Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health
Abstract
1. Introduction
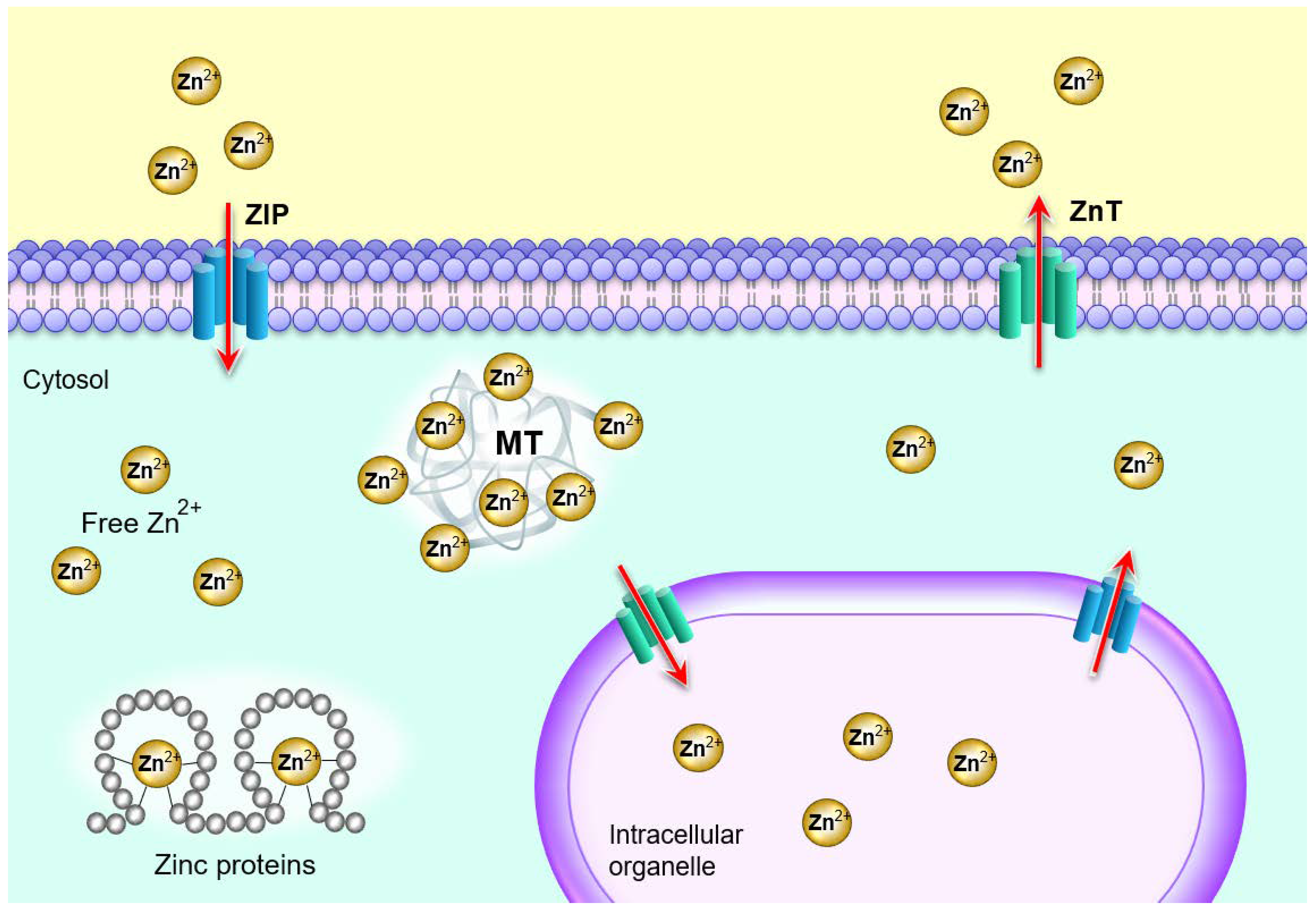
2. Zinc Homeostasis
3. Sources and Recommendation of Zinc
4. Zinc Deficiency
5. Role of Zinc in Oral Health and Disease
6. Effects of Zinc on Dental Caries and Periodontal Tissues
7. Effects of Zinc Deficiency on Oral Malignancy
8. Effects of Zinc on Oral Mucositis
9. Role of Zinc Deficiency in Cleft Lip, Cleft Palate and Salivary Glands
10. Benefits of Zinc Treatment in Oral Diseases
11. Zinc Toxicity
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaur, S.; Agnihotri, R. Trace mineral micronutrients and chronic periodontitis—A review. Biol. Trace Elem. Res. 2017, 176, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.J. Zinc in the mouth, its interactions with dental enamel and possible effects on caries; a review of the literature. Int. Dent. J. 2011, 61 (Suppl. 3), 46–54. [Google Scholar] [CrossRef]
- Sejdini, M.; Begzati, A.; Salihu, S.; Krasniqi, S.; Berisha, N.; Aliu, N. The role and impact of salivary Zn levels on dental caries. Int. J. Dent. 2018, 2018, 8137915. [Google Scholar] [CrossRef] [PubMed]
- Devi, C.B.; Nandakishore, T.; Sangeeta, N.; Basar, G.; Devi, N.O.; Jamir, S.; Singh, M.A. Zinc in human health. IOSR J. Dent. Med Sci. (IOSR-JDMS) 2014, 13, 18–23. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Kowitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Hossain, A.; Pin, C.H.; Yahya, N.A. Zinc and metallothionein in the development and progression of dental caries. Biol. Trace Elem. Res. 2019, 187, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Shams, B.; Afshari, E.; Tajadini, M.; Keikha, M.; Qorbani, M.; Heshmat, R.; Motlagh, M.E.; Kelishadi, R. The relationship of serum vitamin D and Zinc in a nationally representative sample of Iranian children and adolescents: The CASPIAN-III study. Med. J. Islamic Repub. Iran 2016, 30, 430. [Google Scholar]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The metal of life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, J.; Joshi, M.; Giri, P. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
- Zofkova, I.; Davis, M.; Blahos, J. Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiol. Res. 2017, 66, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Alker, W.; Haase, H. Zinc and sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Pawlitzki, M.; Uebelhor, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower serum zinc levels in patients with multiple sclerosis compared to healthy controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef]
- Fatima, T.; Rahim, Z.B.; Lin, C.W.; Qamar, Z. Zinc: A precious trace element for oral health care? JPMA J. Pak. Med. Assoc. 2016, 66, 1019–1023. [Google Scholar]
