1. Introduction
Micronutrient malnutrition affects more than half of the global population, primarily in developing regions [
1,
2]. Iron (Fe), zinc (Zn), and vitamin A deficiencies are prominent health constraints worldwide [
3]. In low-income countries, plants are the significant source of food. In crude cereal and legume foods, the low bioavailability of Fe and Zn leads to metabolic disorders that are associated with these nutritional factors. Hence, increasing the nutritional value of such types of dietary ingredients will contribute to the nutritional status of the target population. Mineral, phosphorous, and phytate content is much higher in bran than whole grain [
4,
5,
6].
Field pea (
Pisum sativum L.) is a main pulse crop grown for human consumption as a source of protein, carbohydrates, minerals, and bioactive plant-origin bioactive compounds, contributing to better metabolic health. In 2014, the global production of peas was 11.2 million tons [
7]. The main component of pea is starch, which includes two polymers of
d-glucose: amylose and amylopectin [
8,
9]. Because of the alterations in physiochemical characteristics between pulses and cereal starches, starch from pulses can deliver some specific features to food systems as high gelation temperature, resistance to shear thinning, increased elasticity, and high concentration of resistant starch [
10].
In addition, field peas include bioactive compounds such as oligosaccharides, polyphenols, and phytate [
11]. Water-soluble carbohydrates in peas comprise mostly disaccharides and oligosaccharides. The raffinose group of oligosaccharides (RFOs) is the most targeted in pea research. These factors include galactose molecules (linked by α-
d-1, 6-glycosidic bonds) attached to sucrose [
12]. Humans lack the essential enzymes that are essential to break down these RFOs, and this results in these oligosaccharides being digested by intestinal bacterial populations via fermentation, leading to elevated short-chain fatty acid production [
13]. Furthermore, a recent study indicated that intra-amniotic administration of raffinose upregulated the expression of brush border membrane (BBM) functional proteins, downregulated the expression of Fe-related proteins (indicating improvement of dietary iron bioavailability), and elevated villus surface area. Furthermore, raffinose increased the richness and composition of probiotic populations, and it reduced that of pathogenic bacterial species. Overall, raffinose improved microbial population, dietary Fe bioavailability, and BBM functionality in vivo [
14].
The main phenolic compounds found in peas comprise condensed tannins, flavonoids, and phenolic acids [
15]. These phenolic compounds are found specifically in the seed coat and are biosynthesized via the phenylpropanoid pathway, with condensed tannin molecules being responsible for the seed-coat coloring [
16]. In dark-colored hulls, tannin and flavonoid compounds are the majority of phenolic compounds; however, in seeds with clear hulls, phenolic acids are the main compounds [
17]. Polyphenols in the seed coat present antioxidant and anti-mutagenic activity, shielding the seed from oxidative stress [
18]. In field conditions, these compounds also deliver chemical resistance against pathogens and insect pests during the growing process of the plant [
19]. Polyphenols in peas appear mostly as insoluble or bound forms, covalently bonded to structural components of the cell wall such as cellulose, hemicellulose, lignin, and pectin [
20,
21]. The polyphenolic composition of peas is predominantly interesting with respect to metabolic health, given their alleged protective properties against oxidative stress [
15,
22]. According to Campos-Vega [
11] and Rochfort [
23], isoflavone polyphenols are linked with biological pathways in the lessening of osteoporosis and cardiovascular disease, the deterrence of cancer, and treating symptoms related to menopause. Phenolic compounds also display anti-nutritional effects, and related research showed a decrease in the bioavailability of proteins triggered by phenolic compounds [
24]. Phytate functions as a storage for phosphate and minerals in seeds that can be recovered during germination process [
25]. Phytate was recognized as an anti-nutrient due to its ability to chelate with multivalent ions, specifically Zn, Ca, and Fe, inhibiting the body’s capability to absorb dietary minerals by limiting their bioavailability [
24]. There is increasing interest in utilizing pulses in wheat-based products with blends [
26]. The demand for gluten-free products led to investigation of the nutritional characteristics of baked products from pulses like chickpea and lentil [
27], as well as peas [
28]. The rheological properties of pea flour, including the gelation properties of starch, may be considered when exploring the potential application of pea flour in baked goods. Recent uses for pulses could increase the demand for pulses with specific nutritional and rheological properties, which will increase the need to investigate the components affecting the nutritional and functional properties of pulses. It was previously demonstrated that low-phytate pea lines had higher Fe bioavailability than regular or standard pea [
29]; in addition, pea varieties which were low-phytate combined with relatively higher carotenoid concentration in some cases resulted in a further increase in Fe bioavailability in vitro [
30].
