Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Taurisolo® Supplement Preparation
2.2. Animals//Ethics Approval
2.3. Experimental Design
2.4. Motor Performance and Coordination in Rotarod Test
2.5. Gastrocnemius Muscle Homogenate
2.6. Total Antioxidant Capacity (FRAP)
2.7. Antioxidant Activities Determination
2.8. Malondialdehyde Assay
2.9. N-Tyrosine Determination
2.10. Gene Expression
2.11. Statistical Analyses
3. Results
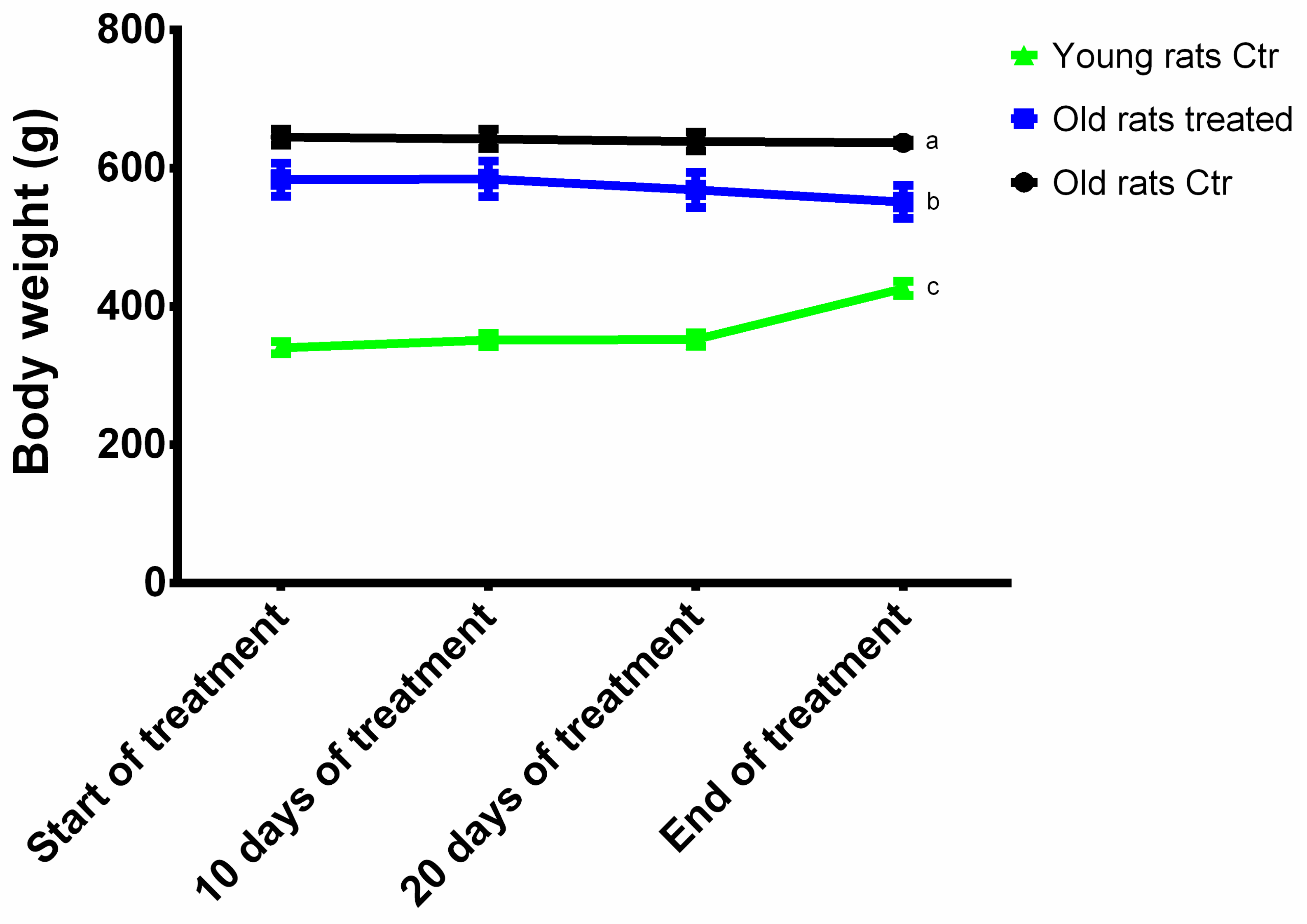
3.1. Animal Body Weight
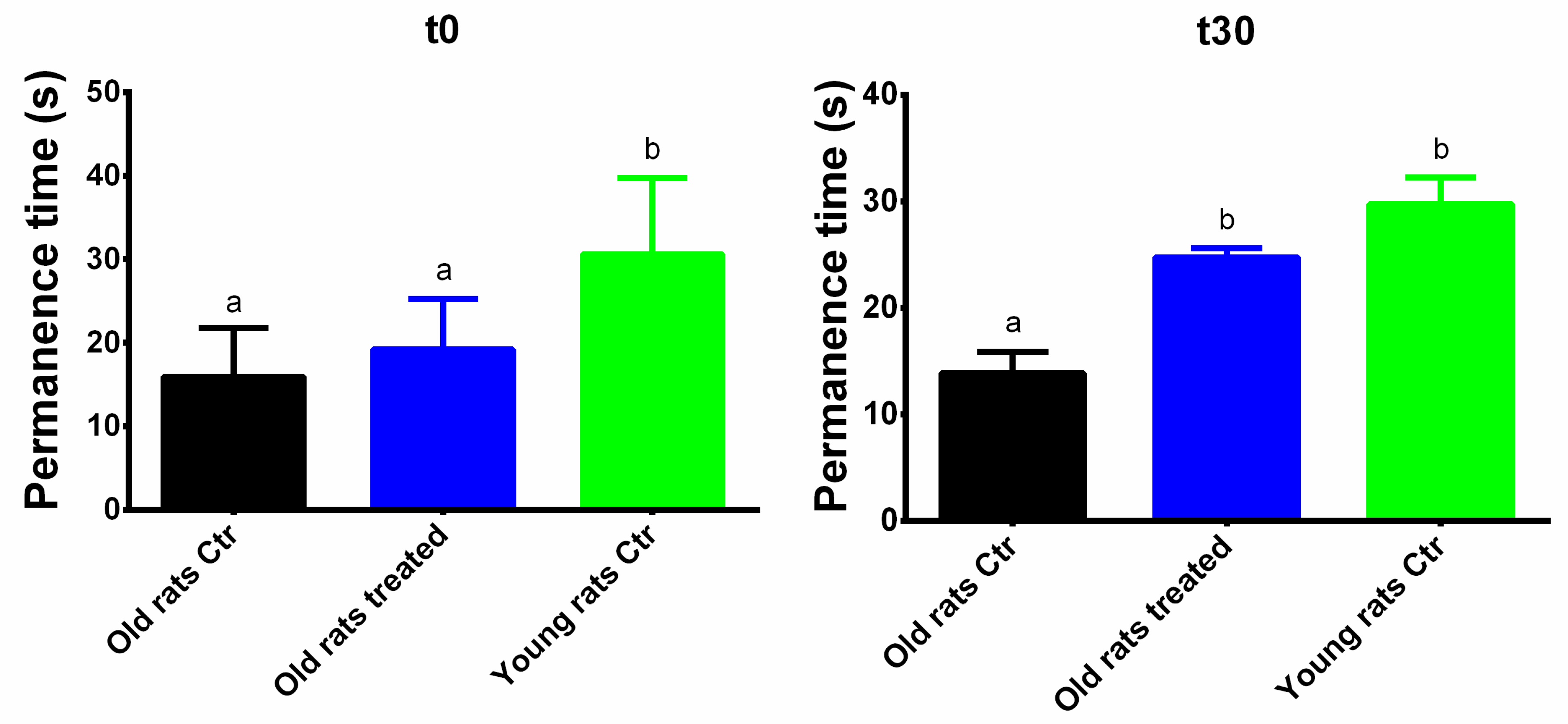
3.2. Taurisolo® Treatment Ameliorates the Motor Performance and Coordination
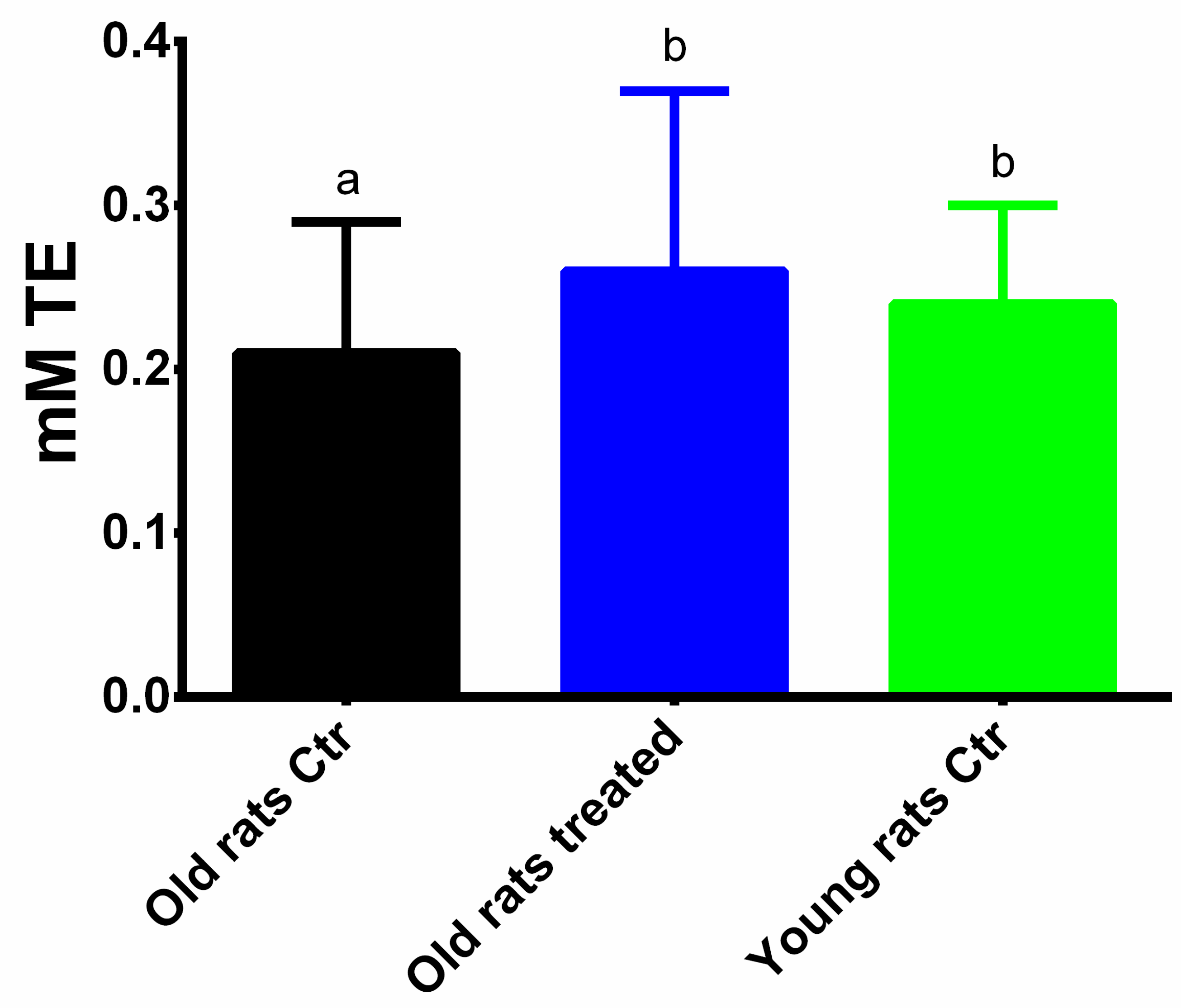
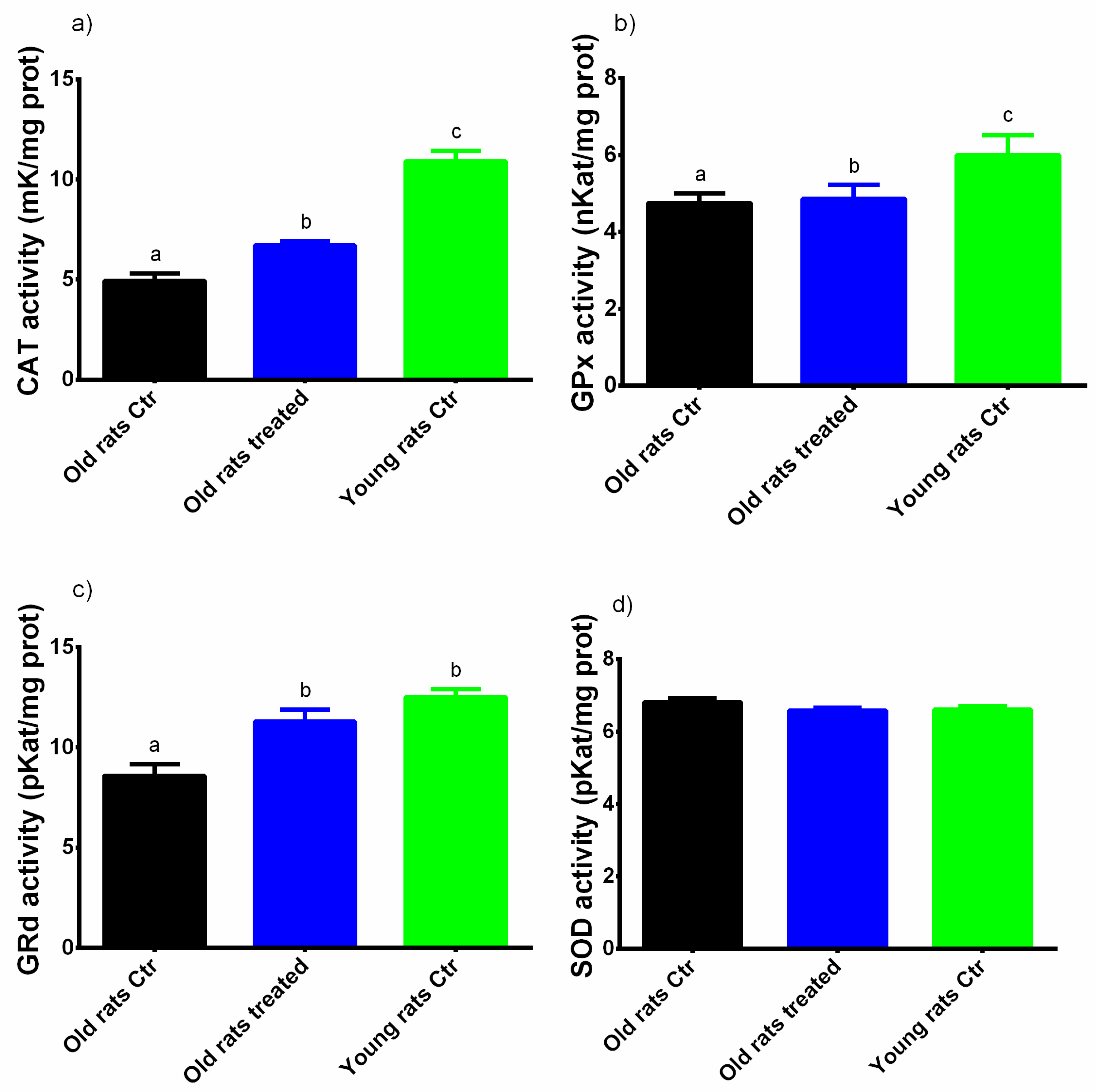
3.3. Taurisolo® Treatment Increases the Total Antioxidant Capacity and the Activity of Antioxidant Enzymes in Muscle
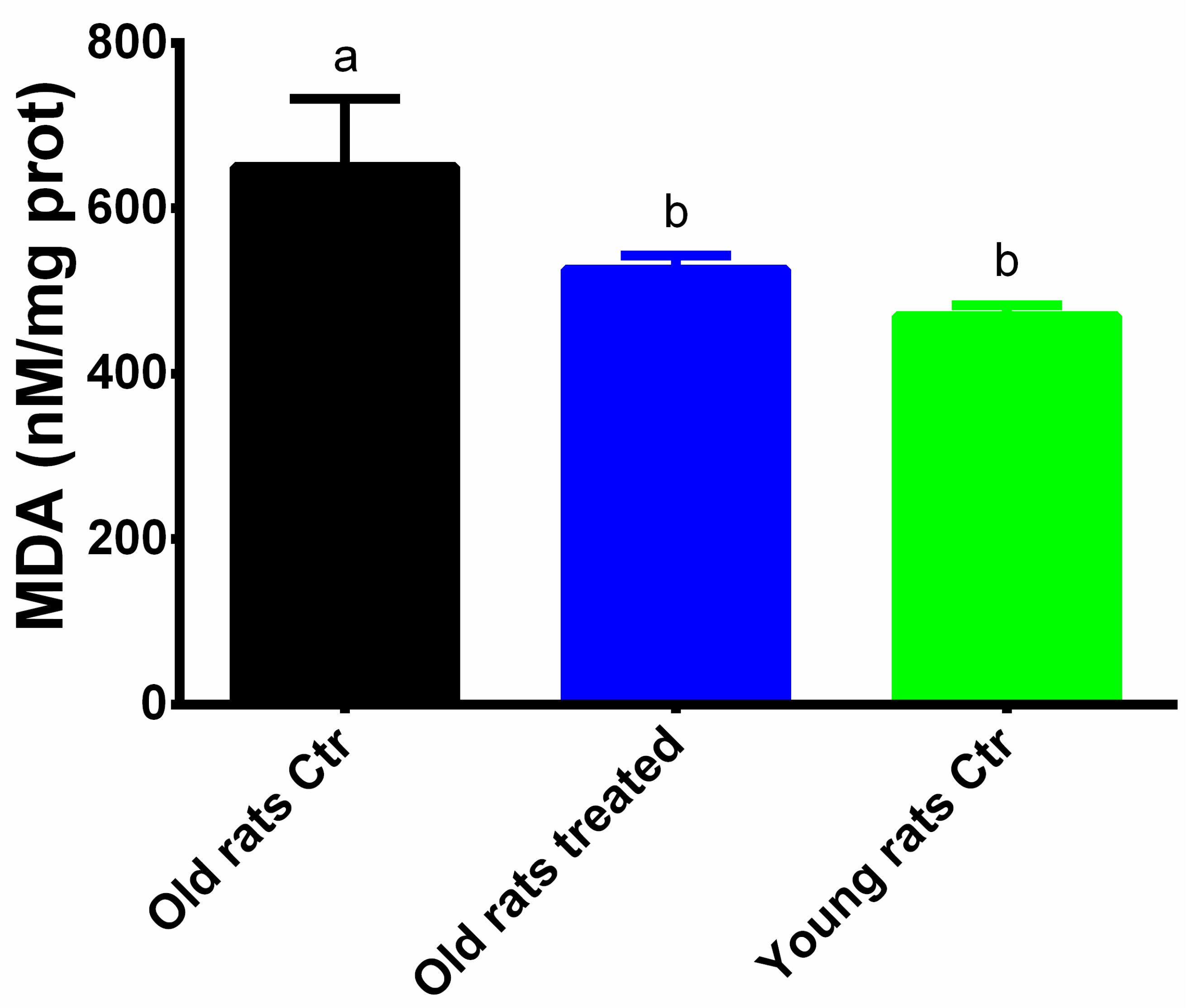
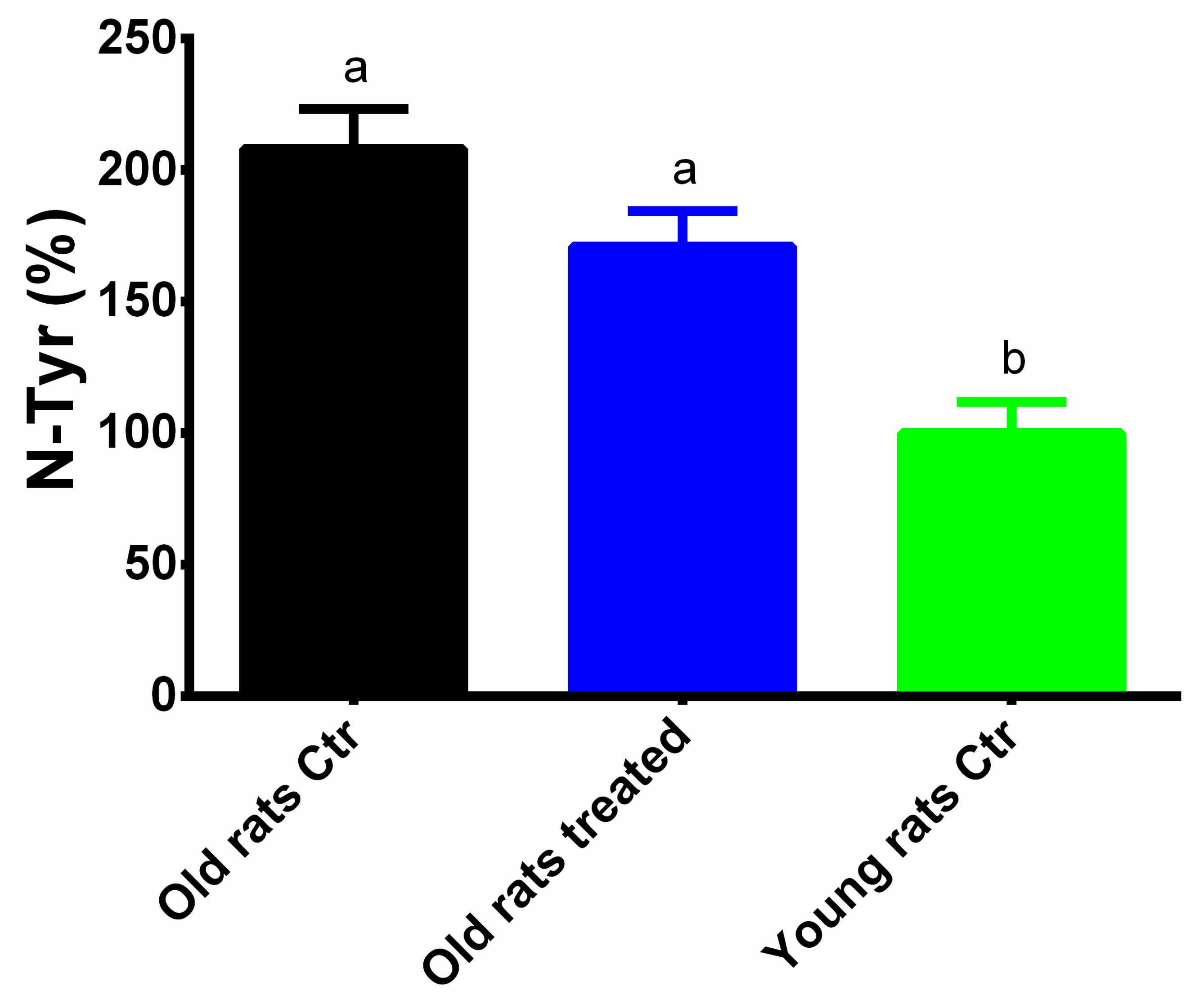
3.4. Taurisolo® Treatment Reduces the Levels of MDA and N-Tyrin Muscle
3.5. Taurisolo® Treatment Modulates the Expression of Oxidative Stress- and Inflammation-Related Genes in Muscle
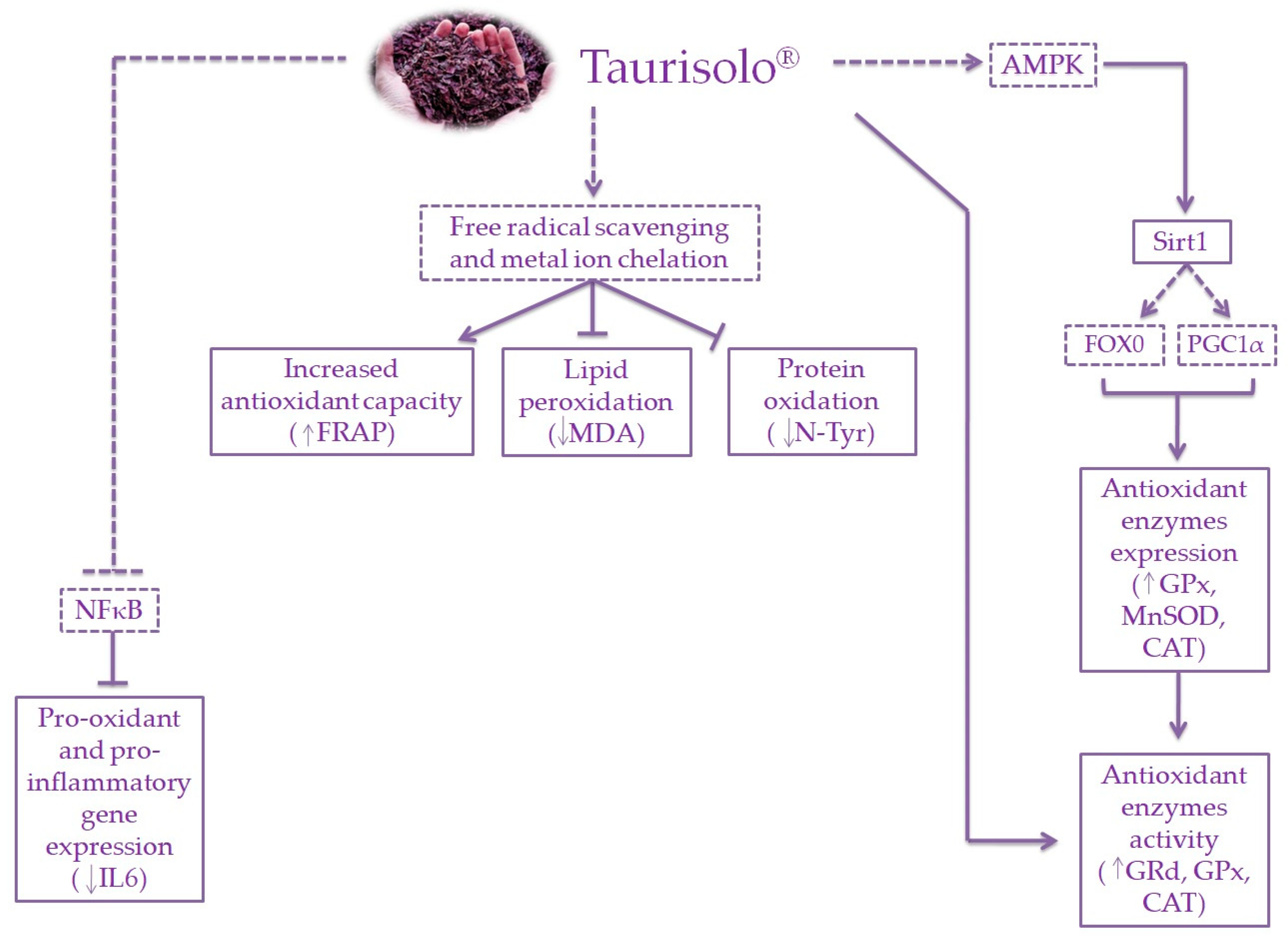
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laurent, C.; Chabi, B.; Fouret, G.; Py, G.; Sairafi, B.; Elong, C.; Gaillet, S.; Cristol, J.P.; Coudray, C.; Feillet-Coudray, C.; et al. Polyphenols decreased liver NADPH oxidase activity, increased muscle mitochondrial biogenesis and decreased gastrocnemius age-dependent autophagy in aged rats. Free Radic. Res. 2012, 46, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging: Effect of free radical reaction inhibitors on the mortality rate of male LAF mice. J. Gerontol. 1968, 23, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Smith, C. Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxid. Med. Cell. Longev. 2016, 2016, 6236309. [Google Scholar] [CrossRef]
