Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources
Abstract
1. Introduction
2. Systematic Review of Major Points
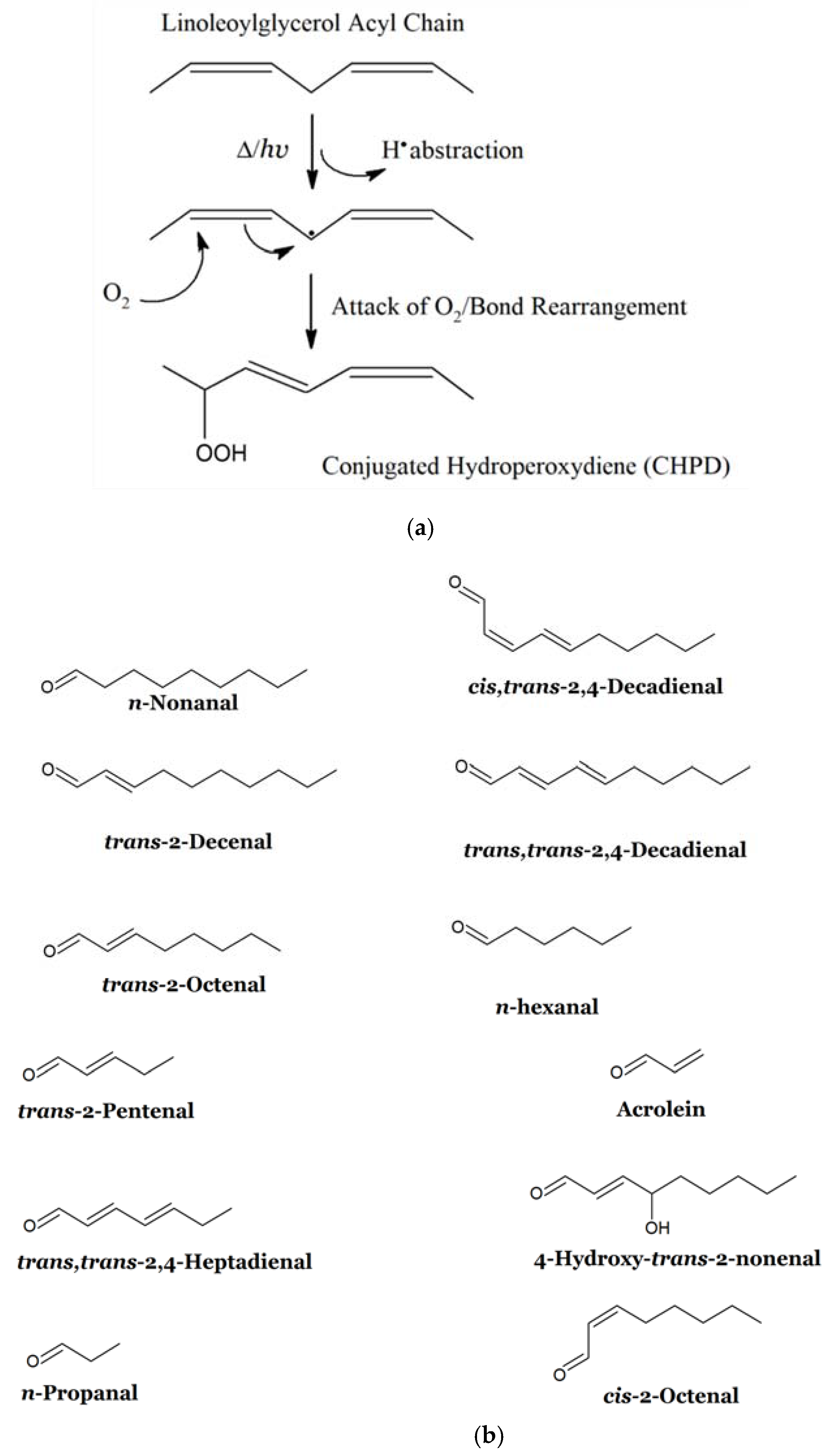
2.1. Lipid Peroxidation Process: Mechanistic Considerations and Relative Susceptibilities of Acylglycerol FAs
2.2. Dietary Sources of LOPs
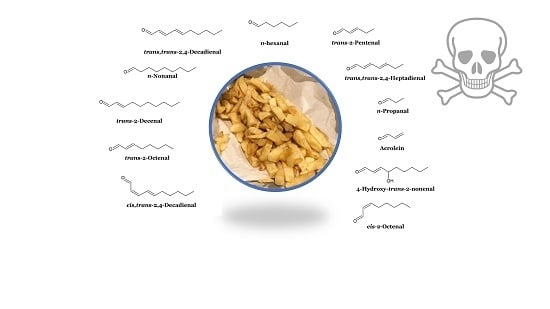
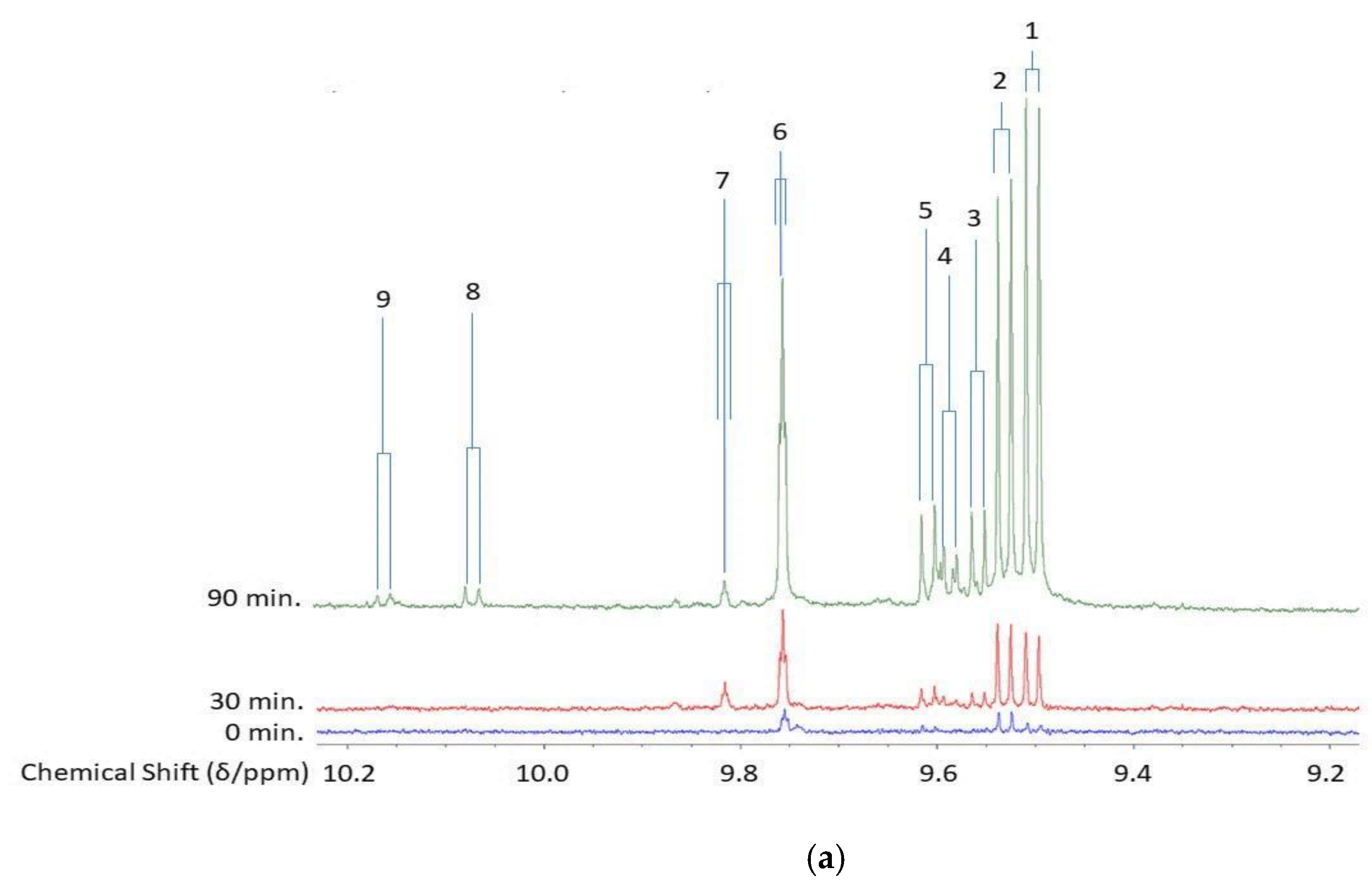
2.3. Fried Food Sources of LOPs
2.4. Exposure of Human Populations to Fried Food Sources of Dietary Aldehydes: Rational Estimates of Their Dietary Ingestion from These Sources
2.4.1. Acrolein
2.4.2. HNE and HHE
2.5. Risk Assessments of Aldehyde Intake in Humans: Estimated Margin of Exposure (MOE) Values
3. Fate and Adverse Health Effects of Primary and Secondary LOPs in Humans and Animal Model Systems Following Dietary Ingestion
3.1. Lipid Hydroperoxide Aldehyde Precursors (CHPDs and HPMs): Gastrointestinal Interactions, Metabolism and Biotransformations, In Vivo Absorption, Toxicity and Deleterious Health Effects
3.2. Secondary Aldehydic LOPs: Dietary Ingestion, Gastrointestinal Fate, In Vivo Absorption, Metabolism and Toxicological Effects
3.2.1. In Vivo Absorption of and Metabolic/Biotransformation Routes for Aldehydic LOPs
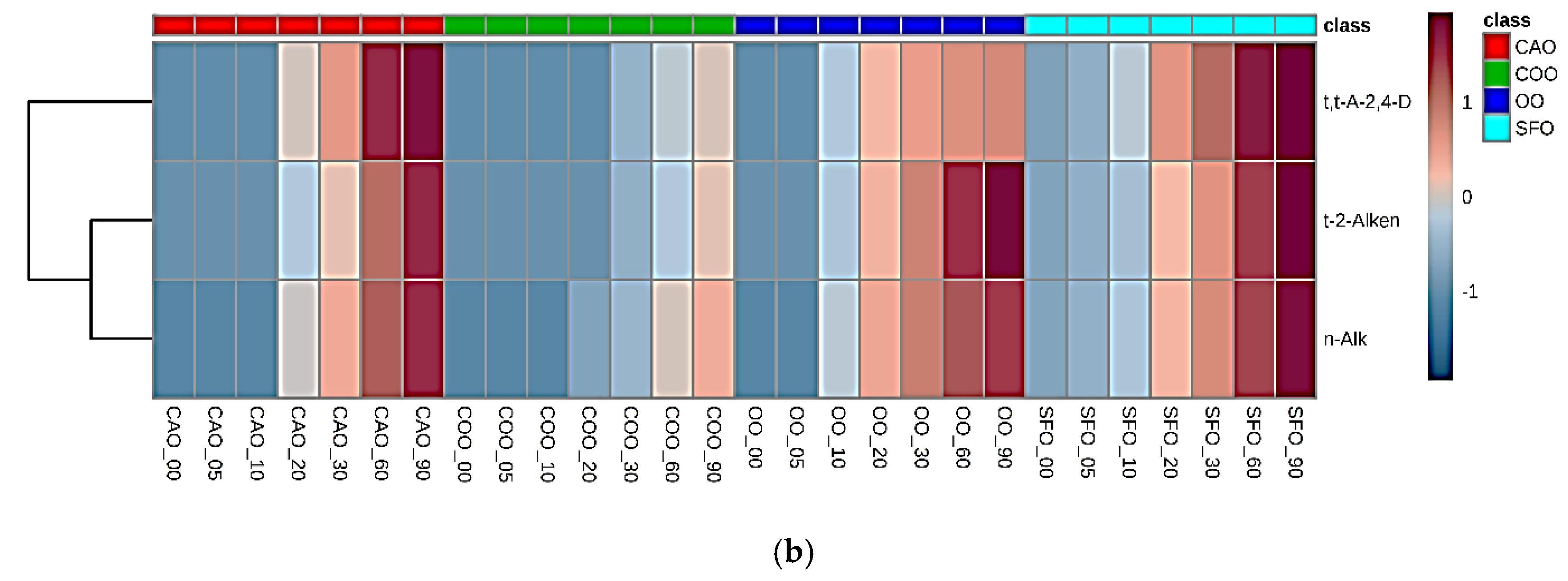
3.2.2. Associations between Dietary Fried Food Aldehyde Concentration Patterns and Those of Human Blood Plasma: Potential Tracking of Dietary LOPS In Vivo?
4. Atherosclerosis and Its Cardiovascular Disease Sequelae
5. Mutagenicity, Genotoxicity and Carcinogenicity of Secondary Aldehydic LOPs, and Potentially Their Dietary/Fried Food Sources
5.1. MDA
5.2. Trans-2-Alkenals
5.3. Acrolein and Chinese Wok Cooking
5.4. Crotonaldehyde
5.5. 4,5-Epoxy-Trans-2-Alkenals
5.6. Acetaldehyde and Formaldehyde
5.7. Impact of Fried Food Intake on Cancer Risks in Humans
6. Potential Mechanisms for the Toxicity and Health Effects of Dietary Aldehydes
6.1. Important Considerations for HNE and HNE
6.1.1. In Vivo Generation of HNE/HHE from High and Sustained Dietary Supplementation with ω-3 PUFAs?
6.1.2. Influence of CO Consumption on Blood Plasma Levels of HNE and HHE
6.1.3. Haem Oxygenase-1 Expression and Dietary Marine Oil Supplementation: Potential Beneficial Role of HHE
6.1.4. Cell Signalling by HHE and HNE
6.1.5. Aldehydes as the Dominant Carcinogens Present in Cigarette Smoke
7. Targeted Nutrition and Potential Interventional Routes for Eliminating or Alleviating Health Risks Associated with Dietary LOP Intake in Humans
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Grootveld, M.; Ruiz-Rodado, V.; Silwood, C.J.L. Detection, monitoring and deleterious health effects of lipid oxidation products generated in COs during thermal stressing episodes. Inform Am. Oil Chem. Soc. 2014, 25, 614–624. [Google Scholar]
- Grootveld, M.; Silwood, C.J.L.; Addis, P.; Claxson, A.W.D.; Bonet Serra, B.; Viana, M. Health effects of oxidised heated oils. Foodserv. Res. Internat. 2001, 13, 39–53. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Grootveld, K.L. Chronic non-communicable disease risks presented by lipid oxidation products in fried foods. Hepatobil. Surg. Nutr. 2018, 7, 305–312. [Google Scholar] [CrossRef]