- Rosing, C.K.; Cavagni, J.; Gaio, E.J.; Muniz, F.; Ranzan, N.; Oballe, H.J.R.; Friedrich, S.A.; Severo, R.M.; Stewart, B.; Zhang, Y.P. Efficacy of two mouthwashes with cetylpyridinium chloride: A controlled randomized clinical trial. Braz. Oral Res. 2017, 31, e47. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Hu, D.Y.; Mateo, L.R.; Morrison, B.M., Jr.; Delgado, E.; Zhang, Y.P. Control of established gingivitis and dental plaque using a 1450 ppm fluoride/zinc-based dentifrice: A randomized clinical study. J. Clin. Dent. 2015, 26, 104–108. [Google Scholar]
- Seyedmajidi, S.A.; Seyedmajidi, M.; Moghadamnia, A.; Khani, Z.; Zahedpasha, S.; Jenabian, N.; Jorsaraei, G.; Halalkhor, S.; Motallebnejad, M. Effect of zinc-deficient diet on oral tissues and periodontal indices in rats. Int. J. Mol. Cell. Med. 2014, 3, 81–87. [Google Scholar]
- Goto, T.; Shirakawa, H.; Furukawa, Y.; Komai, M. Decreased expression of carbonic anhydrase isozyme II, rather than of isozyme VI, in submandibular glands in long-term zinc-deficient rats. Br. J. Nutr. 2008, 99, 248–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komai, M.; Goto, T.; Suzuki, H.; Takeda, T.; Furukawa, Y. Zinc deficiency and taste dysfunction; contribution of carbonic anhydrase, a zinc-metalloenzyme, to normal taste sensation. BioFactors (Oxf. Engl.) 2000, 12, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ozler, G.S. Zinc deficiency in patients with recurrent aphthous stomatitis: A pilot study. J. Laryngol. Otol. 2014, 128, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Orbak, R.; Kara, C.; Ozbek, E.; Tezel, A.; Demir, T. Effects of zinc deficiency on oral and periodontal diseases in rats. J. Periodontal Res. 2007, 42, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Shokrizadeh, M.; Sajadi, F. Dental considerations in acrodermatitis enteropathica: A report of two cases. J. Oral Health Oral Epidemiol. 2019, 8, 104–108. [Google Scholar] [CrossRef]
- Mehdipour, M.; Taghavi Zenooz, A.; Sohrabi, A.; Gholizadeh, N.; Bahramian, A.; Jamali, Z. A comparison of the effect of triamcinolone ointment and mouthwash with or without zinc on the healing process of aphthous stomatitis lesions. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 87–91. [Google Scholar] [CrossRef]
- Yildirimyan, N.; Ozalp, O.; Satir, S.; Altay, M.A.; Sindel, A. Recurrent aphthous stomatitis as a result of zinc deficiency. Oral Health Prev. Dent. 2019, 17, 465–468. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Najim, R.A.; Al-Hayani, R.K.; Al-Nuaimy, A.A.; Maroof, D.M. The therapeutic and prophylactic role of oral zinc sulfate in management of recurrent aphthous stomatitis (ras) in comparison with dapsone. Saudi Med. J. 2008, 29, 734–738. [Google Scholar]
- Bao, Z.X.; Yang, X.W.; Shi, J.; Liu, L.X. Serum zinc levels in 368 patients with oral mucosal diseases: A preliminary study. Med. Oral Patol. Oral y Cir. Bucal 2016, 21, e335–e340. [Google Scholar] [CrossRef]
- Uckardes, Y.; Tekcicek, M.; Ozmert, E.N.; Yurdakok, K. The effect of systemic zinc supplementation on oral health in low socioeconomic level children. Turk. J. Pediatr. 2009, 51, 424–428. [Google Scholar]
