Metabolic Syndrome and Sarcopenia
Abstract
1. Introduction
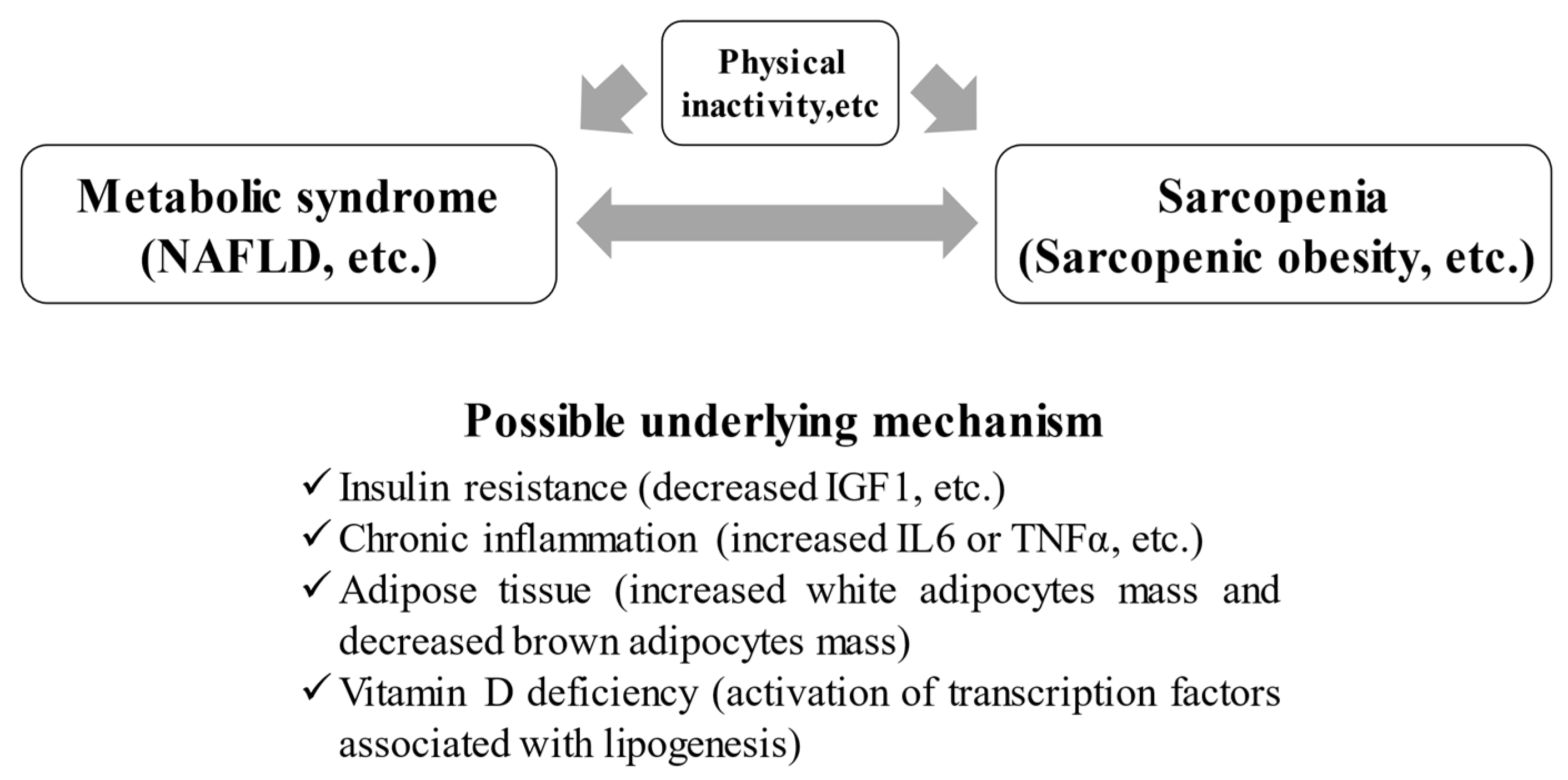
2. Mechanism for Sarcopenia Caused by Metabolic Syndrome
2.1. IR and Sarcopenia in Patients with Met-S
2.2. Adipose Tissue and Sarcopenia in Patients with Met-S
2.3. Persistent Inflammation
2.4. Met-S, Sarcopenia, and Vitamins
3. Met-S and Sarcopenia
3.1. Clinical Evidence of Met-S and Sarcopenia
3.2. Impact of Physical Inactivity
4. Sarcopenic Obesity and Met-S
5. NAFLD, Met-S, and Sarcopenia
6. Interventions for Met-S-Associated Sarcopenia
7. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Available online: https://www.mhlw.go.jp/toukei/list/81-1a.html (accessed on 5 July 2021).