- Estévez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Chapter Two - Health Risks of Food Oxidation. Advanc. Food Nutrit. Res. 2017, 82, 45–81. [Google Scholar]
- Jackson, V.; Penumetcha, M. Dietary oxidised lipids, health consequences and novel food technologies that thwart food lipid oxidation: An update. Intern. J. Food Sci. Technol. 2019, 54, 1981–1988. [Google Scholar] [CrossRef]
- Frankel, E.N. Volatile lipid oxidation-products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Perez-Camino, M.C. Fatty acid composition: A useful tool for the determination of alteration level in heated fats. Rev. Franc. Corps Gras. 1988, 35, 67–70. [Google Scholar]
- Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Haywood, R.M.; Chander, C.L.; Atherton, M.; Lynch, E.J.; Grootveld, M.C. Generation of lipid peroxidation products in culinary oils and fats during episodes of thermal stressing: A high field 1H NMR study. FEBS Lett. 1994, 355, 81–90. [Google Scholar] [CrossRef]
- Haywood, R.M.; Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Coumbarides, G.; Hawkes, J.; Lynch, E.J.; Grootveld, M.C. Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: Investigations using high resolution proton NMR spectroscopy. Free Rad. Res. 1995, 22, 441–482. [Google Scholar] [CrossRef]
- Silwood, C.J.L.; Grootveld, M. Application of high-resolution two-dimensional 1H and 13C nuclear magnetic resonance techniques to the characterization of lipid oxidation products in autoxidized linoleoyl/linolenoyglycerols. Lipids 1999, 34, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Martınez-Yusta, A.; Goicoechea, E.; Guillen, M.D. A Review of thermo-oxidative degradation of food lipids studied by 1H NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from COs during frying practices: Peroxidative resistance of a monounsaturate-rich algae oil. Sci. Rep. 2019, 9, 4125. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–786S. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, Z.; Stevanovic, S.; Ristovski, Z.; Salimi, F.; Gao, J.; Wang, H.; Li, L. Role of Chinese cooking emissions on ambient air quality and human health. Sci. Total Environ. 2017, 589, 173–181. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Comprehen. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; The Oily Press: Dundee, Scotland, UK, 1998; p. 9. [Google Scholar]
- Frankel, E.N. Frying fats. In Lipid Oxidation; Frankel, E.N., Ed.; The Oily Press: Dundee, Scotland, UK, 2005; pp. 227–248. [Google Scholar]
- Neff, W.E.; Byrdwell, W.C. Characterization of model triacylglycerol (triolein, trilinolein and trilinolenin) autoxidation products via high-performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 1998, 818, 169–186. [Google Scholar] [CrossRef]
- Wang, G.W.; Guo, Y.; Vondriska, T.M.; Zhang, J.; Zhang, S.; Tsai, L.L.; Zong, N.C.; Bolli, R.; Bhatnagar, A.; Prabhu, S.D. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 1016–1022. [Google Scholar] [CrossRef]
- Acceptable Daily Intakes for Agricultural and Veterinary Chemicals; Current as of 31 March 2016; Australian Government Department of Health (Commonwealth of Australia, 2016); The Office of Chemical Safety, Department of Health: Canberra, ACT, Australia, 2016.
