Nutrient Adequacy Is Low among Both Self-Declared Lacto-Vegetarian and Non-Vegetarian Pregnant Women in Uttar Pradesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Study Population
2.2. Dependent Variables
2.3. Independent Variable
2.4. Other Variables
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
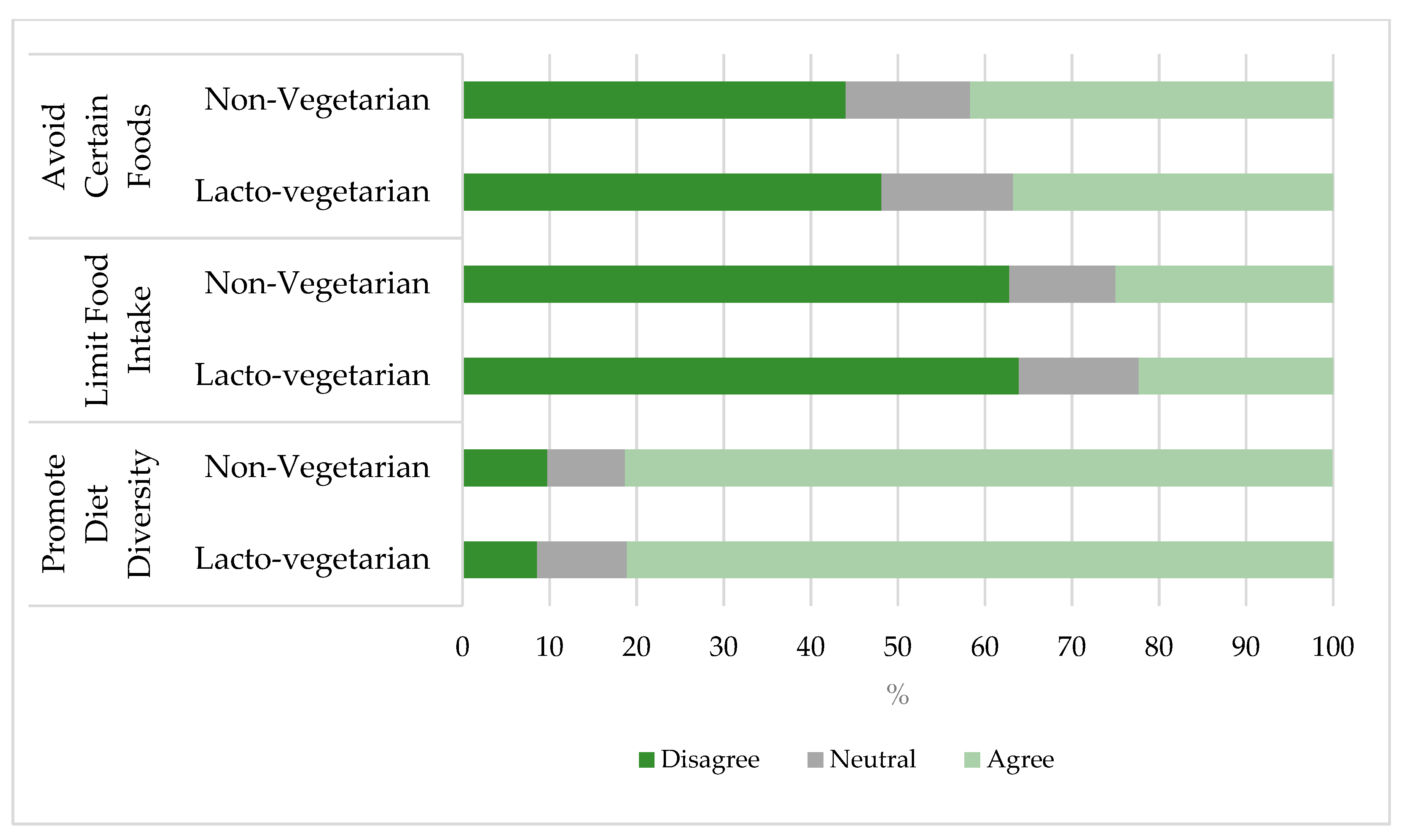
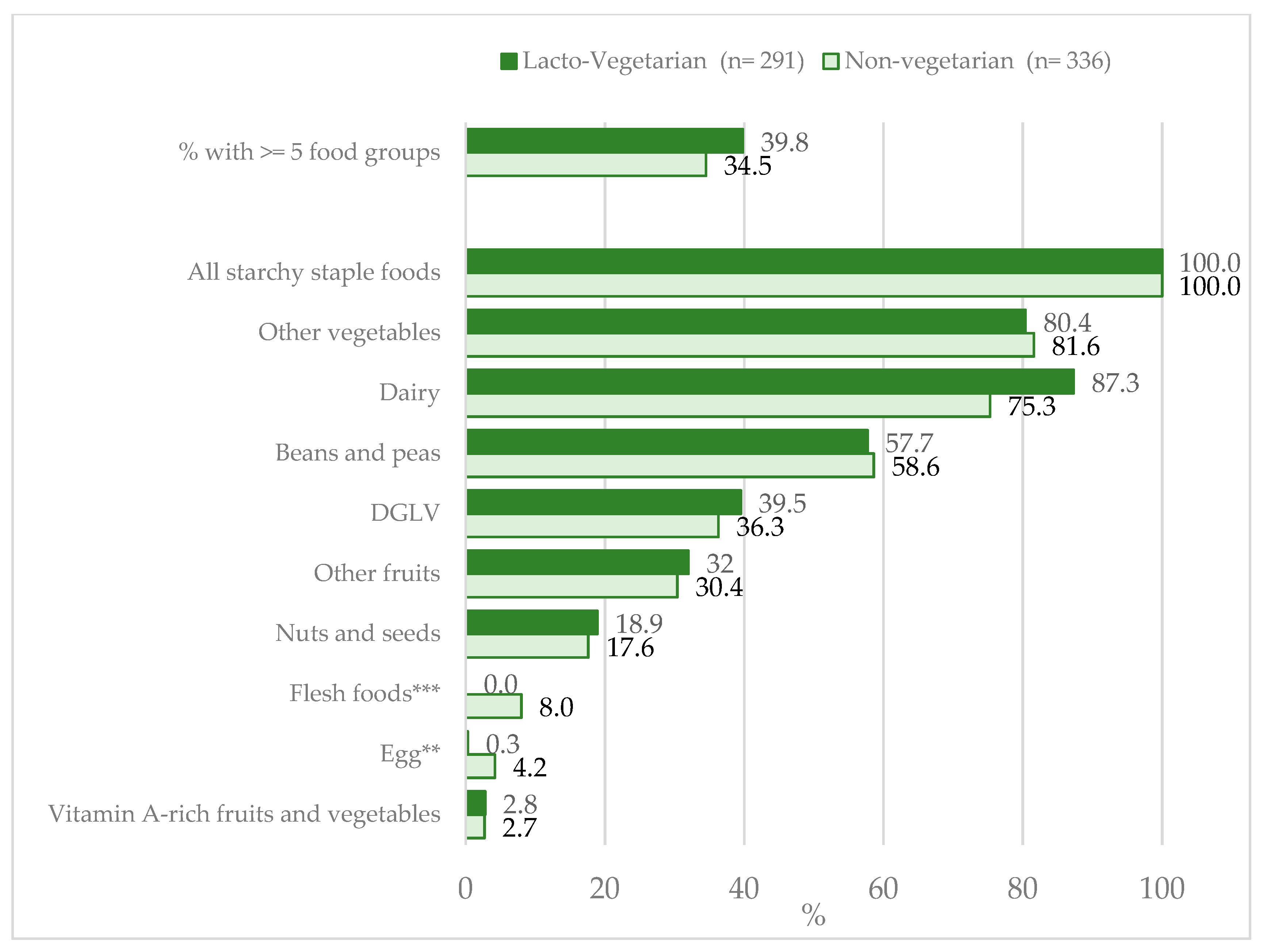
3.2. Dietary Patterns and Diet Diversity
3.3. Energy and Macronutrient Intake
3.4. Micronutrient Intake
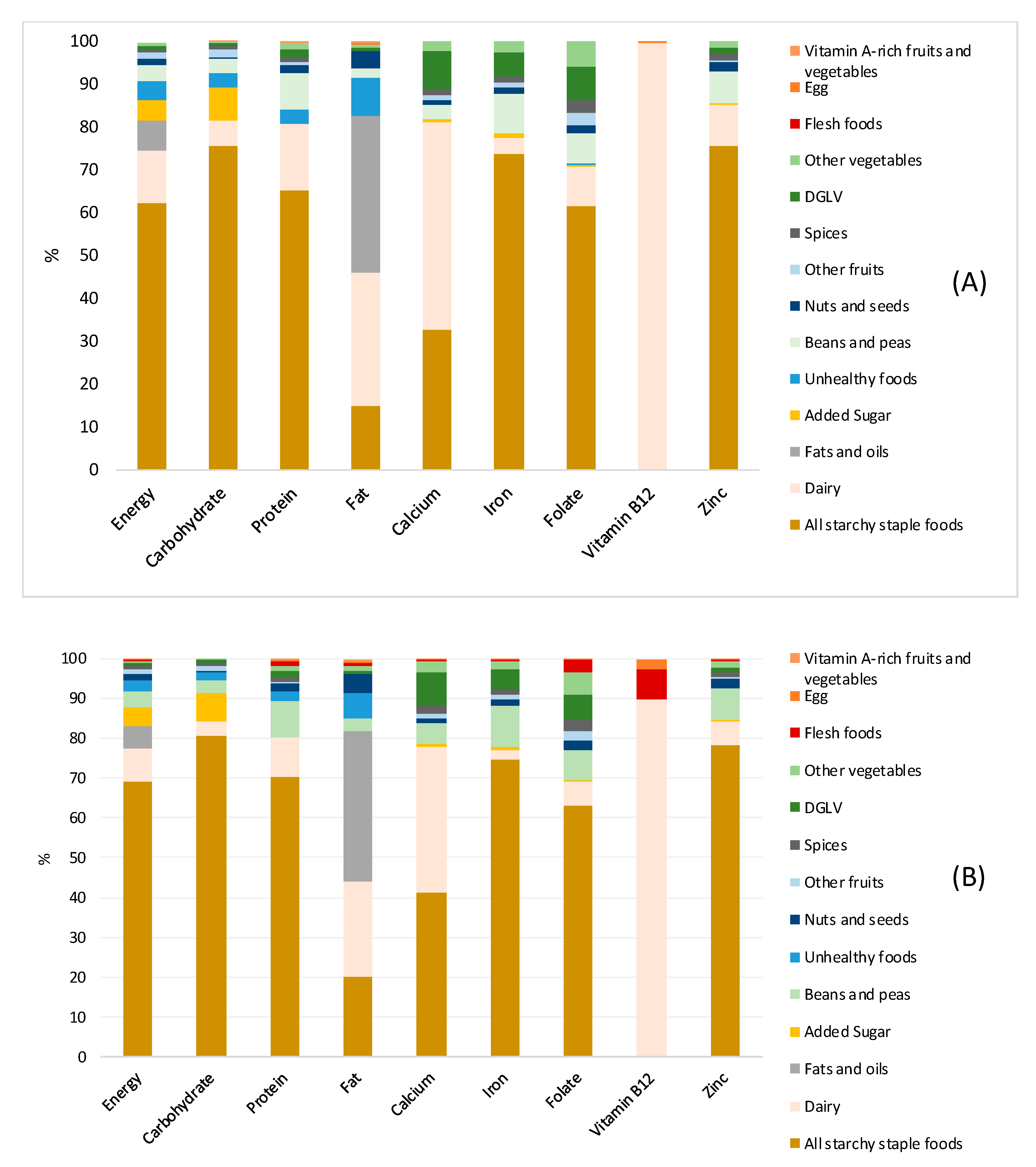
3.5. Contribution of Food Groups to Macro- and Micronutrients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.T.; Deitchler, M.; Arimond, M. Developing Simple Measures of Women’s Diet Quality in Developing Countries: Overview. J. Nutr. 2010, 140, 2048S–2050S. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- International Food Policy Research Institute. Global Nutrition Report 2016 from Promise to Impact Ending Malnutrition by 2030; International Food Policy Research Institute: Washington, DC, USA, 2016. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019. Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; ISBN 978-92-5-131570-5. [Google Scholar]
- WHO. Worldwide Prevalence of Anaemia 1993-2005 of: WHO Global Database on Anaemia; de Benoist, B., McLean, E., Egli, I., Cogswell, M., Eds.; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159665-7. [Google Scholar]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of Women’s Nutrition before and during Early Pregnancy on Maternal and Infant Outcomes: A Systematic Review: Periconceptual nutrition and maternal and infant outcomes. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.P.; Wachs, T.D.; Meeks Gardner, J.; Lozoff, B.; Wasserman, G.A.; Pollitt, E.; Carter, J.A. Child development: Risk factors for adverse outcomes in developing countries. Lancet 2007, 369, 145–157. [Google Scholar] [CrossRef]
