Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Analysis of β-Glucan
2.2. Animals and Study Design
2.3. Biochemical Analysis of the Serum and Concentration of Liver Lipids
2.4. Total Fecal Lipid and β-Glucan Analysis
2.5. Analysis of Short Chain Fatty Acids in Cecal Digesta
2.6. Analysis of Major Microbiota in Cecal Digesta
2.7. Expression Analysis of mRNAs in Liver and Ileum
2.8. Statistical Analysis
3. Results
3.1. Food Intake, Body Weight and Organ Weight
3.2. Fecal Total Fat Excretion and Apparent Absorption of Fat
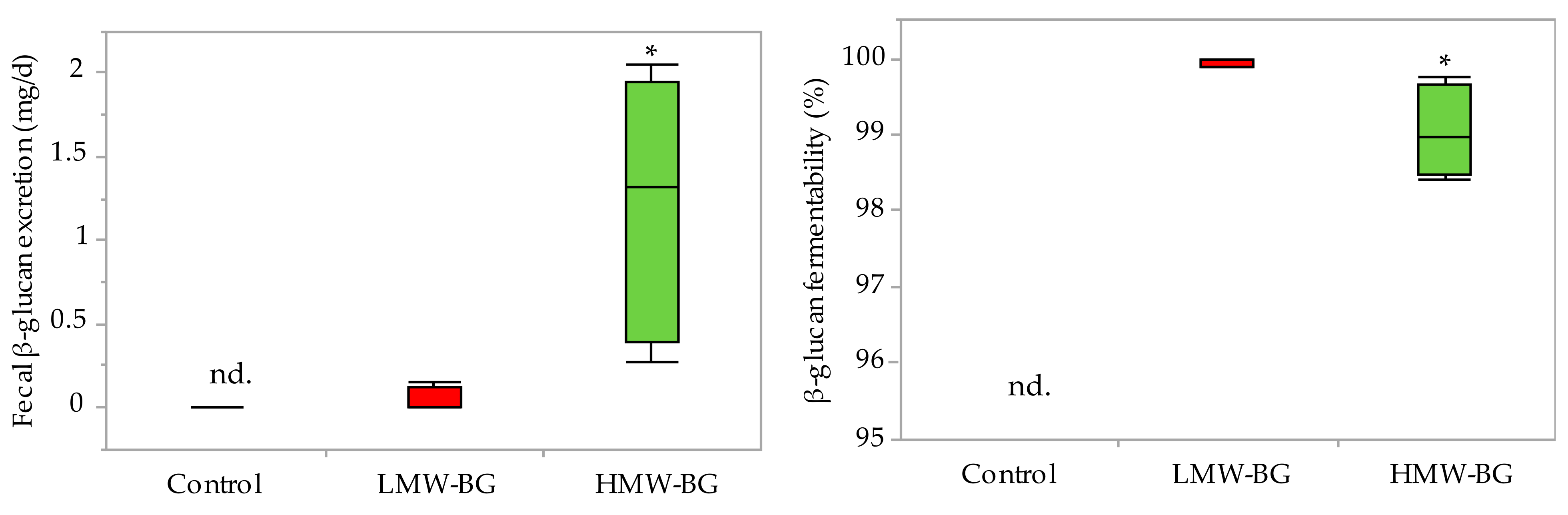
3.3. Fecal β-Glucan Excretion and Fermentability of β-Glucan
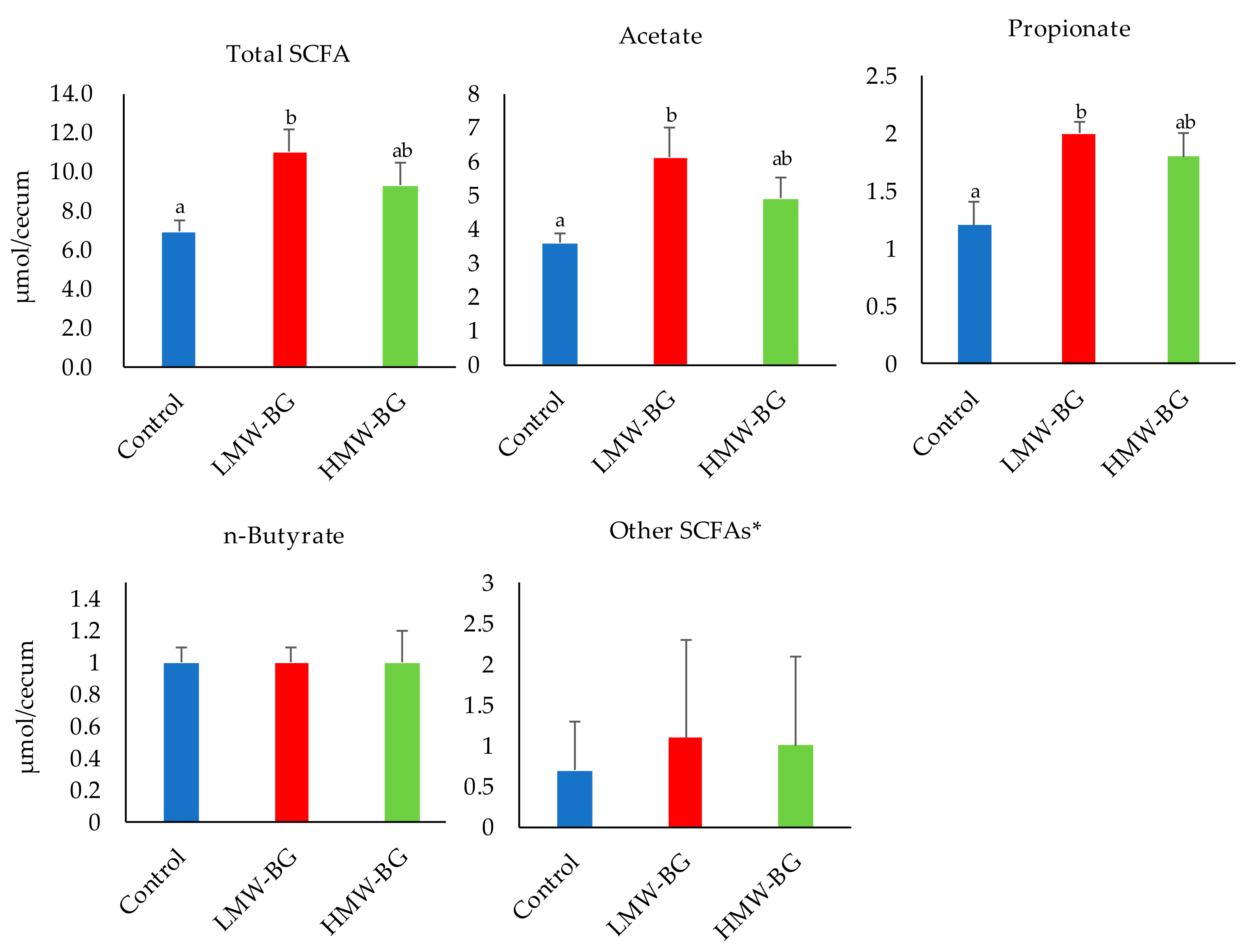
3.4. Short-Chain Fatty Acid (SCFA) Concentrations in Cecal Digesta
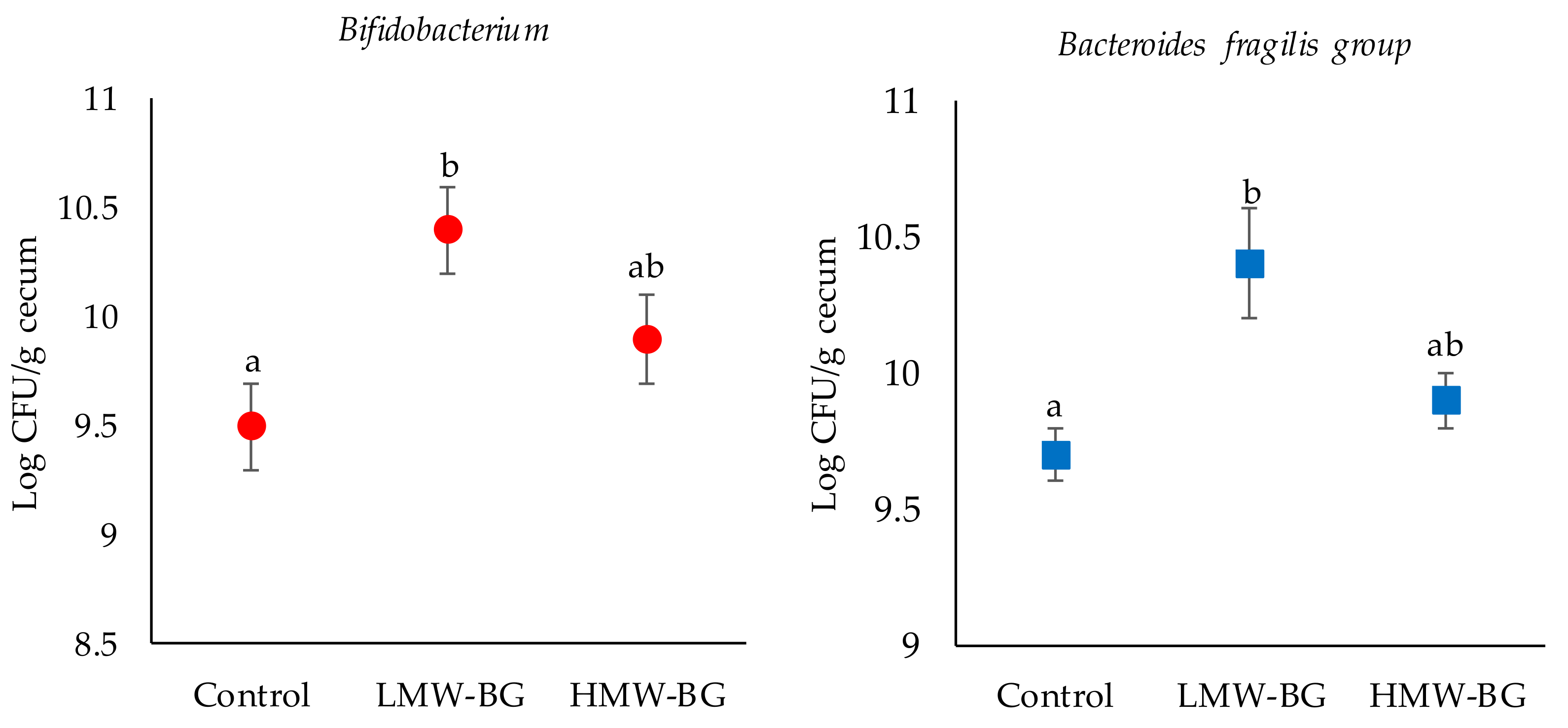
3.5. Bacterial Counts of the Major Microbiota Groups in the Cecal Digesta of Mice Fed the Test Diets
3.6. Biochemical Analysis of the Serum and Liver Lipids
3.7. Expression of mRNAs Related to Liver Lipid Metabolism
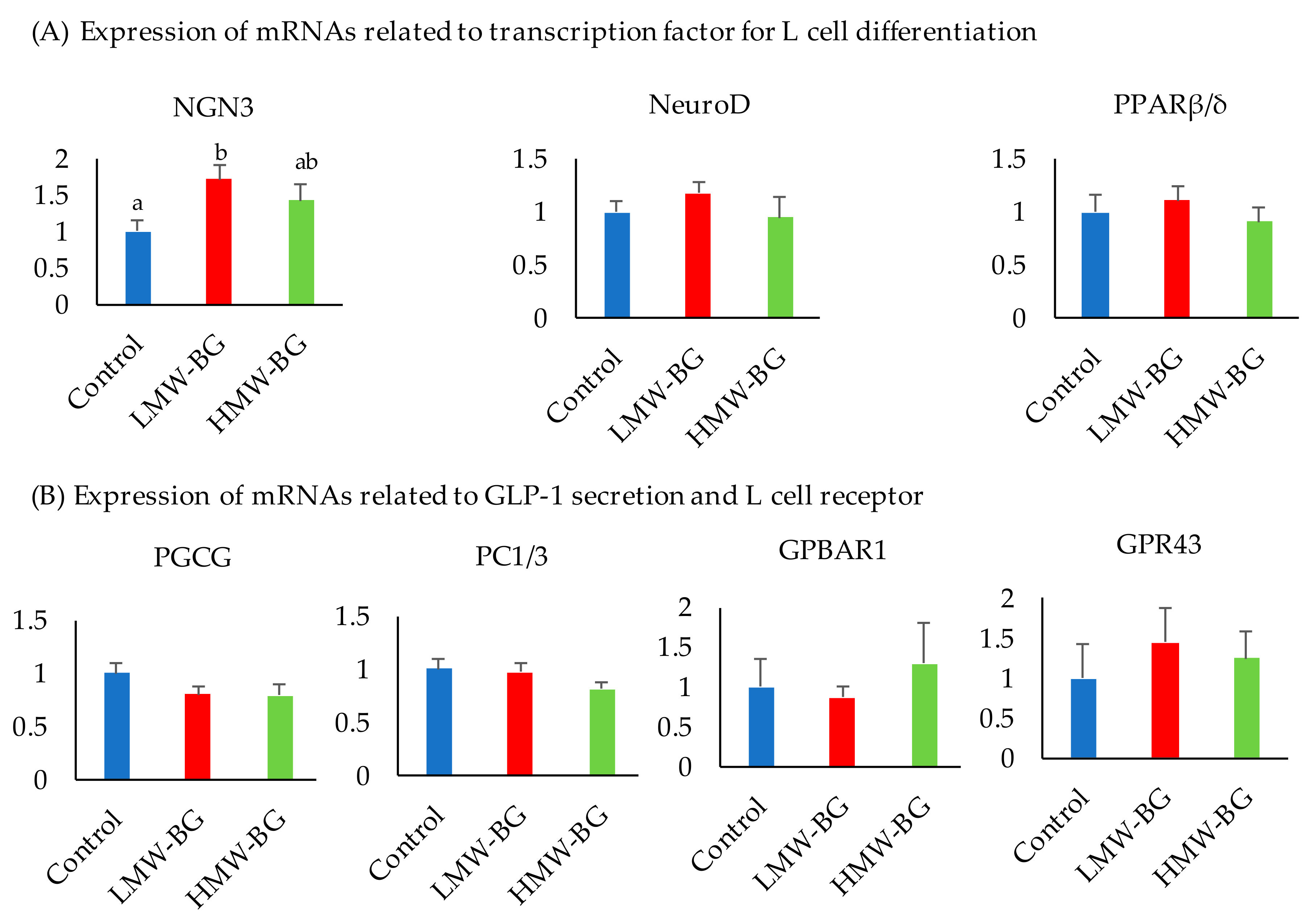
3.8. Expression of mRNAs Related to Ileal L Cell Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Juntunen, K.S.; Niskanen, L.K.; Liukkonen, K.H.; Poutanen, K.S.; Holst, J.J.; Mykkänen, H.M. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am. J. Clin. Nutr. 2002, 75, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Quek, R.Y.; Henry, C.J. Glycemic potency of muffins made with wheat, rice, corn, oat and barley flours: A comparative study between in vivo and in vitro. Eur. J. Nutr. 2015, 54, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Comparison of hormone and glucose responses of overweight women to barley and oats. J. Am. Coll. Nutr. 2005, 2, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Konig, D.; Deibert, P.; Grathwohl, D.; Baumstark, M.W.; Franz, I.W. Effect of an oat bran enriched diet on the atherogenic lipid profile in patients with an increased coronary heart disease risk. A controlled randomized lifestyle intervention study. Ann. Nutr. Metab. 2003, 47, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.A.; Moghadasian, M.H.; Jones, P.J. Cholesterol-lowering effects of oat beta-glucan. Nutr. Rev. 2011, 69, 299–309. [Google Scholar] [CrossRef]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat beta-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley beta-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reduction(i–iv). Eur. J. Clin. Nutr. 2016, 70, 1239–1245. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Macri, L.J.; MacGregor, L.W. Structure and physicochemical properties of barley non-starch polysaccharides—I. Water extractable β-Glucans and arabinoxylans. Carbohydr. Polym. 1998, 35, 249–258. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef]
- Kwong, M.G.; Wolever, T.M.; Brummer, Y.; Tosh, S.M. Increasing the viscosity of oat beta-glucan beverages by reducing solution volume does not reduce glycaemic responses. Br. J. Nutr. 2013, 110, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Ostman, E.; Rossi, E.; Larsson, H.; Brighenti, F.; Björck, I. Glucose and insulin responses in healthy men to barley bread with different levels of (1→3;1→4)-β-glucans; predictions using fluidity measurements of in vitro enzyme digests. J. Cereal Sci. 2006, 43, 230–235. [Google Scholar] [CrossRef]
- Regand, A.; Tosh, S.M.; Wolever, T.M.; Wood, P.J. Physicochemical properties of beta-glucan in differently processed oat foods influence glycemic response. J. Agric. Food. Chem. 2009, 57, 8831–8838. [Google Scholar] [CrossRef] [PubMed]