- Totani, N.; Yawata, M.; Mori, T.; Hammond, E.G. Oxygen content and oxidation in frying oil. J. Oleo Sci. 2013, 62, 989–995. [Google Scholar] [CrossRef][Green Version]
- Beauchamp, R.; Andjelkovich, D.; Klingerman, A.; Morgan, K.; Heck, H. A critical review of the literature on acrolein toxicity. Crit. Rev. Toxicol. 1985, 14, 309–380. [Google Scholar] [CrossRef]
- Hirayama, T.; Yamaguchi, M.; Nakata, T.; Okumura, M.; Yamazaki, T.; Watanabe, T.; Fukui, S. Formation of acrolein by the autooxidation of unsaturated fatty acid methyl esters. Eisei Kagaku 1989, 35, 303–306. [Google Scholar] [CrossRef]
- Lane, R.; Smathers, J. Monitoring aldehyde production during frying by reversed-phase liquid chromatography. J. Assoc. Off. Anal. Chem. 1991, 74, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.; Derraik, J.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci. Rep. 2015, 5, 7928. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Miura, S.; Mori, Y.; Ueta, M.; Tagami, E.; Yoshizawa, T.; Watanabe, T. High-performance liquid chromatographic determination of 2-alkenals in oxidized lipid as their 7-amino-6-methylquinoline derivatives. Chem. Pharm. Bull. 1991, 39, 1253–1257. [Google Scholar] [CrossRef]
- Shields, P.G.; Xu, G.X.; Blot, W.J.; Trivers, G.E.; Weston, A.; Pellizzari, E.D.; Qu, Y.H.; Harris, C. C Volatile emissions of wok cooking oil. Proc. Am. Assoc. Cancer Res. 1993, 34, 118. [Google Scholar]
- Csallany, A.S.; Han, I.; Shoeman, D.W.; Chen, C. 4-Hydroxynonenal (HNE), a toxic aldehyde in French fries from fast food restaurants. Free Rad. Biol. Med. 2012, 53, S250. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α,β-unsaturated aldehydes. Food Chem. 2012, 131, 915–926. [Google Scholar] [CrossRef]
- Suhr, J.; Kwan, H. Estimation of daily exposure to 4-hydroxy-2-alkenals in Korean foods containing n-3 and n-6 polyunsaturated fatty acids. Food Add. Contamin. 2003, 22, 701–708. [Google Scholar]
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Carson, M.L.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Acrolein (August). CAS#: 107-02-8. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=557&tid=102 (accessed on 10 October 2019).
- Lachenmeier, D.W.; Kanteres, F.; Rehm, J. Carcinogenicity of acetaldehyde in alcoholic beverages: Risk assessment outside ethanol metabolism. Addiction 2009, 104, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Jendral, J.A.; Lachenmeier, D.W. The margin of exposure to formaldehyde in alcoholic beverages. Arch. Indust. Hyg. Toxicol. 2012, 63, 227–237. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Nicolli, K.P.; Souza-Silva, É.A.; Manfroi, V.; Zini, C.A.; Welke, J.E. Carbonyl compounds in different stages of vinification and exposure risk assessment through Merlot wine consumption. Food Additiv. Contamin. 2018, 35, 2315–2331. [Google Scholar] [CrossRef] [PubMed]
- Peterle, G.P.; Hernandes, K.C.; Schmidt, L.M.; Hoffmann, J.B.; Zini, C.A.; Welke, J.E. Exposure risk to carbonyl compounds and furfuryl alcohol through the consumption of sparkling wines. Ciência Rural 2019, 49, e20180986. [Google Scholar] [CrossRef]
- WHO Food Additives Series 40, 49th Meeting of JECFA. 1998. Available online: www.inchem.org/documents/jecfa/jecmono/v040je17.htm (accessed on 3 September 2019).
- Chapter 5.8 Formaldehyde. Air Quality Guidelines, 2nd ed.; WHO Regional Office for Europe: Copenhagen, Denmark, 2001.
- World Health Organization (WHO). Human Health Risk Assessment Toolkit: Chemical. 2010. Available online: http://www.who.int/ipcs/methods/harmonization/areas/ra_toolkit/en/ (accessed on 18 February 2020).
- Sottero, B.; Rossin, D.; Poli, G.; Biasi, F. Lipid oxidation products in the pathogenesis of inflammation-related gut diseases. Curr. Med. Chem. 2018, 25, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Privett, O.S. Toxicity of fatty ozonides and peroxides. Lipids 1972, 7, 715–721. [Google Scholar] [CrossRef]
- Wolff, S.P.; Nourooz-Zadeh, I. Hypothesis: UK consumption of dietary lipid hydroperoxides—A possible contributory factor to atherosclerosis. Atherosclerosis 1996, 119, 261–263. [Google Scholar] [CrossRef]
- Kokatnur, M.G.; Bergan, J.G.; Draper, H.H. Chronic toxicity of methyl linoleate hydroperoxide for the rabbit. Proc. Soc. Exp. Biol. Med. 1966, 123, 254–258. [Google Scholar] [CrossRef]
- Hata, K.; Fujimoto, K.; Kaneda, T. Absorption of lipid hydroperoxides in carp. Nihon-suisan-gakkai-shi 1986, 52, 677–684. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ashida, H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochimica et Biophysica Acta (BBA) Lipids Lipid Metab. 1998, 1393, 349–361. [Google Scholar] [CrossRef]
- Hartz, J.W.; Funakoshi, S.; Deutsch, H.F. The levels of superoxide dismutase and catalase in human tissue as determined immunochemically. Clin. Chim. Acta 1973, 46, 125–132. [Google Scholar] [CrossRef]
- Marklund, S.L.; Westman, N.G.; Lundgren, E.; Roos, G. Copper- and zinc-containing superoxide dismutase, manganese-containing superoxide dismutase, catalase, glutathione peroxidase in normal and neoplastic human cell lines and normal human tissues. Cancer Res. 1982, 42, 1955–1961. [Google Scholar]
- Ogasawara, T.; Hoensch, H.; Ohnhaus, E.E. Distribution of glutathione and its related enzymes in small intestinal mucosa of rats. Arch. Toxicol. 1985, 8, 110–112. [Google Scholar]
- Chu, F.F.; Doroshow, J.H.; Esworthy, R.S. Expression, characterisation, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J. Biol. Chem. 1993, 268, 2571–2578. [Google Scholar] [PubMed]
- Wingler, K.; Muller, C.; Schmehl, K.; Florian, S.; Brigelius-Flohe, R. Gastrointestinal glutathione peroxidase prevents transport of lipid hydroperoxides in CaCo-2 cells. Gastroenterology 2000, 119, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Rad. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
- Tullberg, C.; Larsson, K.; Carlsson, N.-G.; Comi, I.; Scheers, N.; Vegarud, G.; Undeland, I. Formation of reactive aldehydes (MDA, HHE, HNE) during the digestion of cod liver oil: Comparison of human and porcine in vitro digestion models. Food and Funct. 2016, 3, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Lyall, K.; Smyth, L.; Fernie, C.E.; Riemersma, R.A. Dietary hydroxy fatty acids are absorbed in humans: Implications for the measurement of ’oxidative stress’ in vivo. Free Rad. Biol. Med. 2002, 32, 162–168. [Google Scholar] [CrossRef]
- Penumetcha, M.; Khan, N.; Parthasarathy, S. Dietary oxidized fatty acids: An atherogenic risk? J. Lipid Res. 2000, 41, 1473–1480. [Google Scholar]
- Kritchevsky, D.; Tepper, S.A. Cholesterol vehicle in experimental atherosclerosis. 9. Comparison of heated corn oil and heated olive oil. J. Atheroscler. Res. 1967, 7, 647–651. [Google Scholar] [CrossRef]
- Staprãns, I.; Rapp, J.H.; Pan, X.-M.; Hardman, D.A.; Feingold, K.R. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 533–538. [Google Scholar] [CrossRef]
- Soffritti, M.; Belpoggi, F.; Lambertini, L.; Lauriola, M.; Padovani, M.; Maltoni, C. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 87–105. [Google Scholar] [CrossRef]
- Stavridis, J.C. Toxicity and carcinogenicity of aldehydes. In Oxidation: The Cornerstone of Carcinogenesis. Oxidation and Tobacco Smoke Carcinogenesis. A Relationship Between Cause and Effect; Stavridis, J.C., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 161–173. [Google Scholar] [CrossRef]
- Emerit, I. Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Rad. Biol. Med. 1994, 36, 99–109. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L.; Rodomonte, A. Structure–activity relationships for the mutagenicity and carcinogenicity of simple and α-β unsaturated aldehydes. Environ. Mol. Mutagen. 2003, 42, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-C.; Chang, L.W.; Lee, H.-L.; Tsai, L.-H.; Liu, V.-C.; Lisn, P. DNA damage induced by trans,trans-2,4-decadienal (tt-DD), a component of cooking oil fume, in human bronchial epithelial cells. Environ. Mol. Mutagen. 2010, 51, 315–321. [Google Scholar] [PubMed]
- Jayaraj, A.P.; Rees, K.R.; Tovey, F.E.I.; White, J.S. A molecular basis of peptic ulceration due to diet. Br. J. Exp. Path. 1986, 67, 149–155. [Google Scholar]
- Benedetti, A.; Ferrali, A.; Casini, A.F.; Peiri, S.; Comporti, M. Foot edema induced by carbonyl compounds originating from the peroxidation of microsomal lipids. Biochem. Pharmacol. 1980, 29, 121–124. [Google Scholar] [CrossRef]
- Indart, A.; Viana, M.; Grootveld, M.; Silwood, C.; Sanchez-Vera, I.; Bonet Serra, B. Teratogenic actions of thermally-stressed COs in rats. Free Rad. Res. 2002, 36, 1051–1058. [Google Scholar] [CrossRef]
- Long, E.K.; Murphy, T.C.; Leiphon, L.J.; Watt, J.; Morrow, J.D.; Milne, G.L.; Howard, J.R.H.; Picklo, M.J., Sr. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008, 105, 714–724. [Google Scholar] [CrossRef]
- Leong, X.-F.; Mustafa, M.R.; Das, S.; Jaarin, K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids Health Dis. 2010, 9, 66. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Shaw, H.M.; Yang, M.F.; Huang, C.Y.; Hsieh, C.H.; Chao, P.M. Dietary oxidised frying oil causes oxidative damage of pancreatic islets and impairment of insulin secretion, effects associated with vitamin E deficiency. Br. J. Nutr. 2011, 105, 1311–1319. [Google Scholar] [CrossRef]
- Bein, K.; Leikauf, G. Acrolein—A pulmonary hazard. Molec. Nutr. Food Res. 2011, 55, 1342–1360. [Google Scholar] [CrossRef]
- Lee, T.; Gany, F. Cooking oil fumes and lung cancer: A review of the literature in the context of the U.S. population. J. Immigr. Minor Health 2013, 15, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Jiang, Y.; Jin, S.; Li, Y. Association between cooking oil fume exposure and lung cancer among Chinese nonsmoking women: A meta-analysis. Onco. Targets Ther. 2016, 9, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Cui, Z.; Guan, P.; Li, X.; Wu, W.; Ren, Y.; He, Q.; Zhou, B. Interaction between polymorphisms in Pre-MiRNA genes and cooking oil fume exposure on the risk of lung cancer in Chinese non-smoking female population. PLoS One. 2015, 10, e0128572. [Google Scholar] [CrossRef]