- Jones, D.P.; Mody, V.C., Jr.; Carlson, J.L.; Lynn, M.J.; Sternberg, P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 2002, 33, 1290–1300. [Google Scholar] [CrossRef]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 2013, 12, 943–949. [Google Scholar] [CrossRef]
- Lee, H.; Lim, J.Y.; Choi, S.J. Oleate prevents palmitate-induced atrophy via modulation of mitochondrial ROS production in skeletal myotubes. Oxid. Med. Cell. Longev. 2017, 2017, 2739721. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial quality control and muscle mass maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Joseph, A.M.; Adhihetty, P.J.; Wawrzyniak, N.R.; Wohlgemuth, S.E.; Picca, A.; Kujoth, G.C.; Prolla, T.A.; Leeuwenburgh, C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS ONE 2013, 8, e69327. [Google Scholar] [CrossRef]
- Lourenco Dos Santos, S.; Baraibar, M.A.; Lundberg, S.; Eeg-Olofsson, O.; Larsson, L.; Friguet, B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015, 5, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Roseno, S.L.; Davis, P.R.; Bollinger, L.M.; Powell, J.J.; Witczak, C.A.; Brault, J.J. Short-term, high-fat diet accelerates disuse atrophy and protein degradation in a muscle-specific manner in mice. Nutr. Metab. (Lond.) 2015, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; McArdle, A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J. Physiol. 2011, 589, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Meyer, A.; Dal-Ros, S.; Auger, C.; Keller, N.; Ramamoorthy, T.G.; Zoll, J.; Metzger, D.; Schini-Kerth, V.; Geny, B.; et al. Polyphenols prevent ageing-related impairment in skeletal muscle mitochondrial function through decreased reactive oxygen species production. Exp. Physiol. 