- Lia, A.; Hallmans, G.; Sandberg, A.S.; Sundberg, B.; Aman, P.; Andersson, H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 1995, 62, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef] [PubMed]
- Baea, I.N.; Lee, N.; Kim, S.M.; Lee, H.G. Effect of partially hydrolyzed oat β-glucan on the weight gain and lipid profile of mice. Food Hydrocoll. 2009, 23, 2016–2021. [Google Scholar] [CrossRef]
- Immerstrand, T.; Andersson, K.E.; Wange, C.; Rascon, A.; Hellstrand, P.; Nyman, M.; Cui, K.E.; Bergenstahl, B.; Tragardh, C.; Oste, R. Effects of oat bran, processed to different molecular weights of beta-glucan, on plasma lipids and caecal formation of SCFA in mice. Br. J. Nutr. 2010, 104, 364–373. [Google Scholar] [CrossRef]
- Mio, K.; Yamanaka, C.; Ichinose, Y.; Kohyama, N.; Yanagisawa, T.; Aoe, S. Effects of barley β-glucan with various molecular weights partially hydrolyzed by endogenous β-glucanase on glucose tolerance and lipid metabolism in mice. Cereal Chem. 2020, 97, 1056–1065. [Google Scholar] [CrossRef]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. [Google Scholar] [CrossRef]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-lowering effect of oats and oat beta glucan: Modes of action and potential role of bile acids and the microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Connolly, M.L.; Tzounis, X.; Tuohy, K.M.; Lovegrove, J.A. Hypocholesterolemic and prebiotic effects of a whole-grain oat-based granola breakfast cereal in a cardio-metabolic “At Risk” population. Front Microbiol. 2016, 7, 1675. [Google Scholar] [CrossRef] [PubMed]