Biofortified staple foods are an effective instrument through which to address micronutrient deficiencies worldwide, with emphasis on Fe and Zn, in numerous target populations [
1,
31,
32,
33,
34,
35]. The in vivo (
Gallus gallus) model was established as an excellent model to assess dietary Fe and Zn bioavailability [
33,
34,
35,
36,
37,
38,
39]. Hence, the objective of the current study was to evaluate the ability of low-phytate pea varieties in the context of a complete meal to improve Fe bioavailability and absorption, physiological status, intestinal BBM functionality, and intestinal microbial populations in vivo (
Gallus gallus). We suggest the further use of in vivo screening model to guide future studies aimed to investigate biofortified staple food crops, as this method will allow proceeding to human efficacy studies with superior confidence and success.
4. Discussion
The objective of the current study was to investigate the effects of low-phytate peas, in the context of a complete meal, on Fe bioavailability, absorption, physiological status, intestinal BBM functionality, and gastrointestinal microbial populations in vivo (Gallus gallus).
In studies of biofortification, the process via which the nutritional quality of food crops is improved through agronomic practices, conventional plant breeding, or modern biotechnology [
2], it is necessary and advantageous to utilize in vivo screening tools that are capable of assessing biofortified varieties of staple crops, as well as in relation to the diet in which they are consumed [
1,
33,
36,
38,
39,
46,
54,
55,
56]. The present study, for the first time, presents a demonstration of how the
Gallus gallus model of Fe (and Zn) bioavailability could be useful in the design of the current study aimed at assessing the potential nutritional benefit of lower-phytate versus standard peas. The chosen dietary composition was specifically formulated in accordance to a potential target population (Indian/Bangladeshi pea-based dal meal), similar to previous in vivo studies aimed at assessing dietary Fe bioavailability in beans [
35,
55] and wheat [
38] (
Table 1). Overall, our data agree with previously published knowledge [
1,
39,
49,
55], demonstrating that this in vivo screening approach is effective in the evaluation process of nutritional qualities of the low-phytate pea varieties. Furthermore, the data suggested that lower-phytate pea-based diets were able to moderately improve Fe physiological status in vivo.
Peas are a common staple food crop consumed worldwide, primarily in India, China, Russia, Ethiopia, and Bangladesh. Global dry pea production increased from 9.9 million tons in 2012 to 16.2 million tons in 2017 [
7]. Currently, the leading producers are Canada, Russia, China, Ukraine, and India. In Canada, a leading producer and exporter of dry peas, pea was grown on 1.6 million ha in western Canada (Saskatchewan, Alberta, and Manitoba) in 2017, indicating a significant alteration in cropping practices from the 300 ha reported in 1967. Pea was the major alternative crop as farmers shifted toward a more diversified crop production. Pea varieties (yellow and green cotyledon) are grown, with an average of 80% production in yellow cotyledon varieties. The five-year (2013–2017) average pea yield in western Canada is 2.6 tons/ha (38 bu/ac) [
57]. As for their nutritional value, it was previously demonstrated that pea seeds are high in protein, carbohydrates, fiber, B vitamins, and minerals (potassium, magnesium, calcium, iron), and they are considered an inexpensive source of energy-dense, nutrient-rich food [
58,
59,
60]. In addition, pea seeds are low in fat and cholesterol-free. Because of these nutritional benefits, worldwide pea utilization is expected to continue to grow.
