Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.1.1. Assessment of Blood Pressure
2.1.2. Nutrient Data
2.1.3. Dietary Indices
2.2. Statistical Analysis
Heritability
3. Results
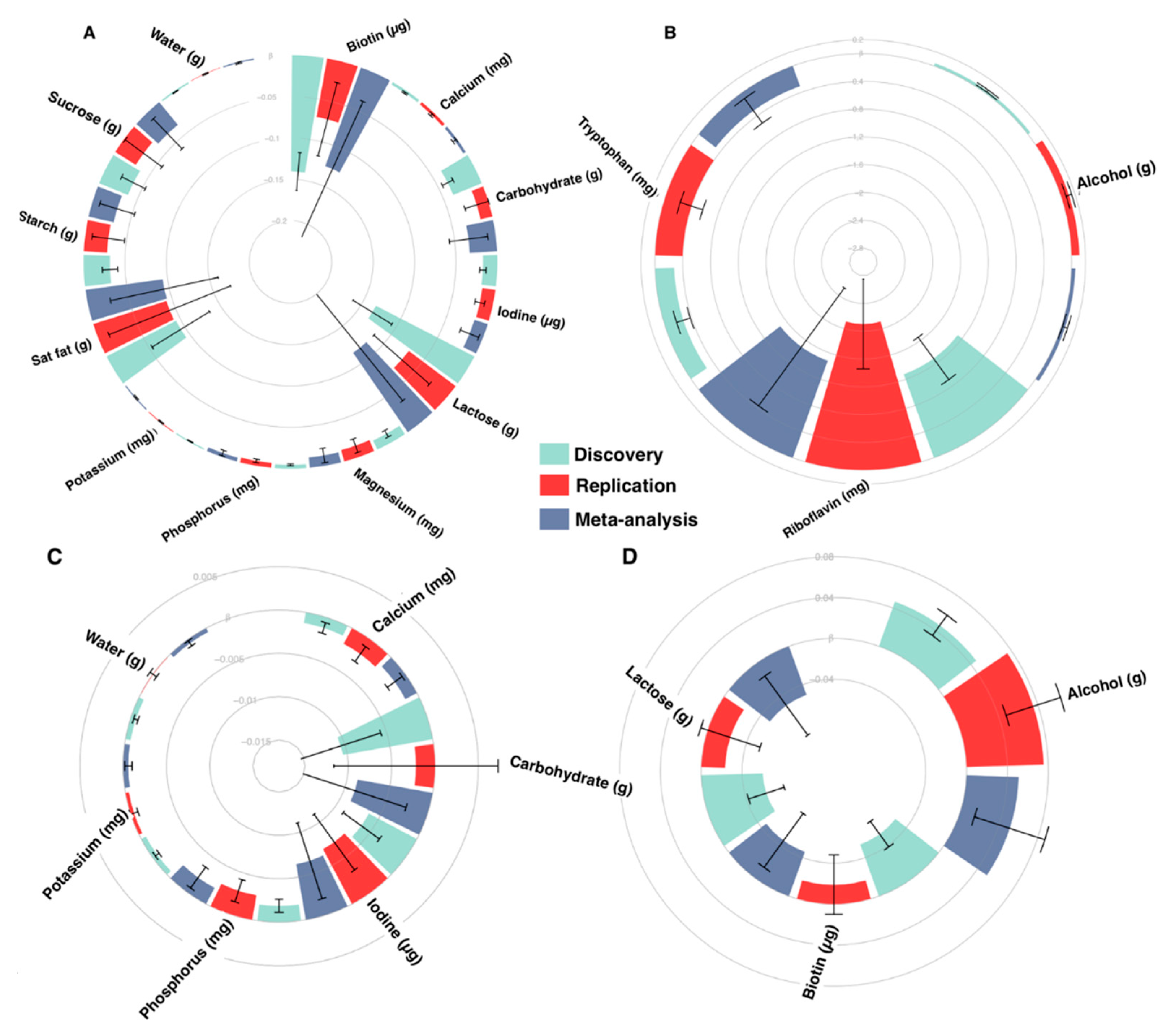
3.1. Nutrient Intake-Blood Pressure Associations
3.2. Dietary Indices and Blood Pressure
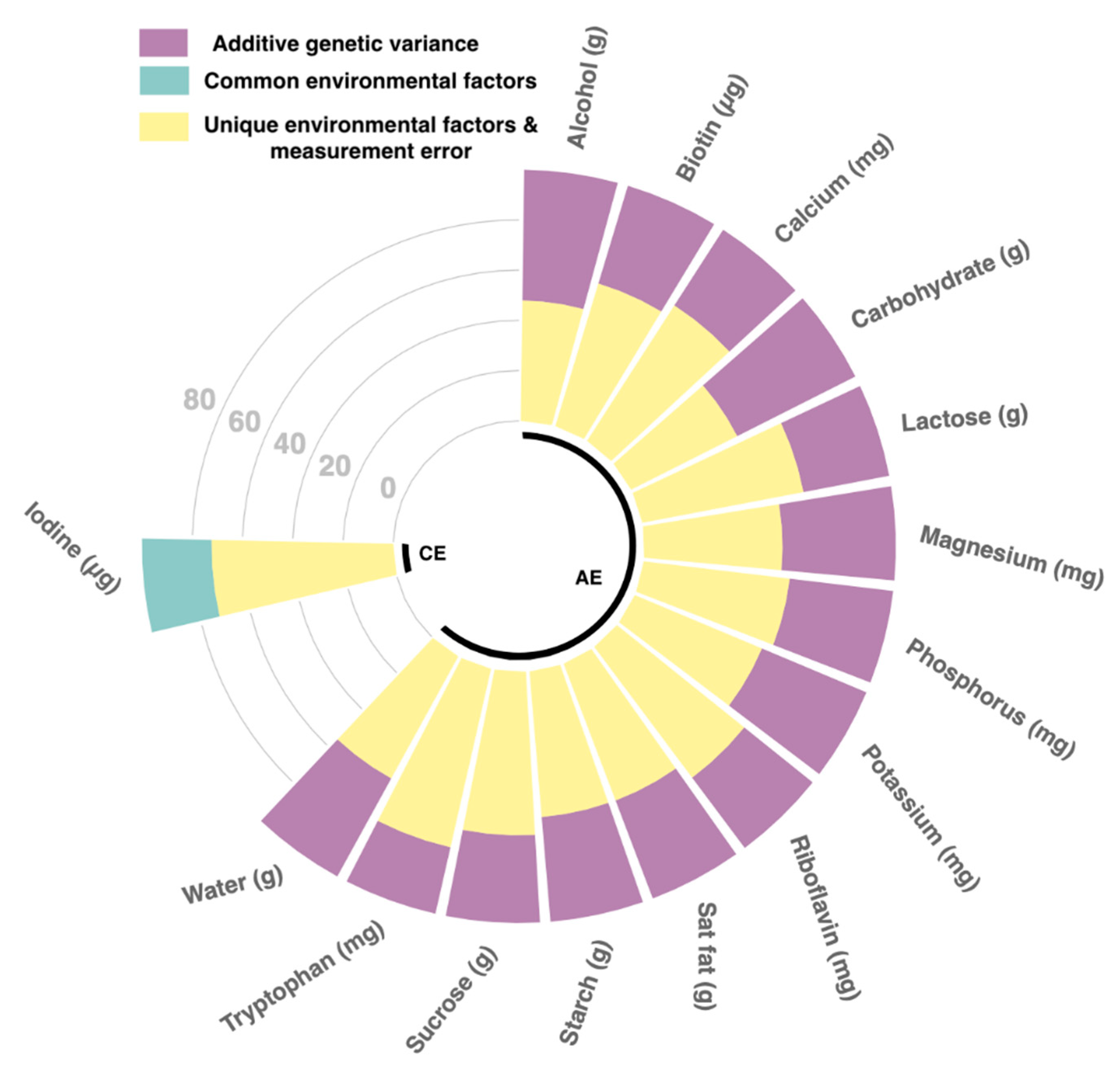
3.3. Heritability
4. Discussion
Nutrients Independently Associated with BP
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017, 2, 775. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; Taylor, J.O.; Hennekens, C.H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- O’Brien, E. The Lancet Commission on hypertension: Addressing the global burden of raised blood pressure on current and future generations. J. Clin. Hypertens. 2017, 19, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Ibekwe, R. Modifiable Risk factors of Hypertension and Socio-demographic Profile in Oghara, Delta State; Prevalence and Correlates. Ann. Med. Health Sci. Res. 2015, 5, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Hinderliter, A.; Watkins, L.L.; Craighead, L.; Lin, P.-H.; Caccia, C.; Johnson, J.; Waugh, R.; Sherwood, A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch. Intern. Med. 2010, 170, 126–135. [Google Scholar] [CrossRef]
- Bhat, S.; Mocciaro, G.; Ray, S. The association of dietary patterns and carotid intima-media thickness: A synthesis of current evidence. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef]
- Whelton, P.K.; He, J.; Cutler, J.A.; Brancati, F.L.; Appel, L.J.; Follmann, D.; Klag, M.J. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997, 277, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Verdi, S.; Abbasian, G.; Bowyer, R.C.E.; Lachance, G.; Yarand, D.; Christofidou, P.; Mangino, M.; Menni, C.; Bell, J.T.; Falchi, M.; et al. TwinsUK: The UK Adult Twin Registry Update. Twin Res. Hum. Genet. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Welch, A.A.; McTaggart, A.; Mulligan, A.A.; Runswick, S.A.; Luben, R.; Oakes, S.; Khaw, K.T.; Wareham, N.; Day, N.E. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr. 2001, 4, 847–858. [Google Scholar] [CrossRef]
- Moayyeri, A.; Hammond, C.J.; Valdes, A.M.; Spector, T.D. Cohort Profile: TwinsUK and healthy ageing twin study. Int. J. Epidemiol. 2013, 42, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef] [PubMed]
- McCance, R.A.; Holland, B.; Widdowson, E.M. McCance and Widdowson’s the Composition of Foods; 5th rev. and extended ed.; Royal Society of Chemistry; Ministry of Agriculture, Fisheries and Food: Cambridge, UK, 1993; ISBN 978-0-85186-391-7. [Google Scholar]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018, 118, 74–100. [Google Scholar] [CrossRef]