- Christian, P.; Lee, S.E.; Donahue Angel, M.; Adair, L.S.; Arifeen, S.E.; Ashorn, P.; Barros, F.C.; Fall, C.H.; Fawzi, W.W.; Hao, W.; et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 2013, 42, 1340–1355. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, Á.N.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Modell, B.; Lawn, J. Folic acid to reduce neonatal mortality from neural tube disorders. Int. J. Epidemiol. 2010, 39, i110–i121. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron Deficiency: Global Prevalence and Consequences. Food Nutr. Bull. 2003, 24, S99–S103. [Google Scholar] [CrossRef]
- Haider, B.A.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- United Nations Children’s Fund. UNICEF’s Approach to Scaling up Nutrition for Mothers and Their Children; Discussion Paper; UNICEF: New York, NY, USA, 2015. [Google Scholar]
- Mayén, A.-L.; Marques-Vidal, P.; Paccaud, F.; Bovet, P.; Stringhini, S. Socioeconomic determinants of dietary patterns in low- and middle-income countries: A systematic review. Am. J. Clin. Nutr. 2014, 100, 1520–1531. [Google Scholar] [CrossRef]
- Na, M.; Mehra, S.; Christian, P.; Ali, H.; Shaikh, S.; Shamim, A.A.; Labrique, A.B.; Klemm, R.D.; Wu, L.S.; West, K.P. Maternal Dietary Diversity Decreases with Household Food Insecurity in Rural Bangladesh: A Longitudinal Analysis. J. Nutr. 2016, 146, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Kavle, J.A.; Landry, M. Addressing barriers to maternal nutrition in low- and middle-income countries: A review of the evidence and programme implications. Matern. Child. Nutr. 2018, 14, e12508. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Global Panel on Agriculture and Food Systems for Nutrition. Food Systems and Diets: Facing the Challenges of the 21st Century; Global Panel on Agriculture and Food Systems for Nutrition: London, UK, 2016; ISBN 978-0-9956228-0-7. [Google Scholar]
- Piccoli, G.; Clari, R.; Vigotti, F.; Leone, F.; Attini, R.; Cabiddu, G.; Mauro, G.; Castelluccia, N.; Colombi, N.; Capizzi, I.; et al. Vegan-vegetarian diets in pregnancy: Danger or panacea? A systematic narrative review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 623–633. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Milner, J.; Joy, E.J.M.; Agrawal, S.; Dangour, A.D. Dietary patterns in India: A systematic review. Br. J. Nutr. 2016, 116, 142–148. [Google Scholar] [CrossRef]