- Yu, I.T.S.; Chiu, Y.-L.; Au, J.S.K.; Wong, T.-W.; Tang, J.-L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006, 66, 4961–4967. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Atherton, M.D.; Sheerin, A.N.; Hawkes, J.; Blake, D.R.; Richens, T.E.; Silwood, C.J.L.; Lynch, E.; Claxson, A.W.D. In Vivo absorption, metabolism, and urinary excretion of α,β-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998, 101, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Kaye, C.M. Biosynthesis of mercapturic acids from allyl alcohol, allyl esters and acrolein. Blochem. J. 1973, 134, 1093–1101. [Google Scholar] [CrossRef]
- McGirr, L.G.; Hadley, M.; Draper, H.H. Identification of N alpha-acetyl-epsilon-(2-propenal)lysine as a urinary metabolite of malondialdehyde. J. Biol. Chem. 1985, 260, 15427–15431. [Google Scholar]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.-A.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochimica et Biophysica Acta 2015, 1852, 826–838. [Google Scholar] [CrossRef]
- Srivastava, S.; Dixit, B.L.; Cai, J.; Sharma, S.; Hurst, H.E.; Bhatnagar, A.; Srivastava, S.K. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: Role of aldose reductase. Free Rad. Biol. Med. 2000, 29, 642–651. [Google Scholar] [CrossRef]
- Mak, S.; Lehotay, D.C.; Yazdanpanah, M.; Azevedo, E.R.; Liu, P.P.; Newton, G.E. Unsaturated aldehydes including 4-OH-nonenal are elevated in patients with congestive heart failure. J. Card. Fail. 2000, 6, 109–114. [Google Scholar] [CrossRef]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A.; Jette, M.; Huot, S. Desrosiers C: Acyloin production from aldehydes in the perfused rat heart: The potential role of pyruvate dehydrogenase. Biochem. J. 1993, 294, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Witz, G. Biological interactions of α,β-unsaturated aldehydes. Free Rad. Biol. Med. 1988, 7, 333–349. [Google Scholar] [CrossRef]
- Srivastava, S.; Chandra, A.; Wang, L.F.; Seifert, W.E.; Dague, B.B.; Ansari, N.H.; Srivastava, S.D.; Bhatnagar, A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J. Biol. Chem. 1998, 273, 10893–10900. [Google Scholar] [CrossRef]
- Kroes, R. WHO Food Additives Series: 42. Furfural. Prepared by the Fifty-first Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 1999; International Program on Chemical Safety (IPCS) INCHEM Home. [Google Scholar]
- Conklin, D.J.; Barski, O.A.; Lesgards, J.F.; Juvan, P.; Rezen, T.; Rozman, D.; Prough, R.A.; Vladykovskaya, E.; Liu, S.; Srivastava, S. Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol. Appl. Pharmacol. 2010, 243, 1–12. [Google Scholar] [CrossRef]
- Conklin, D.J.; Bhatnagar, A. Chapter 6.26 - Aldehydes and Cardiovascular Disease. In Comprehensive Toxicology Reference Work, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 6, pp. 489–512. [Google Scholar]
- Ogihara, T.; Hirano, K.; Morinobu, T.; Kim, H.-S.; Hiroi, M.; Ogihara, H.; Tamai, H. Raised concentrations of aldehyde lipid peroxidation products in premature infants with chronic lung disease. Arch. Dis. Child Fetal Neonatal Ed. 1999, 80, F21–F25. [Google Scholar] [CrossRef]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal. 2010, 13, 39–75. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Rad. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Hoff, H.F.; O’Neil, J.; Wu, Z.; Hoppe, G.; Salomon, R.L. Phospholipid hydroxyalkenals. Biological and chemical properties of specific oxidized lipids present in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 275–282. [Google Scholar] [CrossRef][Green Version]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Modification of low-density lipoprotein that increases its atherogenecity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [PubMed]
- Steinberg, D. Role of oxidized LDL and antioxidants in atherosclerosis. Adv. Exp. Med. Biol. 1995, 369, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Sakai, K.; Itakura, K.; Osawa, T.; Toyokuni, S. Protein modification by lipid peroxidation products: Foration of malondialdehyde-derived Ne-(2-propenal)lysine in proteins. Arch. Biochem. Biophys. 1997, 346, 45–52. [Google Scholar] [CrossRef]
- Requena, J.R.; Fu, M.X.; Ahmed, M.U.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997, 322, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Obama, T.; Kato, R.; Masuda, Y.; Takahashi, K.; Aiuchi, T.; Itabe, H. Analysis of modified apolipoprotein B-100 structures formed in oxidized low-density lipoprotein using LC-MS/MS. Proteomics 2007, 7, 2132–2141. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E.; et al. Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Morimitsu, Y.; Osawa, T.; Noguchi, N.; Niki, E. Acrolein is a product of lipid peroxidation reaction: Ormation of acrolein and its conjugate with lysine residues in oxidized low-density lipoprotein. J. Biol. Chem. 1998, 273, 16058–16066. [Google Scholar] [CrossRef]
- Uchida, K.; Toyokuni, S.; Nishikawa, K.; Kawokishi, S.; Oda, H.; Hiai, H.; Stadtman, E.R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low density lipoproteins: Markers for atherosclerosis. Biochemistry 1994, 33, 12487–12494. [Google Scholar] [CrossRef]
- Tamamizu-Kato, S.; Wong, J.Y.; Jairam, V.; Uchida, K. Modification by acrolein, a component of tobacco smoke and age-related oxidative stress, mediates functional impairment of human apolipoprotein E. Biochemistry 2007, 46, 8392–8400. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakazato, Y.; Saiki, R.; Igarashi, K.; Kitada, M.; Ishii, I. Acrolein-conjugated low-density lipoprotein induces macrophage foam cell formation. Atherosclerosis 2013, 227, 51–57. [Google Scholar] [CrossRef]
- Mali, V.R.; Deshpand, M.; Pan, G.; Thandavarayan, R.A.; Palaniyandi, S.S. Impaired ALDH2 activity decreases the mitochondrial respiration in H9C2 cardiomyocytes. Cell Signal. 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Achanta, S.; Jordt, S.E. TRPA1: Acrolein meets its target. Toxicol. Appl. Pharmacol. 2017, 324, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hill, B.G.; Gu, Y.; Cai, J.; Srivastava, S.; Bhatnagar, A.; Prabhu, S.D. Mechanisms of acrolein-induced myocardial dysfunction: Implications for environmental and endogenous aldehyde exposure. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3673–H3684. [Google Scholar] [CrossRef] [PubMed]
- Ismahil, M.A.; Hamid, T.; Haberzettl, P.; Gu, Y.; Chandrasekar, B.; Srivastava, S.; Prabhu, S.D. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am. J. Physiology. Heart Circ. Physiol. 2011, 301, H2050–H2060. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, C.C.; Chan, D.C.; Chiu, C.Y.; Yang, R.S.; Liu, S.H. Adverse effects of acrolein, a ubiquitous environmental toxicant, on muscle regeneration and mass. J. Cachexia Sarcopenia Muscle 2019, 10, 165–176. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicolog. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried food consumption and cardiovascular health: A review of current evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef]
- Cho, Y.; Song, M.; Kim, T.S.; Ryu, J.C. DNA Methylome analysis of saturated aliphatic aldehydes in pulmonary toxicity. Sci. Rep. 2018, 8, 10497. [Google Scholar] [CrossRef]
- Eckl, P.M.; Bresgen, N. Review Article: Genotoxicity of lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Dung, C.H.; Wu, S.C.; Yen, G.C. Genotoxicity and oxidative stress of the mutagenic compounds formed in fumes of heated soybean oil, sunflower oil and lard. Toxicol. In Vitro. 2006, 20, 439–447. [Google Scholar] [CrossRef]
- Straif, K.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006, 7, 977–978. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Mueller, C.; Straesser, U.; Kamp, H.G.; Eisenbrand, G. α,β-Unsaturated carbonyl compounds: Induction of oxidative DNA damage in mammalian cells. Mutagenesis 2003, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.J.; Til, H.P.; deVrijer, F.; Woutersen, R.A.; Cassee, F.R.; van Bladeren, P.J. Aldehydes: Occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 1991, 259, 363–385. [Google Scholar] [CrossRef]
- Van der Veen, L.A.; Hashim, M.F.; Shyr, Y.; Marnett, L.J. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc. Natl. Acad. Sci. USA 2003, 100, 14247–14252. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D. Worldwide regulation of frying fats and oils. Inform Am. Oil Chem. Soc. 1993, 4, 1366–1371. [Google Scholar]
- Yonei, S.; Furui, H. Lethal and mutagenic effects of malondialdehyde, a decomposition product of peroxidized lipids, on Escherichia coli with different DNA-repair capacities. Mutat. Res./Genet. Toxicol. 1981, 88, 23–32. [Google Scholar] [CrossRef]
- Seto, H.; Okuda, T.; Takesue, T.; Ikemura, T. Reaction of malonaldehyde with nucleic acid. I. Formation of fluorescent pyrimido(1,2-a)purin-10(3H)-one nucleosides. Bull. Chem. Soc. Jpn. 1983, 56, 1799–1802. [Google Scholar] [CrossRef]
- Leuratti, C.; Watson, M.A.; Deag, E.J.; Welch, A.; Singh, R.; Gottschalg, E.; Marnett, L.J.; Atkin, W.; Day, N.E.; Shuker, D.E.G.; et al. Detection of malondialdehyde DNA adducts in human colorectal mucosa. Cancer Epidemiol. Biomark. Prev. 2002, 11, 267–273. [Google Scholar]
- Draper, H.H.; McGirr, L.G.; Hadley, M. The metabolism of malondialdehyde. Lipids 1986, 21, 305. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Malonaldehyde, Sodium Salt; Technical Report; National Institutes of Health: Research Triangle Park, NC, USA, 1988; Volume 331, pp. 5–13.