- Matsunaga, M.; Hurwitz, E.L.; Li, D. Development and Evaluation of a Dietary Approaches to Stop Hypertension Dietary Index with Calorie-Based Standards in Equivalent Units: A Cross-Sectional Study with 24-Hour Dietary Recalls from Adult Participants in the National Health and Nutrition Examination Survey 2007–2010. J. Acad. Nutr. Diet. 2018, 118, 62–73. [Google Scholar] [CrossRef]
- Rumawas, M.E.; Dwyer, J.T.; McKeown, N.M.; Meigs, J.B.; Rogers, G.; Jacques, P.F. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J. Nutr. 2009, 139, 1150–1156. [Google Scholar] [CrossRef]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Kim, H.; Rebholz, C.M.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Caulfield, L.E. Operational Differences in Plant-Based Diet Indices Affect the Ability to Detect Associations with Incident Hypertension in Middle-Aged US Adults. J. Nutr. 2020, 150, 842–850. [Google Scholar] [CrossRef]
- Neale, M.C.; Cardon, L.R. Methodology for Genetic Studies of Twins and Families; Springer: Dordrecht, The Netherlands, 1992; ISBN 978-90-481-4179-1. [Google Scholar]
- Verhulst, B.; Estabrook, R. Using genetic information to test causal relationships in cross-sectional data. J. Theor. Polit. 2012, 24, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Rijsdijk, F. Common Pathway Model. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; p. stat06700. ISBN 978-1-118-44511-2. [Google Scholar]
- Menni, C.; Mangino, M.; Zhang, F.; Clement, G.; Snieder, H.; Padmanabhan, S.; Spector, T.D. Heritability analyses show visit-to-visit blood pressure variability reflects different pathological phenotypes in younger and older adults: Evidence from UK twins. J. Hypertens. 2013, 31, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tuvblad, C.; Raine, A.; Baker, L. Genetic and environmental influences on nutrient intake. Genes. Nutr. 2013, 8, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.F.; O’Connell, D.L.; Roberts, D.C.; Allen, J.R.; Knapp, J.C.; Steele, P.L.; Silove, D. Lifestyle factors in monozygotic and dizygotic twins. Genet. Epidemiol. 1988, 5, 311–321. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Joe, B. Towards Precision Medicine for Hypertension: A Review of Genomic, Epigenomic, and Microbiomic Effects on Blood Pressure in Experimental Rat Models and Humans. Physiol. Rev. 2017, 97, 1469–1528. [Google Scholar] [CrossRef]
- McAuley, E.; McNulty, H.; Hughes, C.; Strain, J.J.; Ward, M. Riboflavin status, MTHFR genotype and blood pressure: Current evidence and implications for personalised nutrition. Proc. Nutr. Soc. 2016, 75, 405–414. [Google Scholar] [CrossRef]