2013, 98, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative protein damage is associated with poor grip strength among older women living in the community. J. Appl. Physiol. (1985) 2007, 103, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Steffl, M.; Bohannon, R.W.; Sontakova, L.; Tufano, J.J.; Shiells, K.; Holmerova, I. Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 835–845. [Google Scholar] [CrossRef]
- Furtado, G.E.; Carvalho, H.M.; Loureiro, M.; Patrício, M.; Uba-Chupel, M.; Colado, J.C.; Hogervorst, E.; Ferreira, J.P.; Teixeira, A.M. Chair-based exercise programs in institutionalized older women: Salivary steroid hormones, disabilities and frailty changes. Exp. Gerontol. 2020, 130, 110790. [Google Scholar] [CrossRef]
- Shadfar, S.; Couch, M.E.; McKinney, K.A.; Weinstein, L.J.; Yin, X.; Rodriguez, J.E.; Guttridge, D.C.; Willis, M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr. Cancer 2011, 63, 749–762. [Google Scholar] [CrossRef]
- Bennett, B.T.; Mohamed, J.S.; Alway, S.E. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, H.; Song, G.; Yang, Y.; Zou, X.; Han, P.; Li, S. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in STZ-induced diabetic mice. Mol. Nutr. Food Res. 2018, 62, e1700941. [Google Scholar] [CrossRef]
- Jackson, J.R.; Ryan, M.J.; Alway, S.E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging (Albany NY) 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: The role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Lapi, D.; Stornaiuolo, M.; Sabatino, L.; Sommella, E.; Tenore, G.; Daglia, M.; Scuri, R.; Di Maro, M.; Colantuoni, A.; Novellino, E.; et al. The pomace extract taurisolo protects rat brain from ischemia-reperfusion injury. Front. Cell. Neurosci. 