- Basu, A.K.; Marne, Â.L.J. Molecular requirements for the mutagenicity of malondialdehyde and related acroleins. Cancer Res. 1984, 44, 2848–2854. [Google Scholar]
- Marnett, L.J.; Hurd, H.K.; Hollstein, M.C.; Levin, D.E.; Esterbauer, H.; Ames, B.N. Naturally occurring carbonyl compounds are mutagens Salmonella tester strain TA104. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1985, 148, 25–34. [Google Scholar] [CrossRef]
- Canonero, R.; Martelli, A.; Marinari, U.M.; Brambilla, G. Mutation induction in Chinese hamster lung V79 cells by five alk-2-enals produced by lipid peroxidation. Mutat. Res. Lett. 1990, 244, 153–156. [Google Scholar] [CrossRef]
- Kiwamoto, R.; Spenkelink, A.; Rietjens, I.M.C.M.; Punt, A. A physiologically based in silico model for trans-2-hexenal detoxification and DNA adduct formation in human including interindividual variation indicates efficient detoxification and a negligible genotoxicity risk. Toxicokinet. Metab. 2013, 87, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Nadasi, E.; Varjas, T.; Pajor, L.; Ember, I. Carcinogenic potential of trans-2-hexenal is based on epigenetic effect. In Vivo 2005, 19, 559–562. [Google Scholar] [PubMed]
- Cohen, S.M.; Garland, E.M.; St John, M.; Okamura, T.; Smith, R.A. Acrolein initiates rat urinary bladder carcinogenesis. Cancer Res. 1992, 52, 3577–3581. [Google Scholar]
- Kawai, Y.; Furuhata, A.; Toyokuni, S.; Aratani, Y.; Uchida, K. Formation of acrolein-derived 2’-deoxyadenosine adduct in an iron-induced carcinogenesis model. J. Biol. Chem. 2003, 278, 50346–50354. [Google Scholar] [CrossRef]
- Nath, R.G.; Ocando, J.E.; Guttenplan, J.B.; Chung, F.-L. N2-Propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998, 58, 581–584. [Google Scholar]
- Lee, H.W.; Wang, H.T.; Weng, M.W.; Hu, Y.; Chen, W.S.; Chou, D.; Liu, Y.; Donin, N.; Huang, W.C.; Lepor, H.; et al. Acrolein- and 4-aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget 2014, 5, 3526–3540. [Google Scholar] [CrossRef]
- Hecht, S.S.; Koh, W.P.; Wang, R.; Chen, M.; Carmella, S.G.; Murphy, S.E.; Yuan, J.M. Elevated levels of mercapturic acids of acrolein and crotonaldehyde in the urine of Chinese women in Singapore who regularly cook at home. PLoS ONE 2015, 10, e0120023. [Google Scholar] [CrossRef]
- Stevens J., F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef]
- Metayera, C.; Wangb, Z.; Kleinermana, R.A.; Wangc, L.; Brennera, A.V.; Cuib, H.; Caob, J.; Lubin, J.H. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Canc. 2002, 35, 111–117. [Google Scholar] [CrossRef]
- Beland, F.A.; Olson, G.R.; Mendoza, M.C.; Marques, M.M.; Doerge, D.R. Carcinogenicity of glycidamide in B6C3F 1 mice and F344/N rats from a two-year drinking water exposure. Food Chem. Toxicol. 2015, 86, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hu, W.; Hu, Y.; Tang, M.-S. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. USA 2006, 103, 15404–15409. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.; Muriseli, H. (Eds.) The Role of Cyclic Nucleic Acid Adducts in Carcinogenesis and Mutagenesis; IARC Scientific Publications; International Agency for Research on Cancer: Lyon, France, 1986; Volume 70. [Google Scholar]
- Smith, R.A.; Cohen, S.M.; Lawson, T.A. Acrolein mutagenicity in the V79 assay. Carcinogenesis 1990, 11, 497–498. [Google Scholar] [CrossRef]
- van Andel, I.; Sleijffers, A.; Schenk, E.; Rambali, E.; Wolterink, G.; van der Werken, G.; van Aerts, L.A.G.J.M.; Vleeming, W.; van Amsterdam, J.G.C. Adverse Health Effects of Cigarette Smoke: Aldehydes Crotonaldehyde, Butyraldehyde, Hexanal, and Malonaldehyde; RIVM report 340630002/2006; Nutrition, Health Protection and Prevention Department, Ministry of Health, Welfare and Sports (VWS) and of the Food and Consumer Product Safety Authority (VWA): Bilthoven, The Netherlands, 2006. [Google Scholar]
- Chung, F.-L.; Tanaka, T.; Hecht, S.S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986, 46, 1285–1289. [Google Scholar] [PubMed]
- Lutz, D.; Eder, E.; Neudecker, T.; Henschler, D. Structure-mutagenicity relationships in unsaturated carbonylic compounds and their corresponding allylic alcohol. Mutat. Res. 1982, 93, 305–315. [Google Scholar] [CrossRef]
- Laskar, A.A.; Younus, H. (2018): Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Revs. 2018, 1–24. [Google Scholar] [CrossRef]
- Silano, V.; Bolognesi, C.; Castle, L.; Cravedi, J.-P.; Engel, K.-H.; Fowler, P.; Franz, R.; Grob, K.; Husøy, T.; Karenlampi, S.; et al. Scientific Opinion on Flavouring Group Evaluation 226 Revision 1 (FGE.226 Rev1): Consideration of genotoxicity data on -unsaturated aldehyde from chemical subgroup 1.1.1(b) of FGE.19 EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). ADOPTED: 4. EFSA J. 2017. [Google Scholar] [CrossRef][Green Version]
- Salaspuro, M. Acetaldehyde and gastric cancer. J. Digest. Dis. 2011, 12, 51–59. [Google Scholar] [CrossRef]
- Matthews, R.F.; Scanlan, R.A.; Libbey, L.M. Autoxidation products of 2,4-decadienal. J. Am. Oil Chem. Soc. 1971, 48, 745–747. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.; Shen, Q.; Saleh, A.S.M. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015, 52, 7683–7696. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Boskou, G.; Dedoussis, G.V.Z.; Chiou, A.; Tzamtzis, V.A.; Papathanasiou, A. A Quality assessment of frying oils and fats from 63 restaurants in Athens, Greece. Food Serv. Techn. 2003, 3, 49–59. [Google Scholar] [CrossRef]
- Tan, S.L.W.; Chadha, S.; Liu, Y.; Gabasova, E.; Perera, D.; Ahmed, K.; Constantinou, S.; Renaudin, X.; Lee, M.; Aebersold, R.; et al. A class of environmental and endogenous toxins induces BRCA2 haploinsufficiency and genome instability. Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (June 2004). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88 (2006): Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. Available online: http://monographs.iarc.fr/ENG/Monographs/vol88/index.phpExitDisclaimer (accessed on 10 June 2011).
- National Toxicology Program. Report on Carcinogens, Twelfth Edition. Department of Health and Human Services, Public Health Service, National Toxicology Program. Available online: http://ntp.niehs.nih.gov/go/roc12 (accessed on 10 June 2011).