- Wilson, C.P.; Ward, M.; McNulty, H.; Strain, J.J.; Trouton, T.G.; Horigan, G.; Purvis, J.; Scott, J.M. Riboflavin offers a targeted strategy for managing hypertension in patients with the MTHFR 677TT genotype: A 4-y follow-up. Am. J. Clin. Nutr. 2012, 95, 766–772. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Iliou, A.; Mikros, E.; Elliott, P. An Overview of Metabolic Phenotyping in Blood Pressure Research. Curr. Hypertens. Rep. 2018, 20, 78. [Google Scholar] [CrossRef]
- Roerecke, M.; Kaczorowski, J.; Tobe, S.W.; Gmel, G.; Hasan, O.S.M.; Rehm, J. The effect of a reduction in alcohol consumption on blood pressure: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e108–e120. [Google Scholar] [CrossRef]
- Pajak, A.; Szafraniec, K.; Kubinova, R.; Malyutina, S.; Peasey, A.; Pikhart, H.; Nikitin, Y.; Marmot, M.; Bobak, M. Binge drinking and blood pressure: Cross-sectional results of the HAPIEE study. PLoS ONE 2013, 8, e65856. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.M.T.; Mill, J.G.; Velasquez-Melendez, G.; Moreira, A.D.; Barreto, S.M.; Viana, M.C.; Molina, M.D.C.B. Consumption of alcohol and blood pressure: Results of the ELSA-Brasil study. PLoS ONE 2018, 13, e0190239. [Google Scholar] [CrossRef]
- Kalim, S.; Clish, C.B.; Deferio, J.J.; Ortiz, G.; Moffet, A.S.; Gerszten, R.E.; Thadhani, R.; Rhee, E.P. Cross-sectional examination of metabolites and metabolic phenotypes in uremia. BMC Nephrol. 2015, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, J. Tryptophan Enhancement/Depletion and Reactions to Failure on a Cooperative Computer Game. Neuropsychopharmacology 1999, 21, 755–764. [Google Scholar] [CrossRef][Green Version]
- Feltkamp, H.; Meurer, K.A.; Godehardt, E. Tryptophan-induced lowering of blood pressure and changes of serotonin uptake by platelets in patients with essential hypertension. Klin. Wochenschr. 1984, 62, 1115–1119. [Google Scholar] [CrossRef]
- Farrar, J.; Manson, P. (Eds.) Manson’s Tropical Diseases: Expertconsult.com Gives You fully Searchable Text and More Online, 23th ed.; Elsevier Saunders: Edinburgh, UK, 2014; Expertconsult.com; ISBN 978-0-7020-5101-2. [Google Scholar]
- Public Health England; Food Standards Agency. NDNS: Time Trend and Income Analyses for Years 1 to 9-Analysis Food and Nutrients; Public Health England: London, UK, 2019.
- Public Health England; Food Standards Agency. NDNS Results from Years 7 and 8 (Combined) -Data Tables; Public Health England: London, UK, 2018.