- Thandapilly, S.J.; Ndou, S.P.; Wang, Y.; Nyachoti, C.M.; Ames, N.P. Barley beta-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct. 2018, 9, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Wilczak, J.; Błaszczyk, K.; Kamola, D.; Gajewska, M.; Harasym, J.P.; Jałosińska, M.; Gudej, S.; Suchecka, D.; Oczkowski, M.; Gromadzka-Ostrowska, J. The effect of low or high molecular weight oat beta-glucans on the inflammatory and oxidative stress status in the colon of rats with LPS-induced enteritis. Food Funct. 2015, 6, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Rodriguez, F.; Storey, M.; Farmakalidis, E.; Prosky, L. Determination of soluble and insoluble dietary fiber in psyllium containing cereal products. J. AOAC Int. 1995, 78, 724–729. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Codd, R. Measurement of (1→3), (1→4)-β-D-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1962, 226, 497–509. [Google Scholar]
- Kojima, M.; Arishima, T.; Shimizu, R.; Kohno, M.; Kida, H.; Hirotsuka, M.; Ikeda, I. Consumption of a structured triacylglycerol containing behenic and oleic acids increases fecal fat excretion in humans. J. Oleo Sci. 2013, 62, 997–1001. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, T.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Matsuda, K.; Tsuji, H.; Asahara, T.; Kado, Y.; Nomoto, K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 2007, 73, 32–39. [Google Scholar] [CrossRef]
- Matsuki, T. Development of quantitative PCR detection method with 16S rRNA gene-targeted genus- and species-specific primers for the analysis of human intestinal microflora and its application. Jpn. J. Bacteriol. 2007, 62, 255–261. [Google Scholar] [CrossRef]