- Hong, J.Y.; Bae, W.J.; Yi, J.K.; Kim, G.T.; Kim, E.C. Anti-inflammatory and anti-osteoclastogenic effects of zinc finger protein A20 overexpression in human periodontal ligament cells. J. Periodontal Res. 2016, 51, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, L.; Misra, N.; Deepak, U.; Shiv Kumar, G.C. Estimation of serum zinc, copper, and iron in the patients of oral submucous fibrosis. Natl. J. Maxillofac. Surg. 2015, 6, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Ayinampudi, B.K.; Narsimhan, M. Salivary copper and zinc levels in oral pre-malignant and malignant lesions. J. Oral Maxillofac. Pathol. JOMFP 2012, 16, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Rathi, P.; Harkare, V. Trace elements as a diagnostic biomarker for premalignant lesions and malignant conditions. SRM J. Res. Dent. Sci. 2019, 10, 40–46. [Google Scholar] [CrossRef]
- Sachdev, P.K.; Freeland-Graves, J.; Beretvas, S.N.; Sanjeevi, N. Zinc, copper, and iron in oral submucous fibrosis: A meta-analysis. Int. J. Dent. 2018, 2018, 3472087. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Hosthor, S.S.; Mahesh, P.; Priya, S.A.; Sharada, P.; Jyotsna, M.; Chitra, S. Quantitative analysis of serum levels of trace elements in patients with oral submucous fibrosis and oral squamous cell carcinoma: A randomized cross-sectional study. J. Oral Maxillofac. Pathol. JOMFP 2014, 18, 46–51. [Google Scholar] [CrossRef]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Tang, C.H.; Huang, S.H.; Tsai, M.H.; Chen, S.Y.; Kao, J.Y.; Lin, C.W. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin. Chim. Acta Int. J. Clin. Chem. 2011, 412, 1357–1365. [Google Scholar] [CrossRef]
- Ciapparelli, L.; Retief, D.H.; Fatti, L.P. The effect of zinc on 9, 10-dimethyl-1, 2-benzanthracene (DMBA) induced salivary gland tumours in the albino rat--a preliminary study. S. Afr. J. Med Sci. 1972, 37, 85–90. [Google Scholar]
- Martinez, J.M.; Pereira, D.; Chacim, S.; Mesquita, E.; Sousa, I.; Martins, A.; Azevedo, T.; Mariz, J.M. Mucositis care in acute leukemia and non-Hodgkin lymphoma patients undergoing high-dose chemotherapy. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer 2014, 22, 2563–2569. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.L.; Pi, Y.P.; Chen, H.; Chen, W.Q. Oral zinc sulfate for prevention and treatment of chemotherapy-induced oral mucositis: a meta-analysis of five randomized controlled trials. Front. Oncol. 2018, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Mehdipour, M.; Chavoshi, S.H.; Kahani, S.; Sadrzadeh-Afshar, M. The effect of orally-administered zinc in the prevention of chemotherapy-induced oral mucositis in patients with acute myeloid leukemia. Int. J. Cancer Manag. 2017, 10, e9252. [Google Scholar] [CrossRef]