Plant seeds, such as pea, contain a high concentration of phosphorus. However, about 60–80% of the total phosphorus in seeds is stored in the form of phytate, a mixed-cation salt of phytic acid [
59]. This introduces a nutritional challenge, as negatively charged sites of phytic acid bind and form salts with K
+, Mg
2+, Ca
2+, Mn
2+, Zn
2+, or Fe
3+ [
61]. Phytate causes multiple difficulties, as non-ruminant animals including pig, poultry, fish, and humans, are unable to digest phytate due to lack of a phytase enzyme [
61]; as a result, important micronutrients (as Zn
2+ and Fe
3+) bound to phytate are also excreted and not absorbed, potentially leading to micronutrient deficiencies [
62]. Recently, the development of cultivars with low-phytate content became an effective approach to potentially reducing nutritional concerns ascending from the consumption of phytate-rich grains. Low-phytate varieties were chemically persuaded in maize (
Zea mays L.) [
63], soybean (
Glycine max (L.) Merr.) [
64], barley (
Hordeum vulgare L.) [
63,
65], rice (
Oryza sativa L.) [
66], wheat (
Triticum aestivum L.) [
67], bean (
Phaseolus vulgaris L.) [
68], and pea [
41]. The concentration of phytate phosphorus is significantly reduced in the mutants with an associated increase in available phosphorus. Wilcox et al. [
65] reported an 80% reduction in phytate phosphorus content in a low-phytate soybean mutant, as compared with its nonmutant sibling, and this reduction was matched by an equal increase in inorganic phosphorus.
It was previously demonstrated that low-phytate crops increase the bioavailability of phosphorus and several important nutritional cations, including Fe. These crops could assist in increasing the health of a large proportion of the global population, which is dietary Fe-deficient, primarily in target regions where dietary peas are consumed regularly. For example, in a previous study focused on the nutritional evaluation of low-phytate pea diets in vivo, it was demonstrated that animals fed the low-phosphorus diets had lower weight gain and feed intake (
p < 0.01) than those fed the higher phosphorus level. Bone strength was higher (
p < 0.01) for animals fed diets based on low-phytate pea than for those fed diets based on normal pea or soybean meal. The authors concluded that increasing the availability of the phosphorus in peas could mean that less inorganic phosphorus would be required in order to meet the nutritional requirements of broilers [
59].
In the context of the current study, the results indicated that, despite Hb levels not being significantly higher in the lower-phytate pea groups, significant differences in total body Hb-Fe, the physiological Fe status biomarker [
33,
34,
35,
36,
39,
46,
55], were observed (
Table 8), representing an enhancement in Fe status in the 4802-8-87Y-L dietary group, relative to CDC Bronco and the no-pea dietary group. In addition, the standard pea variety (CDC Bronco) treatment group had a lower HME (
p < 0.05) ratio compared to the group receiving the lower-phytate pea-based diets (groups 1-2347-144, 4803-4-78G-L) (
Table 8), indicating improved dietary Fe bioavailability and increased absorbable Fe [
36,
46,
54]. The CDC Bronco diet presented a higher PA–Fe ratio compared to the all low-phytate pea-based diets (
Table 1), which was associated with increased dietary Fe bioavailability in these pea-based diets [
69,
70,
71]. These results agree with preceding experiments intended to assess Fe bioavailability in Fe-biofortified legumes, such as black beans [
72], red mottled beans [
33], Carioca beans [
36], and pearl millet [
73], as well as in the context of a complete diet. Thus, several intrinsic factors, including phytates, may influence the bioavailability of Fe from these pea varieties and other crops [
56,
74,
75,
76], potentially limiting their nutritional benefit.