- Fujita, Y.; Cheung, A.T.; Kieffer, T.J. Harnessing the gut to treat diabetes. Pediatr. Diabetes 2004, 5, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Hoste, S.; Guiot, Y.; Delzenne, N.M. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 2007, 98, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.L.; Sorrentino, G.; Egerod, K.L.; Kroone, C.; Mortensen, B.; Knop, F.K.; Reimann, F.; Gribble, F.M.; Drucker, D.J.; de Koning, E.J.P.; et al. L-cell differentiation is induced by bile acids through GPBAR1 and paracrine GLP-1 and serotonin signaling. Diabetes 2020, 69, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Thondre, P.S.; Shafat, A.; Clegg, M.E. Molecular weight of barley β-glucan influences energy expenditure, gastric emptying and glycaemic response in human subjects. Br. J. Nutr. 2013, 110, 2173–2179. [Google Scholar] [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Horton, J.D. Sterol regulatory element-binding proteins: Transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 2002, 30, 1091–1095. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.I.; Kim, T.H.; Im, S.S.; Park, S.K.; Lee, I.K.; Kim, K.S.; Ahn, Y.H. SREBP-1c mediates the insulin-dependent hepatic glucokinase expression. J. Biol. Chem. 2004, 279, 30823–30829. [Google Scholar] [CrossRef]
- Aoe, S.; Watanabe, N.; Yamanaka, C.; Ikegami, S. Effects of barley on carbohydrate metabolism and abdominal fat accumulation in diet-induced obese mice. J. Jpn. Assoc. Diet. Fiber Res. 2010, 14, 55–65. [Google Scholar]
- Aoe, S.; Ichinose, Y.; Kohyama, N.; Komae, K.; Takahashi, A.; Yoshioka, T.; Yanagisawa, T. Effects of β-glucan content and pearling of barley in diet-induced obese mice. Cereal Chem. 2017, 94, 956–962. [Google Scholar] [CrossRef]