- Available online: http://www.e-healthnet.mhlw.go.jp/information/food/e-02-001.html (accessed on 5 July 2021).
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan society of hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J.S.; Morley, J.E.; Rosano, G.; Bernabei, R.; Von Haehling, S.; Kalantar-Zadeh, K. Muscle wasting disease: A proposal for a new disease classification. J. Cachex Sarcopenia Muscle 2014, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Perla, F.M.; Chiesa, C. Sarcopenia and nonalcoholic fatty liver disease: A causal relationship. HepatoBiliary Surg. Nutr. 2019, 8, 144–147. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Available online: http://www.mhlw.go.jp/bunya/kenkou/dl/kenkounippon21_01.pdf (accessed on 5 July 2021).
- Ahuja, V.; ERA JUMP Study Group; Kadowaki, T.; Evans, R.W.; Kadota, A.; Okamura, T.; El Khoudary, S.R.; Fujiyoshi, A.; Barinas-Mitchell, E.; Hisamatsu, T.; et al. Comparison of HOMA-IR, HOMA-β% and disposition index between US white men and Japanese men in Japan: The ERA JUMP study. Diabetologia 2015, 58, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Ito, H.; Ohashi, Y.; Akanuma, Y.; Yamada, N. Japan Diabetes Complication Study Group. Obesity and type 2 diabetes in Japanese patients. Lancet 2003, 361, 85. [Google Scholar] [CrossRef]
- Mukai, N.; Doi, Y.; Ninomiya, T.; Hata, J.; Yonemoto, K.; Iwase, M.; Iida, M.; Kiyohara, Y. Impact of metabolic syndrome compared with impaired fasting glucose on the development of type 2 diabetes in a general Japanese population: The hisayama study. Diabetes Care 2009, 32, 2288–2293. [Google Scholar] [CrossRef][Green Version]
- Sanada, K.; Iemitsu, M.; Murakami, H.; Gando, Y.; Kawano, H.; Kawakami, R.; Tabata, I.; Miyachi, M. Adverse effects of coexistence of sarcopenia and metabolic syndrome in Japanese women. Eur. J. Clin. Nutr. 2012, 66, 1093–1098. [Google Scholar] [CrossRef][Green Version]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between metabolic syndrome and insulin resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, M.; Chi, V.T.Q.; Wang, J.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Bao, X.; Gu, Y.; et al. Handgrip strength is inversely associated with metabolic syndrome and its separate components in middle aged and older adults: A large-scale population-based study. Metabologism 2019, 93, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Walsh, K. Adipokines, myokines and cardiovascular disease. Circ. J. 2009, 73, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Yoon, J.W.; Ha, Y.-C.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, Y.J.; Lim, J.-Y.; Kim, K.W.; Park, K.S.; et al. Hyperglycemia is associated with impaired muscle quality in older men with diabetes: The korean longitudinal study on health and aging. Diabetes Metab. J. 2016, 40, 140–146. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean sarcopenic obesity study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Ryu, S.-Y.; Park, J.; Choi, S.-W. Association of sarcopenia with metabolic syndrome in Korean population using 2009–2010 Korea national health and nutrition examination survey. Metab. Syndr. Relat. Disord. 2019, 17, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Tabara, Y.; Ikegami, H.; Takata, Y.; Kamide, K.; Ikezoe, T.; Kiyoshige, E.; Makutani, Y.; Onuma, H.; Gondo, Y.; et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J. Diabetes Investig. 2019, 10, 1471–1479. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The colors of adipose tissue. Gac. Med. Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef]
- Almind, K.; Manieri, M.; Sivitz, W.; Cinti, S.; Kahn, C.R. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Lin, J.; Lim, W.; Jin, W.; Lee, H.J. Role of brown adipose tissue in metabolic syndrome, aging, and cancer cachexia. Front. Med. 2018, 12, 130–138. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.S.; Lacasa, D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V. Handbook on Metabolic Syndrome. Classification, Risk Factors and Health Impact; Lopez Garcia, C.M., Perez Gonzalez, P.A., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2012; pp. 169–188. [Google Scholar]