2020, 14, 3. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Goldberg, D.M.; Spooner, R. Glutathione reductase. In Methods of Enzymatic Analysis. Enzyme 1: Oxidoreductases, Transferases; Verlagchemie, B., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1984; Volume 3, pp. 258–265. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Hassan, S.T.S.; Tenore, G.C.; Novellino, E. Effect of grape pomace polyphenols with or without pectin on TMAO serum levels assessed by LC/MS-based assay: A preliminary clinical study on overweight/obese subjects. Front. Pharmacol. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Moranta, D.; Pani, G. Dietary polyphenols and neurogenesis: Molecular interactions and implication for brain ageing and cognition. Neurosci. Biobehav. Rev. 2018, 90, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic silymarin, quercetin and naringenin treatments increase monoamines synthesis and hippocampal sirt1 levels improving cognition in aged rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Lambert, K.; Hokayem, M.; Thomas, C.; Fabre, O.; Cassan, C.; Bourret, A.; Bernex, F.; Feuillet-Coudray, C.; Notarnicola, C.; Mercier, J.; et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 2885. [Google Scholar] [CrossRef]
- Matsumura, N.; Takahara, S.; Maayah, Z.H.; Parajuli, N.; Byrne, N.J.; Shoieb, S.M.; Soltys, C.M.; Beker, D.L.; Masson, G.; El-Kadi, A.O.S.; et al. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J. Mol. Cell. Cardiol. 2018, 125, 162–173. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tsutaki, A.; Tamura, Y.; Kouzaki, K.; Sashihara, K.; Nakashima, S.; Tagashira, M.; Tatsumi, R.; Nakazato, K. Dietary apple polyphenols increase skeletal muscle capillaries in Wistar rats. Physiol. Rep. 2018, 6, e13866. [Google Scholar] [CrossRef]