- Guo, R.; Xu, Z.; Wu, S.; Li, X.; Li, J.; Hu, H.; Wu, Y.; Ai, L. Molecular properties and structural characterization of an alkaline extractable arabinoxylan from hull-less barley bran. Carbohydr. Polym. 2019, 15, 250–260. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Possemiers, S.; Druart, C.; Van de Wiele, T.; De Backer, F.; Cani, P.D.; Larondelle, Y.; Delzenne, M.N. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS ONE 2011, 6, e20944. [Google Scholar] [CrossRef] [PubMed]

| (g/kg Diet) | |||
|---|---|---|---|
| Control | LMW-BG | HMW-BG | |
| Casein | 200 | 183 | 200 |
| L-cystin | 3 | 3 | 3 |
| Corn starch | 350.7 | 300.9 | 350.7 |
| Dextrinized corn starch | 132 | 132 | 45.4 |
| Sucrose | 100 | 100 | 100 |
| Soybean oil | 70 | 70 | 70 |
| Lard | 42 | 42 | 42 |
| Cellulose | 54.8 | - | 14.8 |
| LMW-BG | - | 121.6 | - |
| HMW-BG mixed with corn starch | - | - | 126.6 |
| AIN-93G mineral mixture | 35 | 35 | 35 |
| AIN-93 vitamin mixture | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| t-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Control | LMW-BG | HMW-BG | |
|---|---|---|---|
| Initial weight (g) | 20.6 ± 0.5 | 20.6 ± 0.4 | 20.6 ± 0.4 |
| Final weight (g) | 31.3 ± 0.9 | 31.0 ± 0.8 | 28.9 ± 0.7 |
| Body weight gain (g/d) | 0.19 ± 0.01 a | 0.17 ± 0.01 ab | 0.14 ± 0.01 b |
| Food intake (g/d) | 4.1 ± 0.2 a | 3.2 ± 0.1 b | 3.4 ± 0.2 ab |
| Food efficiency ratio (%) | 4.56 ± 0.23 ab | 5.30 ± 0.25 a | 4.01 ± 0.22 b |
| Control | LMW-BG | HMW-BG | |
|---|---|---|---|
| Liver (g) | 1.11 ± 0.03 a | 1.04 ± 0.05 ab | 0.92 ± 0.02 b |
| Cecum with digesta (g) | 0.30 ± 0.03 a | 0.51 ± 0.03 b | 0.44 ± 0.03 b |
| Total abdominal fat | 2.09 ± 0.11 a | 1.54 ± 0.23 ab | 1.25 ± 0.19 b |
| Retroperitoneal fat (g) | 0.46 ± 0.03 a | 0.32 ± 0.06 ab | 0.25 ± 0.05 b |
| Epididymal fat (g) | 1.22 ± 0.06 a | 0.89 ± 0.08 ab | 0.75 ± 0.10 b |