- Stott-Miller, M.; Neuhouser, M.L.; Stanford, J.L. Consumption of deep-fried foods and risk of prostate cancer. Prostate 2013, 73, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. Fried food and prostate cancer risk: Systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2015, 66, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Steineck, G.; Järvinen, R.; Hakulinen, T.; Aromaa, A. Intake of fried meat and risk of cancer: A follow-up study in Finland. Int. J. Cancer 1994, 59, 756–760. [Google Scholar] [CrossRef]
- Bosetti, C.; Talamini, R.; Levi, F.; Negri, E.; Franceschi, S.; Airoldi, L.; La Vecchia, C. Fried foods: A risk factor for laryngeal cancer? Br. J. Cancer 2002, 87, 1230–1233. [Google Scholar] [CrossRef]
- Srivastava, S.M.; Singh, M.; George, J.; Bhui, K.; Shukla, Y. Genotoxic and carcinogenic risks associated with the consumption of repeatedly boiled sunflower oil. J. Agric. Food Chem. 2010, 58, 11179–11186. [Google Scholar] [CrossRef]
- Woutersen, R.A.; Appel, M.J.; van Garderen-Hoetmer, A.; Wijnands, M.V. Dietary fat and carcinogenesis. Mutat. Res. 1999, 443, 111–127. [Google Scholar] [CrossRef]
- Xie, M.Z.; Shoulkamy, M.I.; Salem, A.M.H.; Oba, S.; Goda, M.; Nakano, T.; Ide, H. Aldehydes with high and low toxicities inactivate cells by damaging distinct cellular targets. Mutat. Res./Fund. Molec. Mechan. Mutagenesis 2016, 786, 41–51. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Kleinman, W.; Marina, P.; Abraham, P.; Wynder, E.L.; Muscat, J.E. Blood iron, glutathione, and micronutrient levels and the risk of oral cancer. Nutr. Cancer 2008, 60, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Ertl, A.; Scholz, N. The reaction of cysteine with α,β-unsaturated aldehydes. Tetrahedron 1976, 32, 285–289. [Google Scholar] [CrossRef]
- Gan, J.C.; Oandasan, A.; Ansari, G.A.S. In Vitro covalent modification of serum albumin by acrolein. Chemosphere 1991, 23, 939–947. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular mechanisms of aldehyde toxicity: A chemical perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Vieira, O.; Escargueil-Blanc, I.; Salvayre, R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Mol. Aspects Med. 2003, 24, 251–261. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Jacobs, A.T.; Marnett, L.J. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 2010, 43, 673–683. [Google Scholar] [CrossRef]
- Gentile, F.; Pizzimenti, S.; Arcaro, A.; Pettazzoni, P.; Minelli, R.; D’Angelo, D.; Mamone, G.; Ferrant, P.; Toaldo, C.; Cetrangolo, G.; et al. Exposure of HL-60 human leukaemic cells to 4-hydroxynonenal promotes the formation of adduct(s) with alpha-enolase devoid of plasminogen binding activity. Biochem. J. 2009, 422, 285–294. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Conklin, D.J.; Bhatnagar, A. 6.26—Aldehydes and Cardiovascular Disease. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 489–512. [Google Scholar] [CrossRef]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 12, 1700047. [Google Scholar] [CrossRef]
- Goicoechea, E.; Van Twillert, K.; Duits, M.; Brandon, E.D.; Kootstra, P.R.; Blokland, M.H.; Guillén, M.D. Use of an in vitro digestion model to study the bioaccessibility of 4-hydroxy-2-nonenal and related aldehydes present in oxidized oils rich in omega-6 acyl groups. J. Agric. Food Chem. 2008, 56, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Fish Oil for Human Consumption. EFSA J. 2010, 8, 1874. [Google Scholar] [CrossRef]
- Kenmogne-Domguia, H.B.; Moisan, S.; Viau, M.; Genot, C.; Meynier, A. The initial characteristics of marine oil emulsions and the composition of the media inflect lipid oxidation during in vitro gastrointestinal digestion. Food Chem. 2014, 152, 146–154. [Google Scholar] [CrossRef]
- Steppeler, C.; Haugen, J.E.; Rødbotten, R.; Kirkhus, B. Formation of malondialdehyde, 4-hydroxynonenal, and 4-hydroxyhexenal during in vitro digestion of cooked beef, pork, chicken, and salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Calzada, C.; Colas, R.; Guillot, N.; Guichardant, M.; Laville, M.; Véricel, E.; Lagarde, M. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis 2010, 8, 467–472. [Google Scholar] [CrossRef]
- Van Kuijk, F.J.; Holte, L.L.; Dratz, E.A. 4-hydroxyhexenal: A lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim. Biophys. Acta 1990, 1043, 116–118. [Google Scholar] [CrossRef]
- Nakagawa, F.; Morino, K.; Ugi, S.; Ishikado, A.; Kondo, K.; Sato, D.; Konno, S.; Nemoto, K.-I.; Kusunoki, C.; Sekine, O.; et al. 4-Hydroxy hexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem. Biophys. Res. Commun. 2014, 443, 991–996. [Google Scholar] [CrossRef]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Gutzki, F.M.; Beckmann, B.; Frölich, J.C. Simultaneous GC-MS/MS measurement of malondialdehyde and 4-hydroxy-2-nonenal in human plasma: Effects of long-term L-arginine administration. Anal. Biochem. 2017, 524, 31–44. [Google Scholar] [CrossRef]
- Gindzienska-Sieskiewicz, L.W.; Jarocka-Karpowicz, E.; Andrisic, I.; Sierakowski, L.; Zarkovic, S.; Waeg, N.; Skrzydlewska, G. The onset of lipid peroxidation in rheumatoid arthritis: Consequences and monitoring. Free Radic. Res. 2016, 50, 304–313. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360, 438. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. 4-hydroxynonenal-mediated signaling and aging. Free Radic. Biol. Med. 2017, 111, 219–225. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, G.A. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Dianzani, M.U. 4-hydroxynonenal from pathology to physiology. Mol. Aspects Med. 2003, 24, 263–272. [Google Scholar] [CrossRef]
- Weng, M.-W.; Lee, H.-W.; Park, S.-Y.; Hu, Y.; Wang, H.-T.; Chen, L.-C.; Rom, W.N.; Huang, W.C.; Lepro, H.; Wu, X.-R.; et al. Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E6152–E6161. [Google Scholar] [CrossRef] [PubMed]
- Mirzael, H.O.; Salehi, F.; Garovni, H.; Farhadpour, F.J. Role of MC compounds at less of oil absorption of French fries potato. Nutr. Health Food Eng. 2015, 2, 00075. [Google Scholar]
- Kaminskas, L.M.; Pyke, S.M.; Burcham, P.C. Reactivity of hydrazinophthalazine drugs with the lipid peroxidation products acrolein and crotonaldehyde. Org. Biomol. Chem. 2004, 2, 2578–2584. [Google Scholar] [CrossRef]
- Leung, G.; Sun, W.; Zheng, L.; Brookes, S.; Tully, M.; Shi, R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience. 2011, 173, 150–155. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Technological Options to Improve the Nutritional Attributes of Animal Products. 4. Consumer Concerns and Animal Product Options. In Designing Foods: Animal Product Options in the Marketplace; National Academies Press (US): Washington, DC, USA, 1988. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218169/ (accessed on 2 July 2019).
- World Health Organization. Formaldehyde: Concise International Chemical Assessment Document 40; Wissenschaftliche Verlagsgesellschaft: Geneva, Switzerland, 2002. [Google Scholar]
- Shibamoto, T. Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems. J. Pharm. Biomed. Anal. 2006, 41, 12–25. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Acetaldehyde; 18 September 2012—Revision 11 December 2012; European Commission: Brussels, Belgium, 2012; ISSN 1831-4767. ISBN 978-92-79-30778-2. [Google Scholar] [CrossRef]
- National Toxicology Program; P.H.S. National Institutes of Health; U.S. Department of Health and Human Services. 2,4-Decadienal CAS No. 25152–84–5. Testing Status of Agents at NTP; National Toxicology Program, Research Triangle Park: Durham, NC, USA, 1993.