- Sandoval-Acuna, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Milton-Laskibar, I.; Gonzalez, M.; Portillo, M.P. Antiobesity effects of resveratrol: Which tissues are involved? Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Koenig, R.T.; Dickman, J.R.; Wise, M.L.; Ji, L.L. Avenanthramides are bioavailable and accumulate in hepatic, cardiac, and skeletal muscle tissue following oral gavage in rats. J. Agric. Food Chem. 2011, 59, 6438–6443. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Stephenson, K.K.; Wade, K.L.; Liu, H.; Fahey, J.W. Structure-activity analysis of flavonoids: Direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer 2013, 65, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell. Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H.; Dianzani, M.U.; Poli, G.; Slater, T.F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem. J. 1982, 208, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Tsuchida, M.; Miura, T.; Mizutani, K.; Aibara, K. Fluorescent substances in mouse and human sera as a parameter of in vivo lipid peroxidation. Biochim. Biophys. Acta 1985, 834, 196–204. [Google Scholar]
- Stojanovic, S.; Sprinz, H.; Brede, O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Olas, B.; Nowak, P.; Kolodziejczyk, J.; Ponczek, M.; Wachowicz, B. Protective effects of resveratrol against oxidative/nitrative modifications of plasma proteins and lipids exposed to peroxynitrite. J. Nutr. Biochem. 2006, 17, 96–102. [Google Scholar] [CrossRef]
- Brito, P.; Almeida, L.M.; Dinis, T.C. The interaction of resveratrol with ferrylmyoglobin and peroxynitrite; protection against LDL oxidation. Free Radic. Res. 2002, 36, 621–631. [Google Scholar] [CrossRef]