| Mesenteric fat (g) | 0.42 ± 0.03 a | 0.33 ± 0.05 ab | 0.26 ± 0.05 b |
| Control | LMW-BG | HMW-BG | |
|---|---|---|---|
| Fat intake (mg/d) | 404 ± 1 a | 339 ± 1 b | 344 ± 1 b |
| Fecal fat excretion (mg/d) | 11 ± 1 a | 21 ± 3 ab | 39 ± 10 b |
| Apparent digestibility of fat (%) | 97.2 ± 0.2 a | 93.8 ± 0.7 ab | 88.2 ± 3.2 b |
| Control | LMW-BG | HMW-BG | |
|---|---|---|---|
| Total cholesterol (mmol/L) | 3.42 ± 0.09 a | 2.57 ± 0.23 b | 2.66 ± 0.12 b |
| LDL-cholesterol (mmol/L) | 0.15 ± 0.01 a | 0.09 ± 0.01 b | 0.09 ± 0.01 b |
| HDL-cholesterol (mmol/L) | 1.88 ± 0.05 a | 1.52 ± 0.15 b | 1.54 ± 0.07 ab |
| Triglyceride (mmol/L) | 0.52 ± 0.05 | 0.45 ± 0.07 | 0.31 ± 0.04 |
| NEFA (μmol/L) | 635.9 ± 23.1 ab | 654.9 ± 36.6 a | 533.5 ± 37.1 b |
| Glucose (mmol/L) | 21.1 ± 0.69 a | 17.21 ± 1.17 b | 18.64 ± 0.78 ab |
| Insulin (ng/mL) | 1.20 ± 0.17 a | 1.05 ± 0.24 ab | 0.39 ± 0.13 b |
| Leptin (ng/mL) | 15.30 ± 1.29 a | 9.58 ± 2.05 b | 5.74 ± 1.19 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoe, S.; Mio, K.; Yamanaka, C.; Kuge, T. Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients 2021, 13, 130. https://doi.org/10.3390/nu13010130
Aoe S, Mio K, Yamanaka C, Kuge T. Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients. 2021; 13(1):130. https://doi.org/10.3390/nu13010130
Chicago/Turabian StyleAoe, Seiichiro, Kento Mio, Chiemi Yamanaka, and Takao Kuge. 2021. "Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects" Nutrients 13, no. 1: 130. https://doi.org/10.3390/nu13010130
APA StyleAoe, S., Mio, K., Yamanaka, C., & Kuge, T. (2021). Low Molecular Weight Barley β-Glucan Affects Glucose and Lipid Metabolism by Prebiotic Effects. Nutrients, 13(1), 130. https://doi.org/10.3390/nu13010130