- Wu, S.C.; Yen, G.C.; Sheu, F. Mutagenicity and identification of mutagenic compounds of fumes obtained from heating peanut oil. J. Food Prot. 2001, 64, 240–245. [Google Scholar] [CrossRef]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutat. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef]
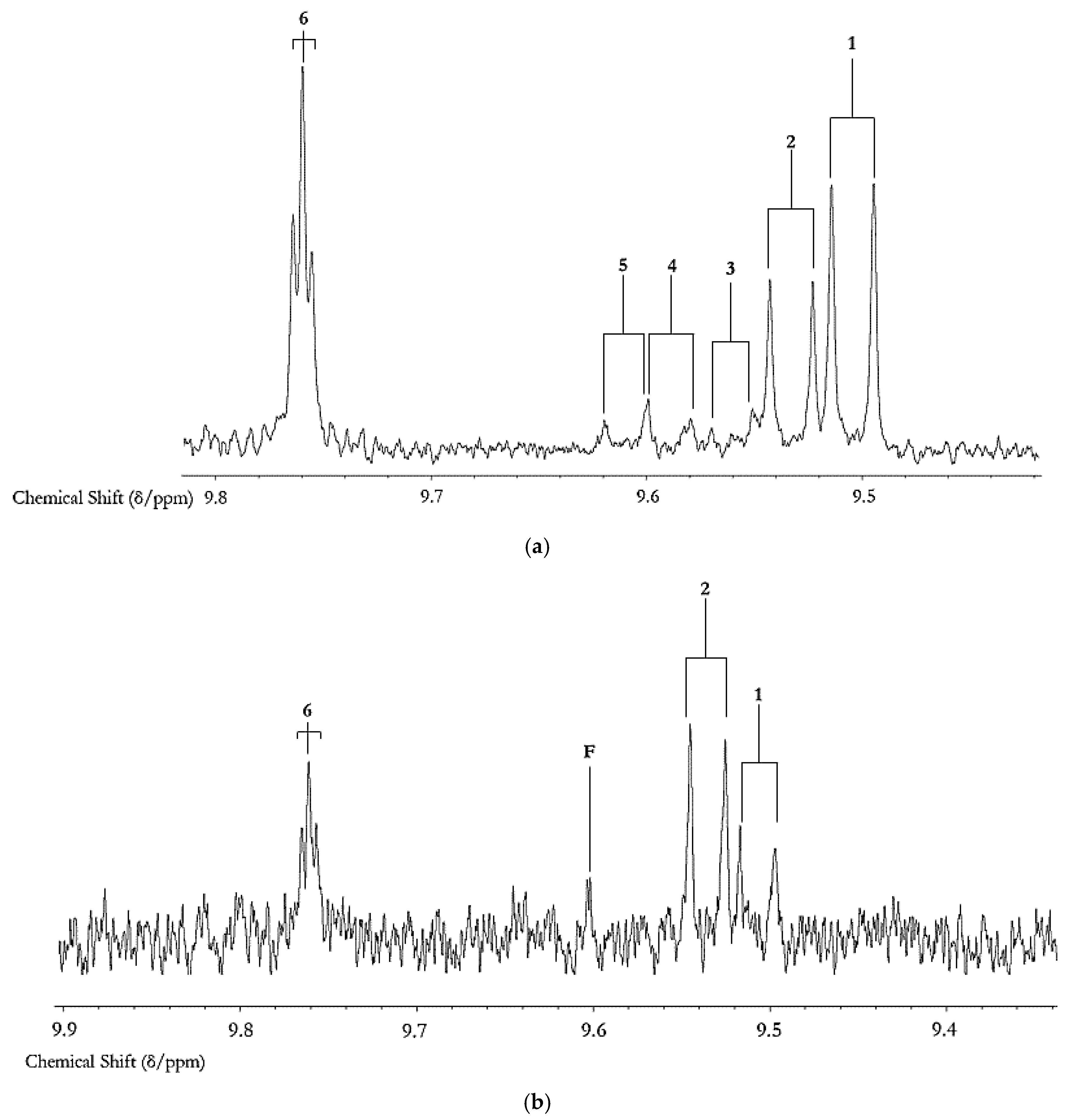
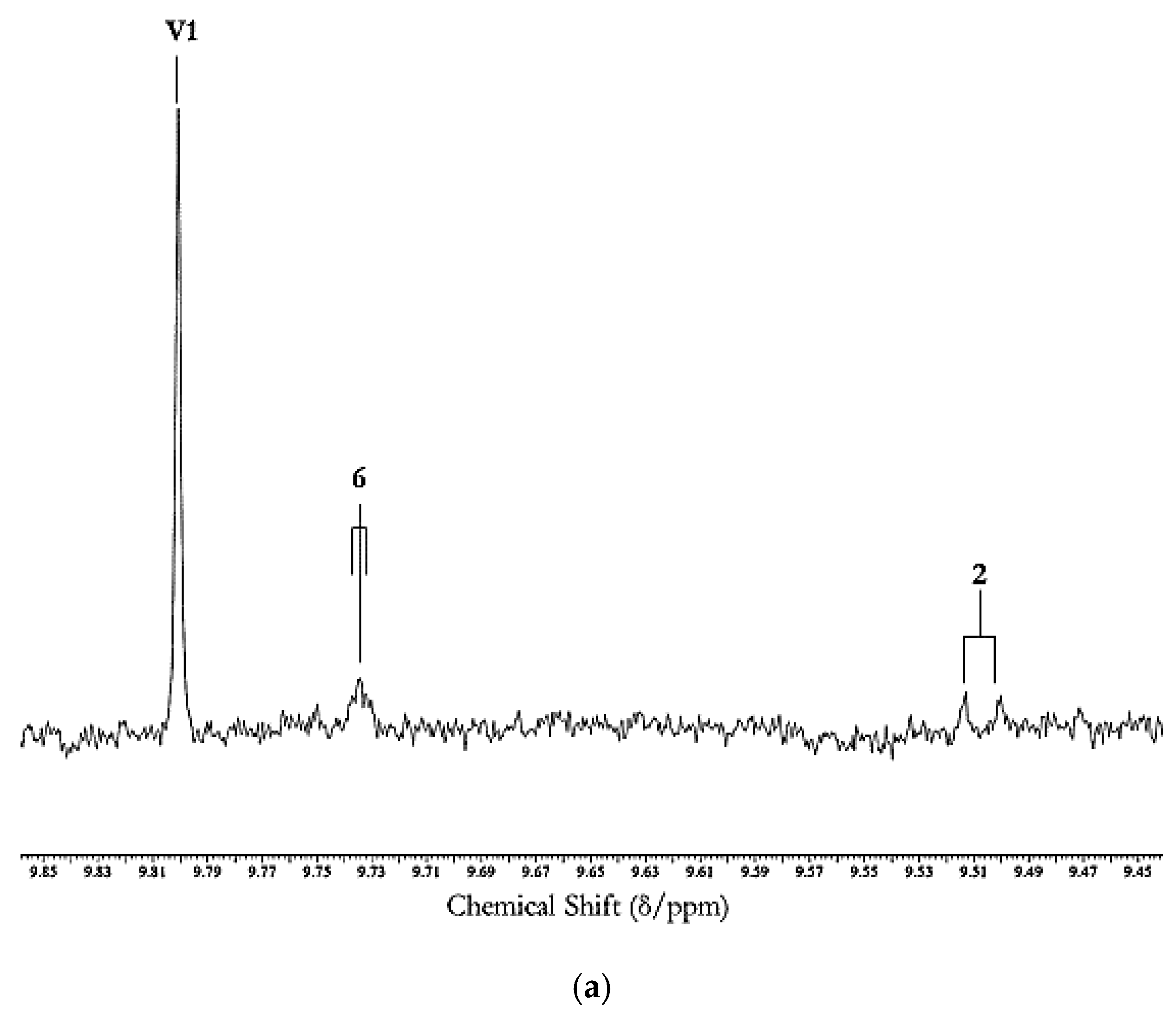
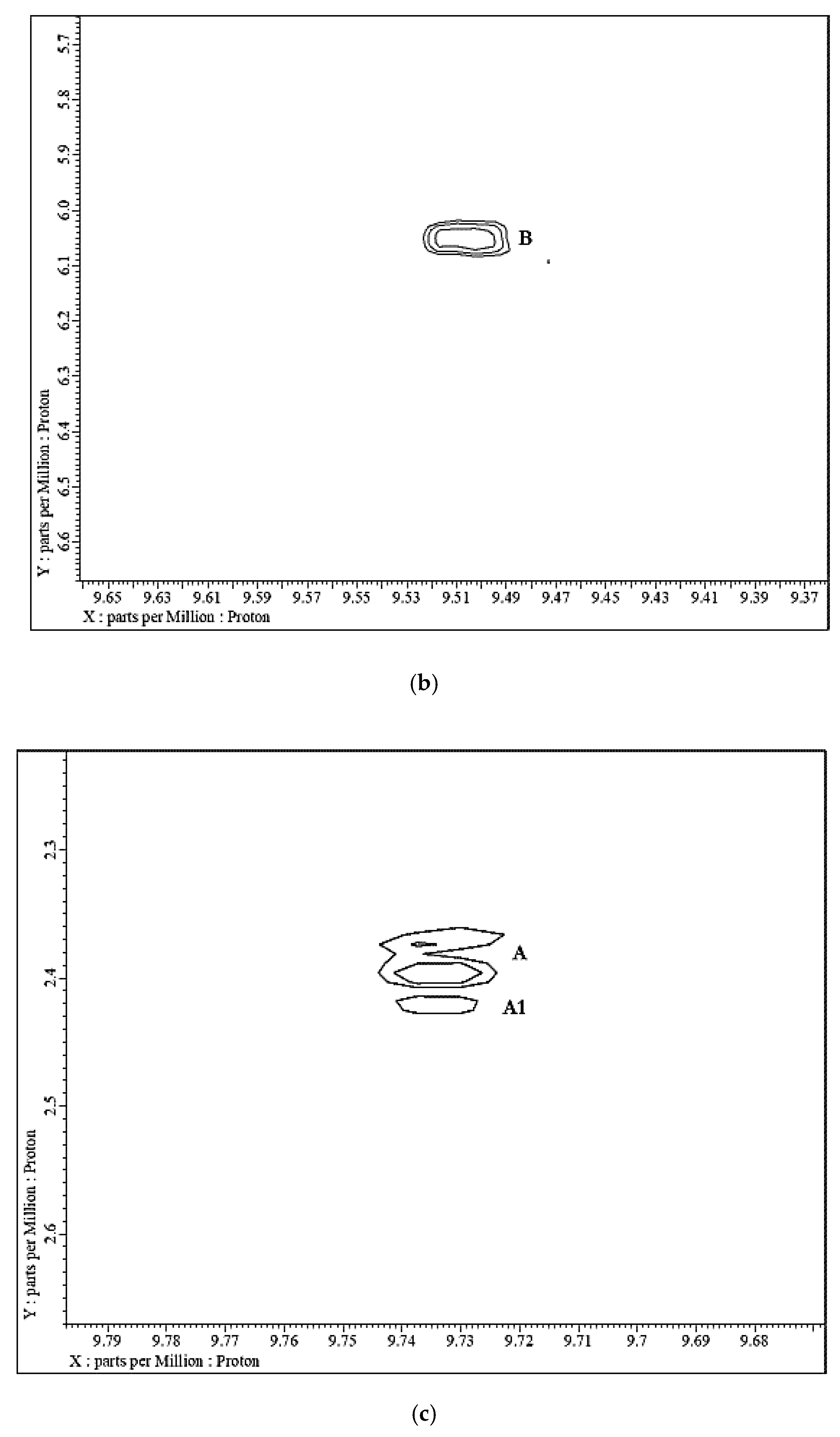
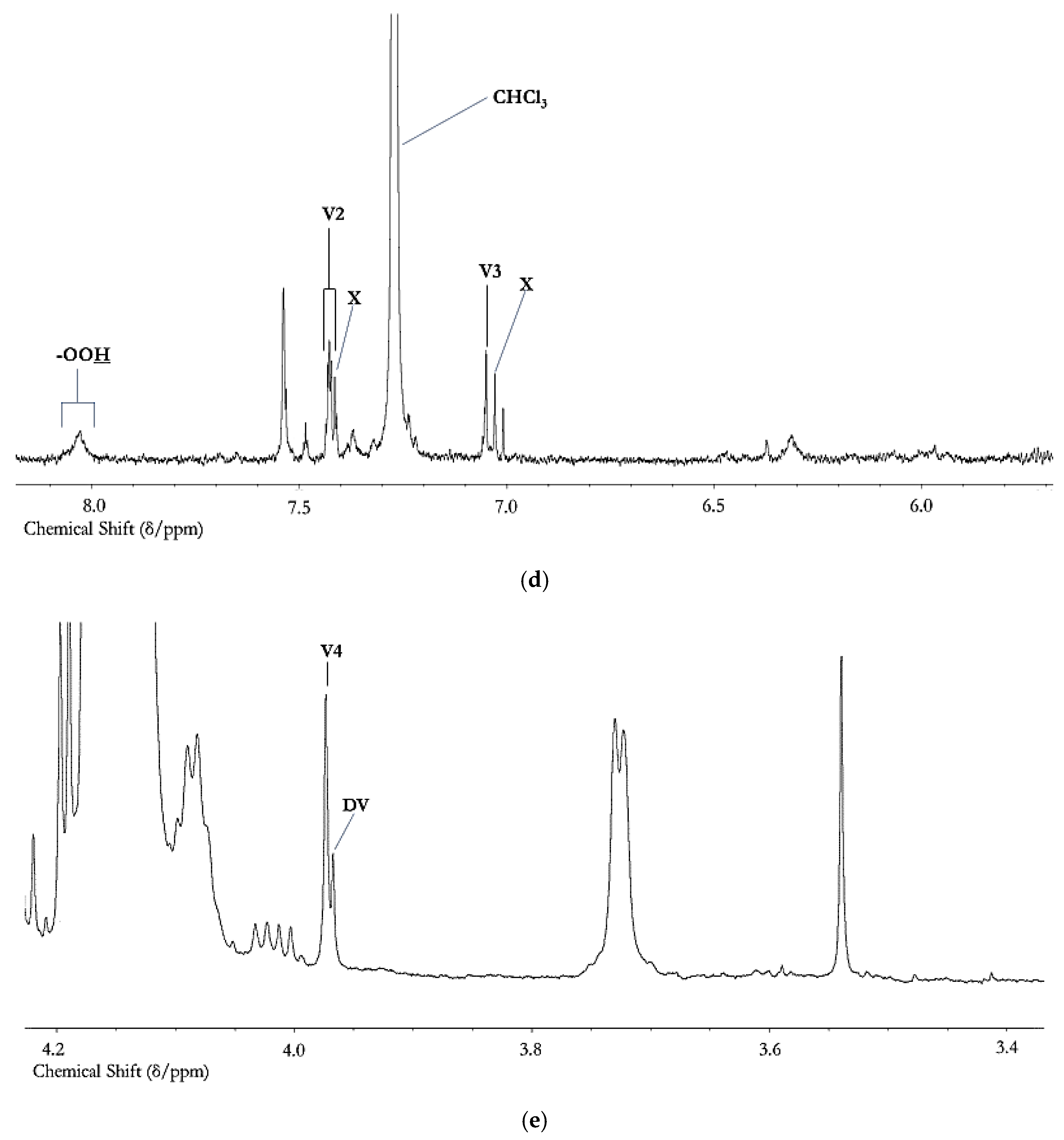

| Source | Aldehyde(s) | Concentration | |
|---|---|---|---|
| Water | Surface water (irrigation canal) | acrolein | 20–200 mg/L |
| Non – carbonated bottled water | Formaldehyde, acetaldehyde, nonanal, and methyl glyoxal | 1.7–57.5 mg/L | |
| Carbonated bottled water | Formaldehyde, acetaldehyde, nonanal, and methyl glyoxal | 3.9–197 mg/L | |
| Ground and surface water | Formaldehyde and acetaldehyde | 4.5–12 mg/L | |
| Ozone – purified water | Formaldehyde and acetaldehyde | 2–20 mg/L | |
| Foods | Fruits | acrolein | 10–50 mg/kg |
| Vegetables | acrolein | 10–590 mg/kg | |
| Donuts | acrolein | 100–900 mg/kg | |
| Codfish fillets | acrolein | 100 mg/kg | |
| Cheese | acrolein | 290–1300 mg/kg | |
| Red wine | acrolein | 3800 mg/kg | |
| Vinegar | acetaldehyde | 1.06 gm/kg | |
| Wheaten bread | butanal | 51 mg/kg | |
| Coffee | furfural | 255 mg/kg | |
| Bread | propanal | 31 mg/kg | |
| banana | 2–hexenal | 2 mg/kg | |
| Heated butter | 2–pentenal | 6 mg/kg | |
| Heated butter | 2,4–nonadienal | 1.5 mg/kg | |
| Vanilla | vanillin | 23 g/kg | |
| Lime, peel oil | citral | 130 g/kg | |
| Anise | anisaldehyde | 25 g/kg | |
| Tangerine, peel oil | 2,4–decadienal | 500 mg/kg | |
| Heated lard | acrolein | 109 mg/L | |
| Sunflower oil | acrolein | 163 mg/L | |
| Cigarettes | Mainstream | acrolein | 10–140 mg/cigarette |
| Mainstream | crotonaldehyde | 18.5 mg/cigarette | |
| Mainstream | acetaldehyde | 619 mg/cigarette | |