- Pandey, K.B.; Mehdi, M.M.; Maurya, P.K.; Rizvi, S.I. Plasma protein oxidation and its correlation with antioxidant potential during human aging. Dis. Markers 2010, 29, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Gouveia, A.M.; Almeida, H. Resveratrol attenuates copper-induced senescence by improving cellular proteostasis. Oxid. Med. Cell. Longev. 2017, 2017, 3793817. [Google Scholar] [CrossRef]
- Lambert, K.; Coisy-Quivy, M.; Bisbal, C.; Sirvent, P.; Hugon, G.; Mercier, J.; Avignon, A.; Sultan, A. Grape polyphenols supplementation reduces muscle atrophy in a mouse model of chronic inflammation. Nutrition 2015, 31, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, G.; Liang, X.; Zhu, M.; Du, M. Grape seed extract prevents skeletal muscle wasting in interleukin 10 knockout mice. BMC Complementary Altern. Med. 2014, 14, 162. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, X.; Jiang, Y.; Zhang, Z.; Bao, L.; Li, Y.; Zhang, F.; Ma, X.; Cai, X.; Jing, L.; et al. Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 365–369. [Google Scholar] [CrossRef]
- Priftis, A.; Goutzourelas, N.; Halabalaki, M.; Ntasi, G.; Stagos, D.; Amoutzias, G.D.; Skaltsounis, L.A.; Kouretas, D. Effect of polyphenols from coffee and grape on gene expression in myoblasts. Mech. Ageing Dev. 2018, 172, 115–122. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Demertzis, N.; Mavridou, P.; Karterolioti, H.; Georgadakis, S.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, L.; Statiri, A.; et al. Effects of polyphenolic grape extract on the oxidative status of muscle and endothelial cells. Hum. Exp. Toxicol. 2014, 33, 1099–1112. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Housmekeridou, A.; Karapouliou, C.