- International Institute for Population Sciences (IIPS). National Family Health Survey (NFHS-4), 2015-2016: India; IIPS: Mumbai, India, 2017. [Google Scholar]
- Nguyen, P.H.; Kachwaha, S.; Avula, R.; Young, M.; Tran, L.M.; Ghosh, S.; Agrawal, R.; Escobar-Alegria, J.; Patil, S.; Menon, P. Maternal nutrition practices in Uttar Pradesh, India: Role of key influential demand and supply factors. Matern. Child. Nutr. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Kachwaha, S.; Tran, L.M.; Mani, S.; Avula, R.; Raj, N.; Patil, S.; Menon, P. Integrating Maternal Nutrition Interventions in Existing Reproductive, Maternal, Newborn and Child Health Services in Uttar Pradesh, India; Alive & Thrive Baseline Survey Report; Alive &Thrive: Washington, DC, USA, 2018. [Google Scholar]
- FAO; FH360. Minimum Dietary Diversity for Women: A Guide to Measurement; FAO: Rome, Italy, 2016; p. 82. [Google Scholar]
- Arimond, M.; Wiesmann, D.; Becquey, E.; Carriquiry, A.; Daniels, M.C.; Deitchler, M.; Fanou-Fogny, N.; Joseph, M.L.; Kennedy, G.; Martin-Prevel, Y.; et al. Simple Food Group Diversity Indicators Predict Micronutrient Adequacy of Women’s Diets in 5 Diverse, Resource-Poor Settings. J. Nutr. 2010, 140, 2059S–2069S. [Google Scholar] [CrossRef]
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Tables; National Institute of Nutrition: Hyderabad, India, 2017. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Butte, N.F.; Wong, W.W.; Treuth, M.S.; Ellis, K.J.; O’Brian Smith, E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 2004, 79, 1078–1087. [Google Scholar] [CrossRef]
- Tooze, J.A.; Krebs-Smith, S.M.; Troiano, R.P.; Subar, A.F. The accuracy of the Goldberg method for classifying misreporters of energy intake on a food frequency questionnaire and 24-h recalls: Comparison with doubly labeled water. Eur. J. Clin. Nutr. 2012, 66, 569–576. [Google Scholar] [CrossRef]