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.; et al. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The effects of dietary tryptophan on affective disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–107. [Google Scholar] [CrossRef]
- Ardiansyah, S.; Shirakawa, H.; Inagawa, Y.; Koseki, T.; Komai, M. Regulation of blood pressure and glucose metabolism induced by L-tryptophan in stroke-prone spontaneously hypertensive rats. Nutr. Metab. Lond. 2011, 8, 45. [Google Scholar] [CrossRef]
- Price, L.H.; Charney, D.S.; Heninger, G.R. Effects of trazodone treatment on serotonergic function in depressed patients. Psychiatry Res. 1988, 24, 165–175. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Fontana, M.; Giusti, A.M.; Pinto, A.; Iannucci, G.; Lenzi, A.; Donini, L.M. Amino Acids and Hypertension in Adults. Nutrients 2019, 11, 1459. [Google Scholar] [CrossRef] [PubMed]
- Wallin, B.G. Interindividual differences in muscle sympathetic nerve activity: A key to new insight into cardiovascular regulation? Acta. Physiol. Oxf. 2007, 190, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, S.; Stuckless, J. Antihypertensive effects of dietary protein and its mechanism. Int. J. Angiol. 2010, 19, e7–e20. [Google Scholar] [CrossRef] [PubMed]
- Taibi, D.M.; Landis, C.A. Valerian and Other CAM Botanicals in Treatment of Sleep Disturbances. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 57–81. ISBN 978-0-12-374228-5. [Google Scholar]
- Aljuraiban, G.S.; Stamler, J.; Chan, Q.; Van Horn, L.; Daviglus, M.L.; Elliott, P.; Oude Griep, L.M. INTERMAP Research Group Relations between dairy product intake and blood pressure: The INTERnational study on MAcro/micronutrients and blood Pressure. J. Hypertens. 2018, 36, 2049–2058. [Google Scholar] [CrossRef]
- Law, M.R.; Frost, C.D.; Wald, N.J. By how much does dietary salt reduction lower blood pressure? III--Analysis of data from trials of salt reduction. BMJ 1991, 302, 819–824. [Google Scholar] [CrossRef]
- Kelly, C.; Geaney, F.; Fitzgerald, A.P.; Browne, G.M.; Perry, I.J. Validation of diet and urinary excretion derived estimates of sodium excretion against 24-h urine excretion in a worksite sample. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 771–779. [Google Scholar] [CrossRef]
- Burggraf, C.; Teuber, R.; Brosig, S.; Meier, T. Review of a priori dietary quality indices in relation to their construction criteria. Nutr. Rev. 2018, 76, 747–764. [Google Scholar] [CrossRef]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724. [Google Scholar] [CrossRef]
