Rep. 2008, 19, 563–566. [Google Scholar] [CrossRef][Green Version]
- Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against experimental reflux esophagitis in rats. Biomol. Ther. 2014, 22, 420–425. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Satia, J.A.; Rabeneck, L. Dietary intake and the risk of gastro-oesophageal reflux disease: A cross sectional study in volunteers. Gut 2005, 54, 11–17. [Google Scholar] [CrossRef]
- Elia, M.; Cummings, J.H. Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur. J. Clin. Nutr. 2007, 61 (Suppl. 1), S40–S74. [Google Scholar] [CrossRef]
- Coleman, H.G.; Murray, L.J.; Hicks, B.; Bhat, S.K.; Kubo, A.; Corley, D.A.; Cardwell, C.; Cantwell, M.M. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: A systematic review and meta-analysis. Nutr. Rev. 2013, 71, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Vannini, S.; Moretti, M.; Villarini, M.; Bragazzi, N.L.; Izzotti, A.; Nucci, D. Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis. J. Nutrigenet. Nutrigenom. 2017, 10, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Corley, D.A. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am. J. Gastroenterol. 2007, 102, 2323–2330. [Google Scholar] [CrossRef]
- De Stefani, E.; Deneo-Pellegrini, H.; Ronco, A.L.; Boffetta, P.; Brennan, P.; Muñoz, N.; Castellsagué, X.; Correa, P.; Mendilaharsu, M. Food groups and risk of squamous cell carcinoma of the oesophagus: A case-control study in Uruguay. Br. J. Cancer 2003, 89, 1209–1214. [Google Scholar] [CrossRef]
- De Stefani, E.; Deneo-Pellegrini, H.; Ronco, A.L.; Boffetta, P.; Correa, P.; Aune, D.; Mendilaharsu, M.; Acosta, G.; Silva, C.; Lando, G.; et al. Meat consumption, cooking methods, mutagens, and risk of squamous cell carcinoma of the esophagus: A case-control study in Uruguay. Nutr. Cancer 2012, 64, 294–299. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; Talamini, R.; Levi, F.; Bosetti, C.; Negri, E.; Franceschi, S.; La Vecchia, C. Role of fried foods and oral/pharyngeal and oesophageal cancers. Br. J. Cancer 2005, 92, 2065–2069. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Knize, M.G.; Salmon, C.P.; Pais, P.; Felton, J.S. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv. Exp. Med. Biol. 1999, 459, 179–193. [Google Scholar] [PubMed]
- Sinha, R.; Rothman, N. Role of well-done, grilled red meat, heterocyclic amines (HCAs) in the etiology of human cancer. Cancer Lett. 1999, 143, 189–194. [Google Scholar] [CrossRef]
- Hakami, R.; Mohtadinia, J.; Etemadi, A.; Kamangar, F.; Nemati, M.; Pourshams, A.; Islami, F.; Nasrollahzadeh, D.; Saberi-Firoozi, M.; Birkett, N.; et al. Dietary intake of benzo(a)pyrene and risk of esophageal cancer in north of Iran. Nutr. Cancer 2008, 60, 216–221. [Google Scholar] [CrossRef]
- Kamangar, F.; Strickland, P.T.; Pourshams, A.; Malekzadeh, R.; Boffetta, P.; Roth, M.J.; Abnet, C.C.; Saadatian-Elahi, M.; Rakhshani, N.; Brennan, P.; et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005, 25, 425–428. [Google Scholar] [PubMed]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Gianfredi, V.; Salvatori, T.; Nucci, D.; Villarini, M.; Moretti, M. Can chocolate consumption reduce cardio-cerebrovascular risk? A systematic review and meta-analysis. Nutrition 2018, 46, 103–114. [Google Scholar] [CrossRef]
- Lohsiriwat, S.; Puengna, N.; Leelakusolvong, S. Effect of caffeine on lower esophageal sphincter pressure in Thai healthy volunteers. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2006, 19, 183–188. [Google Scholar] [CrossRef]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Tehard, B.; Mallet, Y.; Gerber, M.; Norat, T.; Hercberg, S.; Latino-Martel, P. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009, 10, 173–180. [Google Scholar] [CrossRef]