- Joseph, M.L.; Carriquiry, A. A Measurement Error Approach to Assess the Association between Dietary Diversity, Nutrient Intake, and Mean Probability of Adequacy. J. Nutr. 2010, 140, 2094S–2101S. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Huybregts, L.; Sanghvi, T.G.; Tran, L.M.; Frongillo, E.A.; Menon, P.; Ruel, M.T. Dietary Diversity Predicts the Adequacy of Micronutrient Intake in Pregnant Adolescent Girls and Women in Bangladesh, but Use of the 5-Group Cutoff Poorly Identifies Individuals with Inadequate Intake. J. Nutr. 2018, 148, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Jahns, L.; Arab, L.; Carriquiry, A.; Popkin, B.M. The use of external within-person variance estimates to adjust nutrient intake distributions over time and across populations. Public Health Nutr. 2005, 8, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-15742-1. [Google Scholar]
- International Zinc Nutrition Consultative Group (IZiNCG); Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; et al. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S99–S203. [Google Scholar]
- Foote, J.A.; Murphy, S.P.; Wilkens, L.R.; Basiotis, P.P.; Carlson, A. Dietary Variety Increases the Probability of Nutrient Adequacy among Adults. J. Nutr. 2004, 134, 1779–1785. [Google Scholar] [CrossRef]
- Coates, J.C.; Swindale, A.; Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3); FHI 360/FANTA: Washington, DC, USA, 2007. [Google Scholar]
- Filmer, D.; Pritchett, L.H. Estimating Wealth Effects without Expenditure Data—or Tears: An Application to Educational Enrollments in States of India. Demography 2001, 33, 19. [Google Scholar]
- Gadgil, M.S.; Joshi, K.S.; Naik, S.S.; Pandit, A.N.; Otiv, S.R.; Patwardhan, B.K. Association of homocysteine with global DNA methylation in vegetarian Indian pregnant women and neonatal birth anthropometrics. J. Matern. Fetal Neonatal Med. 2014, 27, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe-Rutten, R.A.M.; de Vries, J.H.M.; de Bree, A.; van der Put, N.; van Staveren, W.A.; de Groot, L.C.P.G.M. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef]