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, A.L.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D.; et al. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Med. 2015, 36, 433–441. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Spanidis, Y.; Liosi, M.; Apostolou, A.; Priftis, A.; Haroutounian, S.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D.; et al. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015, 12, 5846–5856. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Zhu, W.; Chen, C.; Sun, Z.; Tan, Z.; Kang, J. Alteration of antioxidant enzymes and associated genes induced by grape seed extracts in the primary muscle cells of goats in vitro. PLoS ONE 2014, 9, e107670. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; Always, S.E. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1572–R1581. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence | Temperature (°C) |
|---|---|---|
| Β-Actin | Fw: 5′-AGG GAAATCGTGCGTGAC-3′ | 95 °C 15 Seg 60 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-CGCTCATTGCCGATAGTC-3′ | ||
| Mn-SOD | Fw: 5′-GGCCAAGGGAGATGTTACAA-3′ | 95 °C 15 Seg 60 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-GCTTGATAGCCTCCAGCAAC-3′ | ||
| IL-10 | Fw: 5′-GGCTCAGCACTGCTATGTTGCC-3′ | 95 °C 15 Seg 60 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-AGCATGTGGGTCTGGCTGACT-3′ | ||
| IL-6 | Fw: 5′-GCCACTGCCTTCCCTACTTCA-3′ | 95 °C 15 Seg 60 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-GACAGTGCATCGCTGTTCA-3′ | ||
| GPx | Fw: 5′-GCTCATGACCGACCCCAAGT-3′ | 95 °C 15 Seg 65 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-GCCAGCCATCACCAAGCCAATA-3′ | ||
| Catalase | Fw: 5′-TGGCCTCCGAGATCTTTTCAATG-3′ | 95 °C 15 Seg 63 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-GCGCTGAAGCTGTTGGGGTAGTA-3′ | ||