- Nucci, D.; Gianfredi, V.; Minelli, L.; Realdon, S. Alcohol consumption and risk of Barrett’s Esophagus. Mini-review of recent literature. Prog. Nutr. 2018, 20, 313–317. [Google Scholar] [CrossRef]
- Lubin, J.H.; Cook, M.B.; Pandeya, N.; Vaughan, T.L.; Abnet, C.C.; Giffen, C.; Webb, P.M.; Murray, L.J.; Casson, A.G.; Risch, H.A.; et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012, 36, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Murray, L.J.; Kamangar, F.; Abnet, C.; Cook, M.B.; Nyrén, O.; Ye, W.; Wu, A.H.; Bernstein, L.; Brown, L.M.; et al. Alcohol intake and risk of oesophageal adenocarcinoma: A pooled analysis from the BEACON Consortium. Gut 2011, 60, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Pelucchi, C.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; Boffetta, P.; La Vecchia, C.; Negri, E. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann. Oncol. 2012, 23, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Kamangar, F.; Whiteman, D.C.; Freedman, N.D.; Gammon, M.D.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: A pooled analysis from the international BEACON consortium. J. Natl. Cancer Inst. 2010, 102, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Margetts, B.M.; Vorster, H.H.; Venter, C.S. Evidence-based nutrition—Review of nutritional epidemiological studies. S. Afr. J. Clin. Nutr. 2002, 15, 68–74. [Google Scholar]
- Coughlin, S.S. Recall bias in epidemiologic studies. J. Clin. Epidemiol. 1990, 43, 87–91. [Google Scholar] [CrossRef]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Sinha, M.K.; Montori, V.M. Reporting bias and other biases affecting systematic reviews and meta-analyses: A methodological commentary. Exp. Rev. Pharm. Outcomes Res. 2006, 6, 603–611. [Google Scholar] [CrossRef]
| Parameter | Inclusion | Exclusion |
|---|---|---|
| Population | Adult population, Male and female, Focusing on EAC alone. | Population with ESCC and EAC combined, esophageal and gastric cardia adenocarcinoma combined, only considered ESCC |
| Intervention | Administration of questionnaire evaluating, food frequency, dietary pattern, BMI, physical activity, smoking habit, alcohol consumption, sociodemographic characteristics | Medication or other intervention intended to reduce EAC risk |
| Control/ Comparison | Stratification according to dietary habits, dietary pattern, BMI, physical activity, smoking habit, alcohol consumption, sociodemographic characteristics | None |
| Outcomes | Risk of EAC | Risk of EAC and ESCC combined or ESCC alone Risk of EAC and gastric cardia adenocarcinoma combined |
| Study design | Epidemiologic studies (case-control, cross-sectional, or cohort studies), pooled analysis, meta-analysis. | Review article, expert opinion, commentary, article with no quantitative information or details, experimental animal models, genetic or immune-histochemical studies |
| Language filter | Only article in English language | Any other language |
| Time filter | From inception until May 2021 | None |
| High Quality (NOS ≥ 7) [References] | Medium/Low Quality (NOS < 7) [References] | |||||
|---|---|---|---|---|---|---|
| Beneficial Effect | Detrimental Effect | No Effect | Beneficial Effect | Detrimental Effect | No Effect | |
| Anthropometric measures | ||||||
| Overweight/obesity (BMI ≥ 25) | - | [38,39,40,42,53,86,95,97,103,104,119,121,122,125,130,133] | [110,116,131] | - | [57,63,75,80,81,88,93,123] | - |
| Abdominal obesity | ||||||