| Total aldehyde | 777 mg/cigarette | ||
| Side – stream | acrolein | 100–1700 mg/cigarette | |
| Ambient | Urban air | acrolein | 0002–0.035 mg/m3 |
| Smoky interiors | acrolein | 0.01–0.05 mg/m3 |
| Aldehyde Classification | |||
|---|---|---|---|
| Potato Chip Serving Size (g): | trans-2-Octenal | trans,trans-Deca-2,4-dienal | n-Hexanal |
| 71 g | 1.09 (0.48) MOE: 52.5 | 1.73 (0.64) MOE: 39.4 | 0.88 (0.50) MOE: 784 |
| 154 g | 2.37 (1.04) MOE: 22.7 | 3.76 (1.40) MOE: 16.9 | 1.91 (1.08) MOE: 363 |
| 300 g | 4.61 (2.01) MOE: 12.5 | 7.32 (2.72) MOE: 9.2 | 3.73 (2.10) MOE; 187 |
| Estimated Aldehyde Content (ppm): | 15.3 (6.8) | 24.4 (9.0) | 12.5 (7.0) |
| Non-CHF Controls (nmol/L) | CHF Disease (nmol/L) | Fried Potato Chips (μmol/kg) | |
|---|---|---|---|
| Long-Chain n-Alkanals (7) | 69–573 | 42–339 | 19–560 |
| Short-Chain n-Alkanals (1) | 67 | 91 | nd |
| trans-2-Alkenals (4) | 106–527 | 163–874 | 0–430 |
| 4-Hydroxy-trans-2-Alkenals (4) | 33–211 | 16–434 | 0.5–2.1 [68] |
| trans,trans-2,4-Alkadienals (2) | 152–180 | 148–420 | 0–443 |
| Malondialdehyde (MDA) | 96 | 101 | 0–6 * |
| Furfural | 2450 | 4060 | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients 2020, 12, 974. https://doi.org/10.3390/nu12040974
Grootveld M, Percival BC, Leenders J, Wilson PB. Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients. 2020; 12(4):974. https://doi.org/10.3390/nu12040974
Chicago/Turabian StyleGrootveld, Martin, Benita C. Percival, Justine Leenders, and Philippe B. Wilson. 2020. "Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources" Nutrients 12, no. 4: 974. https://doi.org/10.3390/nu12040974
APA StyleGrootveld, M., Percival, B. C., Leenders, J., & Wilson, P. B. (2020). Potential Adverse Public Health Effects Afforded by the Ingestion of Dietary Lipid Oxidation Product Toxins: Significance of Fried Food Sources. Nutrients, 12(4), 974. https://doi.org/10.3390/nu12040974