- Chong, M.S.; Lim, J.P.; Leung, B.; Ding, Y.Y.; Tay, L.; Ismail, N.H.; Yeo, A.; Yew, S. Monocyte chemoattractant protein-1: A proinflammatory cytokine elevated in sarcopenic obesity. Clin. Interv. Aging 2015, 10, 605–609. [Google Scholar] [CrossRef]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor- with muscle mass and muscle strength in elderly men and women: The health ABC study. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Molloy, G.J. Association of C-reactive protein and muscle strength in the English longitudinal study of ageing. AGE 2009, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Harwood, H.J. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012, 63, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.; Shachar, S.; Nyrop, K.; Muss, H.; Malpica, L.; Williams, G. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Crit. Rev. Oncol. 2020, 145, 102839. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Mirenayat, F.S.; Saneei, P. Serum vitamin D levels in relation to metabolic syndrome: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13223. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.A.; Bellerba, F.; Corso, F.; Gandini, S. Vitamin D receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2020, 1268, 53–114. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.-I.; Sakai, J.; Nagasawa, K.; et al. Vitamin D metabolite, 25-hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Luben, R.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Elliott, P.; Houston, D.; et al. Causal relationship between obesity and vitamin D status: Bi-directional mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Kashino, I.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Kabe, I.; Mizoue, T. Serum 25-hydroxyvitamin D and metabolic syndrome in a Japanese working population: The furukawa nutrition and health study. Nutrition 2017, 36, 26–32. [Google Scholar] [CrossRef]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and sarcopenia: Potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D deficiency and sarcopenia in older persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Walsh, M.C.; Hunter, G.R.; Livingstone, M.B. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos. Int. 2006, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.D. Muscle mass, survival, and the elderly ICU patient. Nutrition 1996, 12, 456–458. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Gao, T.; Zhong, F.; Cai, J.; Sun, Y.; Ma, A. Association between sarcopenia and metabolic syndrome in middle-aged and older non-obese adults: A systematic review and meta-analysis. Nutrients 2018, 10, 364. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Akishita, M.; Ouchi, Y.; Tuji, T.; Iijima, K. Metabolic syndrome, sarcopenia and role of sex and age: Cross-sectional analysis of kashiwa cohort study. PLoS ONE 2014, 9, e112718. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.-W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between sarcopenia level and metabolic syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.-E.; Jun, J.E.; Lee, Y.-B.; Ahn, J.; Bae, J.C.; Jin, S.-M.; Hur, K.Y.; Jee, J.H.; Lee, M.-K.; et al. Increase in relative skeletal muscle mass over time and its inverse association with metabolic syndrome development: A 7-year retrospective cohort study. Cardiovasc. Diabetol. 2018, 17, 23. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W. Transitional states of sarcopenia: The trajectory of fat accumulation and glucose fluctuation on risk of metabolic syndrome. Ann. N. Y. Acad. Sci. 2021. [Google Scholar] [CrossRef]
- Alexandre, T.D.S.; Aubertin-Leheudre, M.; Carvalho, L.P.; Máximo, R.D.O.; Corona, L.P.; de Brito, T.R.P.; Nunes, D.P.; Santos, J.L.F.; Duarte, Y.A.D.O.; Lebrão, M.L. Dynapenic obesity as an associated factor to lipid and glucose metabolism disorders and metabolic syndrome in older adults—Findings from SABE Study. Clin. Nutr. 2018, 37, 1360–1366. [Google Scholar] [CrossRef]
- Shen, C.; Lu, J.; Xu, Z.; Xu, Y.; Yang, Y. Association between handgrip strength and the risk of new-onset metabolic syndrome: A population-based cohort study. BMJ Open 2020, 10, e041384. [Google Scholar] [CrossRef]