- Gautam, V.P.; Taneja, D.K.; Sharma, N.; Gupta, V.K.; Ingle, G.K. Dietary aspects of pregnant women in rural areas of Northern India. Matern. Child. Nutr. 2008, 4, 86–94. [Google Scholar] [CrossRef]
- Henjum, S.; Torheim, L.E.; Thorne-Lyman, A.L.; Chandyo, R.; Fawzi, W.W.; Shrestha, P.S.; Strand, T.A. Low dietary diversity and micronutrient adequacy among lactating women in a peri-urban area of Nepal. Public Health Nutr. 2015, 18, 3201–3210. [Google Scholar] [CrossRef]
- Institute of Medicine; National Research Council. Weight Gain during Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009; ISBN 978-0-309-13113-1. [Google Scholar]
- Kachwaha, S.; Nguyen, P.H.; Defreese, M.; Avula, R.; Cyriac, S.; Girard, A.W.; Menon, P. Assessing the Economic Feasibility of Assuring Nutritionally Adequate Diets for Vulnerable Populations in Uttar Pradesh, India: Findings from A ‘Cost of the Diet’ Analysis. Curr. Dev. Nutr. 2019. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chakrabarti, A. Food taboos in pregnancy and early lactation among women living in a rural area of West Bengal. J. Fam. Med. Prim. Care 2019, 8, 86. [Google Scholar] [CrossRef]
- Dizon, F.; Herforth, A. The Cost of Nutritious Food in South Asia; Policy Research Working Papers; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Coughlin, S.S. Recall bias in epidemiologic studies. J. Clin. Epidemiol. 1990, 43, 87–91. [Google Scholar] [CrossRef]
- Imdad, A.; Bhutta, Z.A. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC Public Health 2011, 11, S17. [Google Scholar] [CrossRef] [PubMed]
| All | Lacto-Vegetarian | Non-Vegetarian | p-Values | |
|---|---|---|---|---|
| N = 627 | N = 291 | N = 336 | ||
| Participant characteristics | ||||
| Maternal age, year | 25.0 ± 4.1 | 25.0 ± 3.9 | 25.0 ± 4.3 | 0.90 |
| Religion as Hindus, % | 92.7 | 97.9 | 88.1 | <0.001 |
| Caste category, % | ||||
| SC/ST | 42.1 | 26.5 | 55.7 | <0.001 |
| OBC | 40.8 | 50.2 | 32.8 | |
| General | 17.1 | 23.4 | 11.6 | |