| High WC | - | [40,110,118,121,126,130,131] | - | - | [65] | - |
| High WHR | - | [121,126,130] | [32] | - | - | - |
| High hip-circumference | [131] | - | - | - | - | - |
| Physical activity | [89] | - | [110,111] | - | - | - |
| Dietary patterns | ||||||
| “Healthy diet” | [117] | - | [33] | - | - | - |
| “Western diet” | - | [33,36,50] | - | - | - | - |
| “High carbohydrates” | - | - | - | [56,94] | - | - |
| “High fat” | - | - | [70,74,120] | - | [56] | - |
| “High proteins” | - | - | - | - | - | [56] |
| “High Dietary inflammation index” | - | [63,64] | - | - | - | - |
| “Plant-based” | [66,70,71] | - | - | - | - | - |
| “Animal-based” | - | [66,71] | - | - | - | - |
| Foods of plants origin and dietary fiber | ||||||
| Vegetables | [84,87,129] | - | [106,108] | [80] | - | - |
| Fruits | [32,38] | - | [106,108,129] | - | - | - |
| Dietary fiber | [66,87,96] | - | - | [35,58] | - | - |
| Animal products | ||||||
| Red meat | - | [72,112] | [96,101] | [92] | [90] | - |
| White meat | - | - | [101,112] | - | - | - |
| Processed meat | [109] | [112] | [96,101,109] | - | [90] | - |
| Total meat | - | - | [96,109] | - | - | - |
| Nonalcoholic beverages | - | - | - | - | - | - |
| Carbonated soft drink | - | [59] | [55,67,124] | - | - | - |
| Hot beverages | - | - | [124,135] | - | [48] | - |
| Vitamins, minerals, and other nutrients | ||||||
| Polyphenols | [61,132] | - | [79] * | - | - | [62] |
| Vitamin C | [66,69,83,87] | - | - | [34] | - | - |
| Vitamin E | [51] | - | [69,83,99] | [34] | - | - |
| Vitamin A and Carotenoids | [66,83,87] | - | [69] | [35] | - | - |
| Folates | [66,82] | - | [134] | [35] | - | - |
| B Vitamins | [66] | - | [82,134] | - | - | - |
| Vitamin D and calcium | - | [68] + | - | - | - | - |
| Heme iron | - | [73,112] | [115] | [92] | ||
| Nonheme iron | [73,115] | - | - | - | - | - |
| Other compounds (Magnesium, Zinc, Selenium) | [128] | - | [41,69] | [35,94] | - | - |
| Dietary supplements | [51,69,87] | - | [99,102] | [54] | - | - |
| Cooking process and chemical modification during cooking | ||||||
| Frying/broiling | - | - | - | - | - | [90] |
| Grilling/barbecuing | - | - | - | - | [90] | - |
| Acrylamide | - | - | [60] | - | - | - |
| Heterocyclic amines | - | [101] | - | - | - | [85] |
| Alcohol | - | - | [31,45,55,63,95,105,107,127] | - | [37,48] | [47,49,77,88] |
| Smoking | - | [32,45,46,63,95,103,105,110,127,136] | [107] | - | [37,44,47,48,76,78,80,88,93] | [49,54,77] |
| High socioeconomic factors | [46,52] | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nucci, D.; Marino, A.; Realdon, S.; Nardi, M.; Fatigoni, C.; Gianfredi, V. Lifestyle, WCRF/AICR Recommendations, and Esophageal Adenocarcinoma Risk: A Systematic Review of the Literature. Nutrients 2021, 13, 3525. https://doi.org/10.3390/nu13103525
Nucci D, Marino A, Realdon S, Nardi M, Fatigoni C, Gianfredi V. Lifestyle, WCRF/AICR Recommendations, and Esophageal Adenocarcinoma Risk: A Systematic Review of the Literature. Nutrients. 2021; 13(10):3525. https://doi.org/10.3390/nu13103525
Chicago/Turabian StyleNucci, Daniele, Alessio Marino, Stefano Realdon, Mariateresa Nardi, Cristina Fatigoni, and Vincenza Gianfredi. 2021. "Lifestyle, WCRF/AICR Recommendations, and Esophageal Adenocarcinoma Risk: A Systematic Review of the Literature" Nutrients 13, no. 10: 3525. https://doi.org/10.3390/nu13103525
APA StyleNucci, D., Marino, A., Realdon, S., Nardi, M., Fatigoni, C., & Gianfredi, V. (2021). Lifestyle, WCRF/AICR Recommendations, and Esophageal Adenocarcinoma Risk: A Systematic Review of the Literature. Nutrients, 13(10), 3525. https://doi.org/10.3390/nu13103525