- Churilla, J.R.; Summerlin, M.; Richardson, M.R.; Boltz, A.J. Mean combined relative grip strength and metabolic syndrome: 2011–2014 national health and nutrition examination survey. J. Strength Cond. Res. 2020, 34, 995–1000. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prospective urban rural epidemiology (PURE) study investigators. Prognostic value of grip strength: Findings from the prospective urban rural epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Inoue, M.; Iso, H.; Yamamoto, S.; Kurahashi, N.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Daily total physical activity level and premature death in men and women: Results from a large-scale population-based cohort study in Japan (JPHC Study). Ann. Epidemiol. 2008, 18, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Heber, D.; Ingles, S.; Ashley, J.M.; Maxwell, M.H.; Lyons, R.F.; Elashoff, R.M. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am. J. Clin. Nutr. 1996, 64, 472S–477S. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J. Cardio Metab. Syndr. 2007, 2, 183–189. [Google Scholar] [CrossRef]
- Lu, C.-W.; Yang, K.-C.; Chang, H.-H.; Lee, L.-T.; Chen, C.-Y.; Huang, K.-C. Sarcopenic obesity is closely associated with metabolic syndrome. Obes. Res. Clin. Pr. 2013, 7, e301–e307. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Nam, G.E.; Han, K.D.; Choi, S.W.; Jung, S.W.; Bok, A.R.; Kim, Y.H.; Lee, K.S.; Han, B.D.; Kim, D.H. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: The 2008–2010 Korea national health and nutrition examination survey. J. Endocrinol. Investig. 2014, 37, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Park, Y.-M.; Kwon, H.-S.; Ko, S.-H.; Lee, S.-H.; Yim, H.W.; Lee, W.-C.; Park, Y.-M.; Kim, M.K. Sarcopenia as a determinant of blood pressure in older Koreans: Findings from the Korea national health and nutrition examination surveys (KNHANES) 2008–2010. PLoS ONE 2014, 9, e86902. [Google Scholar] [CrossRef]
- Oh, C.; Jho, S.; No, J.-K.; Kim, H.-S. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr. Res. 2015, 35, 1–6. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Koyanagi, A.; Olaya, B.; Ayuso-Mateos, J.L.; Miret, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Haro, J.M. The role of muscle mass and body fat on disability among older adults: A cross-national analysis. Exp. Gerontol. 2015, 69, 27–35. [Google Scholar] [CrossRef]
- Tamura, Y. Ectopic fat, insulin resistance and metabolic disease in non-obese Asians: Investigating metabolic gradation. Endocr. J. 2019, 66, 1–9. [Google Scholar] [CrossRef]
- Kitamoto, T.; Kitamoto, A.; Yoneda, M.; Hyogo, H.; Ochi, H.; Nakamura, T.; Teranishi, H.; Mizusawa, S.; Ueno, T.; Chayama, K.; et al. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Qual. Life Res. 2013, 132, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2015, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Nafld, J.; Hyogo, H.; Ono, M.; Mizuta, T.; Ono, N.; Fujimoto, K.; Chayama, K.; Saibara, T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012, 47, 586–595. [Google Scholar] [CrossRef]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef]
- Koda, M.; Kawakami, M.; Murawaki, Y.; Senda, M. The impact of visceral fat in nonalcoholic fatty liver disease: Cross-sectional and longitudinal studies. J. Gastroenterol. 2007, 42, 897–903. [Google Scholar] [CrossRef]
- Habig, G.; Smaltz, C.; Halegoua-DeMarzio, D. Presence and implications of sarcopenia in non-alcoholic steatohepatitis. Metabolites 2021, 11, 242. [Google Scholar] [CrossRef]
- Yu, R.; Shi, Q.; Liu, L.; Chen, L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: A meta-analysis. BMC Gastroenterol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Loomba, R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Qu, K.; Liu, C.; Zhang, J.-Y.; Liu, S.-S. Sarcopenia and nonalcoholic fatty liver disease: New evidence for low vitamin D status contributing to the link. Hepatology 2016, 63, 675. [Google Scholar] [CrossRef]
- Eliades, M.; Spyrou, E.; Agrawal, N.; Lazo, M.; Brancati, F.L.; Potter, J.J.; Koteish, A.A.; Clark, J.M.; Guallar, E.; Hernaez, R. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Panjawatanan, P.; Thongprayoon, C.; Jaruvongvanich, V.; Ungprasert, P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J. Gastroenterol. 2018, 24, 12–17. [Google Scholar] [CrossRef]
- Pan, X.; Han, Y.; Zou, T.; Zhu, G.; Xu, K.; Zheng, J.-N.; Zheng, M.-H.; Cheng, X. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease-related fibrosis: A meta-analysis. Dig. Dis. 2018, 36, 427–436. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E.; Lee, Y.B.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.M.; Hur, Y.; Jee, J.H.; Lee, M.K.; et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A 7-year longitudinal study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.J.; Plank, L.D. Association of grip strength with non-alcoholic fatty liver disease: Investigation of the roles of insulin resistance and inflammation as mediators. Eur. J. Clin. Nutr. 2020, 74, 1401–1409. [Google Scholar] [CrossRef]