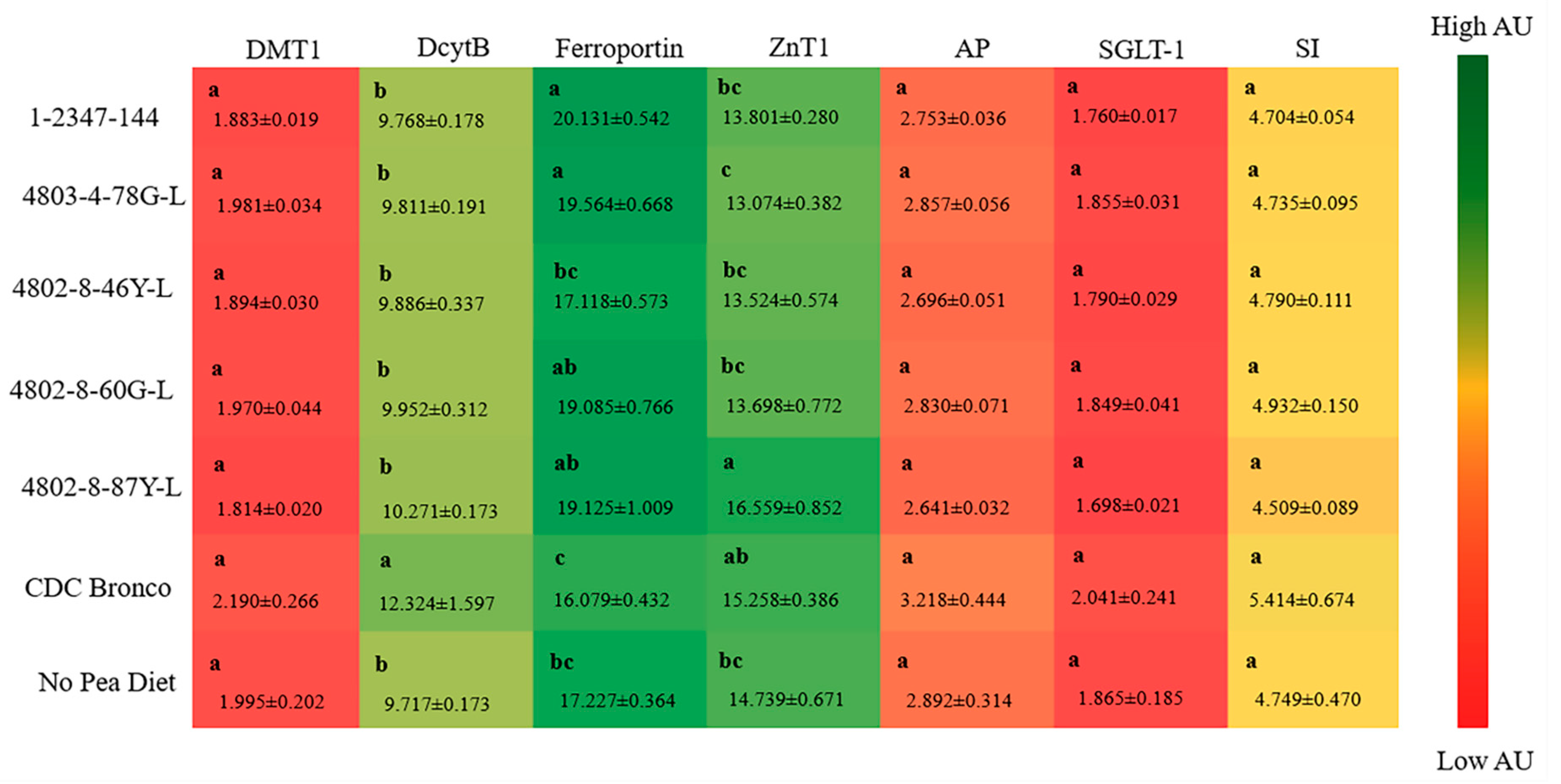
Previous research suggested that increased Fe content alone in biofortified crops may not be adequate to produce a significant physiological improvement in Fe status and in Fe-deficient populations [
36,
55,
76]. In the current study, it appears that, although Fe contents of all tested pea varieties were similar, the consumption of lower-phytate peas was able to moderately improve Fe status and storage, as further suggested by the hepatic ferritin contents of lower-phytate groups relative to CDC Bronco and no-pea diets. Furthermore, the duodenal brush border membrane (BBM) gene expression of ferroportin (FPN) was significantly upregulated, while DcytB was downregulated in the groups receiving the lower-phytate pea-based diets, relative to the CDC Bronco dietary group (
p < 0.05,
Figure 2). However, no significant alterations in the expression of BBM functional proteins were detected amongst treatment groups. Previous studies showed a downregulation of the gene expression of Fe-related BBM proteins (DMT-1, FPN, and Dcytb) in Fe-biofortified diets compared to the Fe-standard diets [
36,
46,
55]. Ferroportin is the Fe exporter that transfers Fe across the enterocyte’s basolateral membrane [
77]. Hence, since the lower-phytate pea-based dietary groups had a higher expression of FPN, more Fe could be transported from the enterocyte into the blood and target tissue; therefore, this mechanism indicates the potential increased amount of absorbable Fe and, hence, the total body Hb-Fe increased in some of the low phytate groups compared to the CDC Bronco and no-pea dietary groups.