| Education, % | ||||
| No schooling | 25.0 | 17.2 | 31.9 | <0.001 |
| Elementary school | 14.8 | 11.0 | 18.2 | |
| Middle school | 21.2 | 21.3 | 21.1 | |
| ≥High school | 38.9 | 50.5 | 28.9 | |
| Occupation as housewives, % | 90.8 | 91.4 | 90.7 | 0.78 |
| Number of previous pregnancies, n | 2.1 ± 1.9 | 1.9 ± 1.8 | 2.3 ± 2.0 | 0.003 |
| Current gestational age in trimester, % | ||||
| Second trimester | 43.2 | 40.2 | 45.8 | 0.16 |
| Third trimester | 56.8 | 59.8 | 54.2 | |
| Household factors | ||||
| Number of people in household, n | 5.1 ± 2.1 | 5.2 ± 2.0 | 5.1 ± 2.1 | 0.33 |
| Household food insecurity, % | 15.9 | 10.7 | 20.5 | 0.001 |
| Household SES index | 3.0 ± 1.4 | 3.3 ± 1.4 | 2.7 ± 1.4 | <0.001 |
| Lacto-Vegetarian | Non-Vegetarian | Unadjusted p-Values | Adjusted p-Values c | |
|---|---|---|---|---|
| N = 291 | N = 336 | |||
| Energy, kcal/day | 2051.5 ± 724.6 | 1949.0 ± 730.4 | 0.08 | 0.78 |
| EER a, kcal/day | 2254.4 ± 147.1 | 2221.2 ± 143.1 | 0.004 | 0.31 |
| Energy intake < 85% of EER, % | 45.7 | 53.3 | 0.06 | 0.34 |
| Carbohydrate | ||||
| Amount consumed, g (SD) | 346.7 ± 124.7 | 346.4 ± 137.3 | 0.98 | 0.48 |
| % energy | 68.0 ± 8.5 | 71.1 ± 8.3 | <0.001 | 0.003 |
| Percent insufficient intake b | 1.0 | 0.9 | 0.90 | 0.82 |
| Percent in optimal range | 35.4 | 20.8 | <0.001 | 0.01 |
| Percent excessive intake | 63.5 | 78.3 | <0.001 | 0.009 |
| Fat | ||||
| Amount consume, g | 45.9 ± 27.2 | 35.1 ± 23.4 | <0.001 | 0.004 |
| % energy | 19.8 ± 8.6 | 16.2 ± 8.2 | <0.001 | 0.001 |
| Percent insufficient intake b | 51.9 | 70.2 | <0.001 | 0.004 |
| Percent in optimal range | 43.6 | 26.8 | <0.001 | 0.001 |
| Percent excessive intake | 4.5 | 3.0 | 0.32 | 0.87 |
| Protein | ||||
| Amount consume, g | 59.9 ± 22.3 | 57.3 ± 22.2 | 0.15 | 0.82 |
| % energy | 11.7 ± 1.5 | 11.8 ± 1.6 | 0.40 | 0.78 |
| Percent insufficient intake b | 11.7 | 10.4 | 0.60 | 0.62 |
| Percent in optimal range | 88.3 | 89.6 | 0.60 | 0.62 |
| Percent excessive intake | 0.0 | 0.0 | 1.0 | 1.0 |