| Sirt-1 | Fw: 5′-TGGAGCAGGTTGCAGGAATCCA-3′ | 95 °C 15 Seg 60 °C 30 Seg 72 °C 30 Seg |
| Rev: 5′-TGGCTTCATGATGGCAAGTGGC-3′ |
| Young Rats Ctr | Old Rats Ctr | Old Rats Treated | |
|---|---|---|---|
| CAT | 1.00 ± 0.36 a | 0.18 ± 0.04 b | 0.68 ± 0.32 a |
| GPx | 1.00 ± 0.19 ab | 0.68 ± 0.09 a | 1.47 ± 0.38 b |
| IL6 | 1.00 ± 0.24 a | 4.84 ± 1.46 b | 1.20 ± 0.25 a |
| Sirt1 | 1.00 ± 0.21 ab | 0.73 ± 0.13 a | 1.72 ± 0.17 ab |
| Mn-SOD | 1.00 ± 0.21 | 0.79 ± 0.06 | 1.58 ± 0.58 |
| IL10 | 1.00 ± 0.16 | 1.44 ± 0.09 | 1.39 ± 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annunziata, G.; Jimenez-García, M.; Tejada, S.; Moranta, D.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Sureda, A.; Novellino, E.; Capó, X. Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats. Nutrients 2020, 12, 1280. https://doi.org/10.3390/nu12051280
Annunziata G, Jimenez-García M, Tejada S, Moranta D, Arnone A, Ciampaglia R, Tenore GC, Sureda A, Novellino E, Capó X. Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats. Nutrients. 2020; 12(5):1280. https://doi.org/10.3390/nu12051280
Chicago/Turabian StyleAnnunziata, Giuseppe, Manuel Jimenez-García, Silvia Tejada, David Moranta, Angela Arnone, Roberto Ciampaglia, Gian Carlo Tenore, Antoni Sureda, Ettore Novellino, and Xavier Capó. 2020. "Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats" Nutrients 12, no. 5: 1280. https://doi.org/10.3390/nu12051280
APA StyleAnnunziata, G., Jimenez-García, M., Tejada, S., Moranta, D., Arnone, A., Ciampaglia, R., Tenore, G. C., Sureda, A., Novellino, E., & Capó, X. (2020). Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats. Nutrients, 12(5), 1280. https://doi.org/10.3390/nu12051280