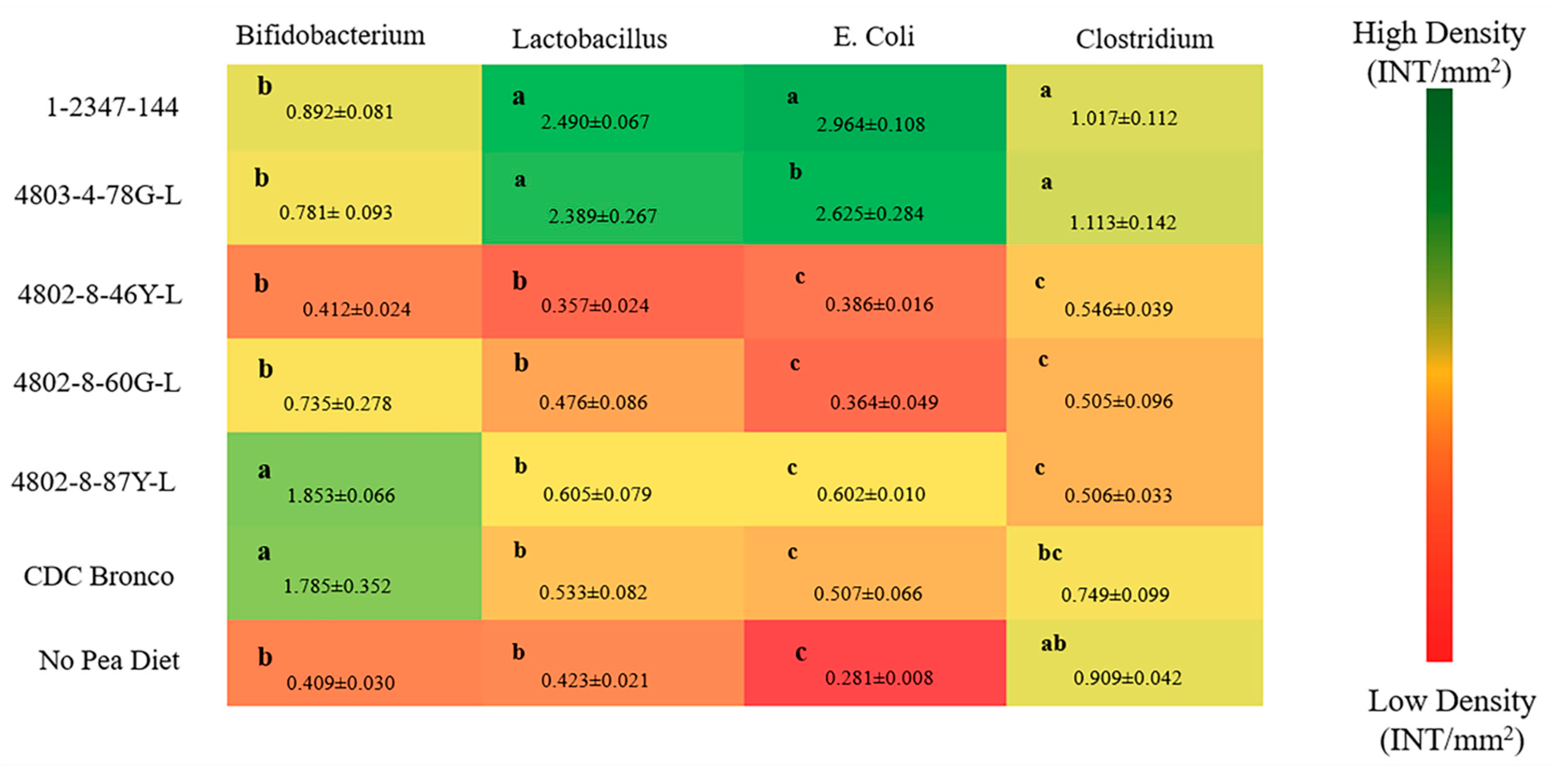
Similar to humans and most animals, the
Gallus gallus model harbors a complex and active intestinal microbiota [
78], significantly and directly influenced by host genetics, environment, and diet [
79]. There is a significant resemblance at the phylum level between the gut microbiota of
Gallus gallus and humans, with Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria representing the dominant bacterial phyla in both [
80]. In this study, a genus- and species-level bacterial population delineation among the low-phytate, standard (CDC Bronco), and no-pea dietary groups was observed. Results indicated that the abundance of
Bifidobacterium was significantly higher (
p < 0.05) in the 4802-8-87Y-L and CDC Bronco groups relative to all other treatment groups. Furthermore, the abundance of
Lactobacillus was significantly higher (
p < 0.05) in the 1-2347-144 and 4803-4-78G-L treatment groups relative to all other treatment groups (
Figure 3). These results suggest that the above lower-phytate pea-based diets may potentially improve the host overall gut health by promoting the abundance of beneficial bacterial populations. Moreover, some of the low-phytate pea varieties (as 1-2347-144) presented a higher (
p < 0.05) total fiber content (soluble and insoluble) compared to the standard CDC Bronco pea (
Table 3). It was previously demonstrated that soluble fiber can increase villi height by elevating intestinal cell proliferation [
81]. In the current study, some of the low-phytate pea dietary groups (such as 4803-4-78G-L, 4802-8-46Y-L, and 4802-8-87Y-L) presented higher (
p < 0.05) protein content compared to the standard CDC Bronco pea (
Table 3), where a higher dietary protein content was shown to increase villi height and intestinal cell proliferation [
82]. Furthermore, indigested dietary proteins and fibers are fermented in the lower intestine, and this action produces short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. Production of SCFAs affects metabolism and gastrointestinal health [
83]. Acetate and propionate are energy substrates for peripheral tissues, and butyrate is referentially used as an energy source by colonocytes [
84,
85].