| EAR (Mean ± SD) | Median (IRQ) | Probability of Adequacy (Mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lacto-Vegetarian | Non-Vegetarian | Unadjusted p-Values | Adjusted p-Value ** | Lacto-Vegetarian | Non-Vegetarian | Unadjusted p-Values | Adjusted p-Value ** | ||
| N = 291 | N = 336 | N = 291 | N = 336 | ||||||
| MPA | 19.9 ± 15.3 | 17.2 ± 13.7 | |||||||
| Calcium, mg * | 800.0 ± 100 | 414.6 (232.1, 727.2) | 270.7 (174.1, 522.6) | <0.001 | <0.001 | 31.5 ± 26.6 | 20.1 ± 21.2 | <0.001 | <0.001 |
| Iron, mg | 24.9 ± 2.3 | 12.9 (9.2, 16.9) | 12.5 (8.7, 17.1) | 0.14 | 0.27 | 4.3 ±16.9 | 2.4 ± 11.8 | 0.10 | 0.28 |
| Zinc, mg | 8.0 ± 1.0 | 8.4 (6.2, 11.1) | 8.4 (5.7, 10.7) | 0.18 | 0.51 | 51.9 ± 38.2 | 50.0 ± 39.5 | 0.53 | 0.92 |
| Vitamin C, mg | 70.0 ± 7.0 | 40.2 (24.3, 61.5) | 33.7 (18.8, 56.4) | 0.11 | 0.54 | 15.4 ± 33.9 | 13.0 ± 32.0 | 0.38 | 0.90 |
| Thiamin, mg | 1.2 ± 0.1 | 1.3 (1.0, 1.7) | 1.2 (0.9, 1.7) | 0.11 | 0.38 | 57.6 ± 42.2 | 51.9 ± 43.3 | 0.10 | 0.33 |
| Riboflavin, mg | 1.2 ± 0.1 | 0.8 (0.6, 1.2) | 0.7 (0.5, 1.0) | 0.02 | 0.49 | 18.5 ± 34.7 | 12.1 ± 29.1 | 0.01 | 0.27 |
| Niacin, mg | 14.0 ± 2.1 | 12.7 (9.3, 16.1) | 12.3 (9.1, 17.1) | 0.73 | 0.61 | 35.8 ± 36.5 | 37.0 ± 38.1 | 0.70 | 0.58 |
| Vitamin B6, mg | 1.6 ± 0.2 | 0.7 (0.4, 1.0) | 0.6 (0.4, 0.8) | 0.003 | 0.17 | 1.0 ± 7.3 | 0.9 ± 7.7 | 0.85 | 0.84 |
| Folate, mcg | 520.0 ± 52.0 | 211.3 (154.8, 283.5) | 203.3 (143.6, 280.5) | 0.60 | 0.91 | 0.4 ± 5.8 | 1.1 ± 8.9 | 0.21 | 0.15 |
| Vitamin B12, mcg | 2.2 ± 0.2 | 0.6 (0.2, 1.4) | 0.3 (0.1, 1.0) | <0.001 | 0.04 | 2.4 ± 11.9 | 0.2 ± 2.0 | <0.001 | 0.02 |
| Vitamin A, mcg RAE | 550.0 ± 55.0 | 31.8 (17.6, 103.1) | 32.4 (15.3, 73.5) | 0.44 | 0.60 | 0.1 ± 0.9 | 0.1 ± 1.2 | 0.86 | 0.60 |
| Unadjusted | Not Adjusted for Energy | Energy Adjusted | ||||
|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p-Values | Coefficient (95% CI) | p-Values | Coefficient (95% CI) | p-Values |
| N = 627 | N = 627 | N = 627 | ||||
| Lacto-vegetarian | 0.04 (0.01, 0.07) | 0.01 | 0.02 (−0.01, 0.05) | 0.29 | 0.01 (−0.01, 0.03) | 0.28 |
| Food Insecure | −0.02 (−0.06, 0.02) | 0.36 | −0.003 (−0.03, 0.02) | 0.82 | ||