- Hart, S.A.; Taylor, J.; Schatschneider, C. There Is a World Outside of Experimental Designs: Using Twins to Investigate Causation. Assess. Eff. Interv. 2013, 38, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.C. Use of twins to study environmental effects. Environ. Health Perspect. 1981, 42, 103–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Institute of Medicine (US). Committee on Dietary Risk Assessment in the WIC Program Dietary Risk Assessment in the WIC Program; National Academies Press (US): Washington, DC, USA, 2002; ISBN 978-0-309-08284-6. [Google Scholar] [CrossRef]
- Cade, J.E.; Warthon-Medina, M.; Albar, S.; Alwan, N.A.; Ness, A.; Roe, M.; Wark, P.A.; Greathead, K.; Burley, V.J.; Finglas, P.; et al. DIET@NET: Best Practice Guidelines for dietary assessment in health research. BMC Med. 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
| Phenotype | n | % | |
|---|---|---|---|
| N | 3889 | ||
| Female, n (%) | 3808 | 97.2 | |
| MZ pairs | 1326 | 34.1 | |
| Mean | SD | ||
| Age, yrs. | 54.9 | 12.8 | |
| BMI, kg/m2 | 25.3 | 4.4 | |
| SBP, mmHg | 121.3 | 16.1 | |
| DBP, mmHg | 76 | 10.5 | |
| Nutrients * | Mean | SD | % energy |
| Water, g | 2719.24 | 780.97 | |
| Alcohol, g | 9.54 | 13.56 | 4.43 |
| Carbohydrates, g | 247.97 | 77.8 | 51.16 |
| Starch, g | 121.49 g | 44.09 | 25.07 |
| Total sugars, g | 123.82 | 45.92 | 25.55 |
| Sucrose, g | 48.56 | 22.1 | 10.02 |
| Maltose, g | 3.8 | 2.27 | 0.58 |
| Lactose, g | 20.27 | 10.6 | 4.18 |
| Saturated fats, g | 25.47 | 10.43 | 11.82 |
| Potassium, mg | 3920 | 1058.97 | |
| Calcium, mg | 1096.2 | 386.02 | |
| Magnesium, mg | 340.48 | 96.4 | |
| Phosphorus, mg | 1494.38 | 421.83 | |
| Iodine, µg | 214.46 | 82.34 | |
| Riboflavin, mg | 2.38 | 0.88 | |
| Tryptophan, mg | 17.06 | 4.76 | |
| Biotin, µg | 47.39 | 14.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louca, P.; Mompeo, O.; Leeming, E.R.; Berry, S.E.; Mangino, M.; Spector, T.D.; Padmanabhan, S.; Menni, C. Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort. Nutrients 2020, 12, 2130. https://doi.org/10.3390/nu12072130
Louca P, Mompeo O, Leeming ER, Berry SE, Mangino M, Spector TD, Padmanabhan S, Menni C. Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort. Nutrients. 2020; 12(7):2130. https://doi.org/10.3390/nu12072130
Chicago/Turabian StyleLouca, Panayiotis, Olatz Mompeo, Emily R. Leeming, Sarah E. Berry, Massimo Mangino, Tim D. Spector, Sandosh Padmanabhan, and Cristina Menni. 2020. "Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort" Nutrients 12, no. 7: 2130. https://doi.org/10.3390/nu12072130
APA StyleLouca, P., Mompeo, O., Leeming, E. R., Berry, S. E., Mangino, M., Spector, T. D., Padmanabhan, S., & Menni, C. (2020). Dietary Influence on Systolic and Diastolic Blood Pressure in the TwinsUK Cohort. Nutrients, 12(7), 2130. https://doi.org/10.3390/nu12072130