In summary, the current study focused on the performance of low-phytate pea varieties in chicken diets. Phytate phosphorus concentration was reduced by approximately 40% in these varieties. The low-phytate pea variety-based diets were able to moderately improve the Fe status in vivo, suggesting that low-phytate field pea has the potential to improve Fe bioavailability in human diets, particularly in the Indian subcontinent, as one of the major importing regions for Canadian peas, and a region where dietary Fe deficiency is a major health concern. Furthermore, as the abolition of micronutrient malnutrition remains a widespread global health problem in developing countries, the current study suggests that increasing micronutrient intake in food through food-based approaches is a sustainable method for the potential prevention of micronutrient deficiencies. Biofortification offers a long-term, sustainable, food-based solution for a world population, and breeding programs may aim to improve grain Zn and Fe concentrations; however, as previously suggested, improving Fe or Zn content may not necessarily result in the desired outcome (i.e., breeding toward increased mineral content may also lead to increased potential dietary inhibitors) and, hence, may not be as effective. In low-income countries, breeding for mineral solidity may remain the only agricultural involvement available to improve the nutritional content of staple crops, and, as suggested in the current study, the genetic improvement of staple food crops, specifically the development of low-phytate pea verities, resulted in improved nutritional quality and dietary Fe bioavailability, including in a complete diet context.
Additionally, as previously demonstrated, the current study presents a cost-effective approach designed to assess the effectiveness of biofortified pea varieties in vivo, as these varieties were developed with an aim to reduce the inhibitory effect of dietary phytate on Fe bioavailability. Therefore, our findings suggest that the use of lower-phytate biofortified peas may be an effective and sustainable approach to decreasing the global abundance of Fe deficiency, with added improvements in intestinal bacterial population structure and intestinal BBM functionality.