| Caste | ||||||
| General | Reference | Reference | ||||
| SC/ST | −0.02 (−0.07, 0.03) | 0.40 | −0.007 (−0.04, 0.02) | 0.65 | ||
| OBC | −0.0004 (−0.04, 0.04) | 0.98 | −0.001 (−0.03, 0.03) | 0.92 | ||
| Maternal Education | ||||||
| No Schooling | Reference | Reference | ||||
| Primary School | 0.03 (−0.02, 0.08) | 0.27 | −0.03 (−0.06, 0.004) | 0.09 | ||
| Secondary and above | 0.04 (0.00, 0.08) | 0.05 | −0.01 (−0.04, 0.01) | 0.41 | ||
| Parity | −0.005 (−0.01, 0.004) | 0.29 | −0.002 (−0.01, 0.003) | 0.38 | ||
| SES | ||||||
| 1 (Lowest Tertile) | Reference | Reference | ||||
| 2 | −0.005 (−0.04, 0.03) | 0.80 | −0.02 (−0.04, 0.003) | 0.09 | ||
| 3 (Highest Tertile) | 0.02 (−0.02, 0.07) | 0.50 | −0.005 (−0.03, 0.02) | 0.69 | ||
| Energy (kcal/day) ** | 0.41 (0.39, 0.44) | <0.001 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellows, A.L.; Kachwaha, S.; Ghosh, S.; Kappos, K.; Escobar-Alegria, J.; Menon, P.; Nguyen, P.H. Nutrient Adequacy Is Low among Both Self-Declared Lacto-Vegetarian and Non-Vegetarian Pregnant Women in Uttar Pradesh. Nutrients 2020, 12, 2126. https://doi.org/10.3390/nu12072126
Bellows AL, Kachwaha S, Ghosh S, Kappos K, Escobar-Alegria J, Menon P, Nguyen PH. Nutrient Adequacy Is Low among Both Self-Declared Lacto-Vegetarian and Non-Vegetarian Pregnant Women in Uttar Pradesh. Nutrients. 2020; 12(7):2126. https://doi.org/10.3390/nu12072126
Chicago/Turabian StyleBellows, Alexandra L., Shivani Kachwaha, Sebanti Ghosh, Kristen Kappos, Jessica Escobar-Alegria, Purnima Menon, and Phuong H. Nguyen. 2020. "Nutrient Adequacy Is Low among Both Self-Declared Lacto-Vegetarian and Non-Vegetarian Pregnant Women in Uttar Pradesh" Nutrients 12, no. 7: 2126. https://doi.org/10.3390/nu12072126
APA StyleBellows, A. L., Kachwaha, S., Ghosh, S., Kappos, K., Escobar-Alegria, J., Menon, P., & Nguyen, P. H. (2020). Nutrient Adequacy Is Low among Both Self-Declared Lacto-Vegetarian and Non-Vegetarian Pregnant Women in Uttar Pradesh. Nutrients, 12(7), 2126. https://doi.org/10.3390/nu12072126