- Meng, G.; Wu, H.; Fang, L.; Li, C.; Yu, F.; Zhang, Q.; Liu, L.; Du, H.; Shi, H.; Xia, Y.; et al. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large-scale adult population. Sci. Rep. 2016, 6, 33255. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; Goulart, R.D.A.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical exercise and myokines: Relationships with sarcopenia and cardiovascular complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Barajas-Galindo, D.E.; Arnáiz, E.G.; Vicente, P.F.; Ballesteros-Pomar, M.D. Effects of physical exercise in sarcopenia. A systematic review. Endocrinol. Diabetes Nutr. 2021, 68, 159–169. [Google Scholar] [CrossRef]
- Pérez, E.A.; Olivares, V.M.; Martínez-Espinosa, R.M.; Vila, M.D.M.; García-Galbis, M.R. New insights about how to make an intervention in children and adolescents with metabolic syndrome: Diet, exercise vs. changes in body composition. A systematic review of RCT. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.; Koşar, Ş.; Güzel, Y.; Tin, H.; Yan, X. The role of exercise, diet, and cytokines in preventing obesity and improving adipose tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown adipose tissue controls skeletal muscle function via. the secretion of myostatin. Cell Metab. 2018, 28, 631–643.e3. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Rasmussen, B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.T.; Barnette, K.G.; Bohl, C.E.; Hancock, M.L.; Rodriguez, D.; Dodson, S.T.; Morton, R.A.; Steiner, M.S. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachex Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Lowe, D.A.; Brown, M. Estrogen replacement and skeletal muscle: Mechanisms and population health. J. Appl. Physiol. 2013, 115, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Prado, C.M.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study design and rationale for the phase 3 clinical development program of enobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.; Clark, B. The vicious cycle of myostatin signaling in sarcopenic obesity: Myostatin role in skeletal muscle growth, insulin signaling and implications for clinical trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [PubMed]
- Zhou, L.-S.; Xu, L.-J.; Wang, X.-Q.; Huang, Y.-H.; Xiao, Q. Effect of angiotensin-converting enzyme inhibitors on physical function in elderly subjects: A systematic review and meta-analysis. Drugs Aging 2015, 32, 727–735. [Google Scholar] [CrossRef]
- Tabrizi, R.; Hallajzadeh, J.; Mirhosseini, N.; Lankarani, K.B.; Maharlouei, N.; Akbari, M.; Asemi, Z. The effects of vitamin D supplementation on muscle function among postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2019, 18, 591–603. [Google Scholar]
| Joint Statement [49] | Japanese Criteria | |
|---|---|---|
| Definition | Three or more of the following items apply | Required item and at least two items in other items |
| WC | Each country adopts its own standards | <Required item> Male: ≥85 cm, female: ≥90 cm |
| TG | ≥150 mg/dL or under treatment | ≥150 mg/dL or under treatment |
| HDL cholesterol | <40 mg/dL or under treatment (Male) <50 mg/dL or under treatment (Female) | <40 mg/dL or under treatment |
| Blood pressure | ≥130 mmHg (systolic) and/or ≥85 mmHg (diastolic) or under treatment | ≥130 mmHg (systolic) and/or ≥85 mmHg (diastolic) or under treatment |
| FBG | ≥100 mg/dL or under treatment | ≥110 mg/dL or under treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. https://doi.org/10.3390/nu13103519
Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic Syndrome and Sarcopenia. Nutrients. 2021; 13(10):3519. https://doi.org/10.3390/nu13103519
Chicago/Turabian StyleNishikawa, Hiroki, Akira Asai, Shinya Fukunishi, Shuhei Nishiguchi, and Kazuhide Higuchi. 2021. "Metabolic Syndrome and Sarcopenia" Nutrients 13, no. 10: 3519. https://doi.org/10.3390/nu13103519
APA StyleNishikawa, H., Asai, A., Fukunishi, S., Nishiguchi, S., & Higuchi, K. (2021). Metabolic Syndrome and Sarcopenia. Nutrients, 13(10), 3519. https://doi.org/10.3390/nu13103519