- Shuai, T.; Tian, X.; Shi, B.; Chen, H.; Liu, X.L.; Yi, L.J.; Chen, W.Q.; Li, X.E. Prophylaxis with oral zinc sulfate against radiation induced oral mucositis in patients with head and neck cancers: A systematic review and meta-analysis of four randomized controlled trials. Front. Oncol. 2019, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Hadjibabaie, M.; Iravani, M.; Shamshiri, A.R.; Hayatshahi, A.; Javadi, M.R.; Khoee, S.H.; Alimoghaddam, K.; Ghavamzadeh, A. The effect of zinc sulfate in the prevention of high-dose chemotherapy-induced mucositis: A double-blind, randomized, placebo-controlled study. Hematol. Oncol. 2012, 30, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Pasyar, N.; Ramzi, M. The effect of zinc sulfate on prevention, incidence, and severity of mucositis in leukemia patients undergoing chemotherapy. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2018, 33, 14–21. [Google Scholar] [CrossRef]
- Arbabi-kalati, F.; Arbabi-kalati, F.; Deghatipour, M.; Ansari Moghadam, A. Evaluation of the efficacy of zinc sulfate in the prevention of chemotherapy-induced mucositis: A double-blind randomized clinical trial. Arch. Iran. Med. 2012, 15, 413–417. [Google Scholar]
- De Meneses, A.G.; Normando, A.G.C.; Porto de Toledo, I.; Reis, P.E.D.; Guerra, E.N.S. Effects of oral supplementation in the management of oral mucositis in cancer patients: A meta-analysis of randomized clinical trials. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2019, 49, 117–125. [Google Scholar] [CrossRef]
- Hozyasz, K.K.; Kaczmarczyk, M.; Dudzik, J.; Bulska, E.; Dudkiewicz, Z.; Szymanski, M. Relation between the concentration of zinc in maternal whole blood and the risk of an infant being born with an orofacial cleft. Br. J. Oral Maxillofac. Surg. 2009, 47, 466–469. [Google Scholar] [CrossRef]
- Hozyasz, K.K.; Ruszczynska, A.; Bulska, E. Low zinc and high copper levels in mothers of children with isolated cleft lip and palate. Wiad. Lek. (Wars. Pol. 1960) 2005, 58, 382–385. [Google Scholar]
- Tamura, T.; Munger, R.G.; Corcoran, C.; Bacayao, J.Y.; Nepomuceno, B.; Solon, F. Plasma zinc concentrations of mothers and the risk of nonsyndromic oral clefts in their children: A case-control study in the Philippines. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 612–616. [Google Scholar] [CrossRef]
- Jara-Palacios, M.A.; Cornejo, A.C.; Narvaez-Caicedo, C.; Moreano, G.; Vasquez, K.P.; Moreno-Izquierdo, C.; Romero-Sandoval, N. Plasma zinc levels in Ecuadorian mothers of infants with nonsyndromic cleft lip with or without cleft palate: A case series. Birth Defects Res. 2018, 110, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, W.; Yu, J.; Li, Z.; Jin, L.; Liu, J.; Zhang, Y.; Wang, L.; Ren, A. Association between selected essential trace element concentrations in umbilical cord and risk for cleft lip with or without cleft palate: A case-control study. Sci. Total Environ. 2019, 661, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kostecka-Sochon, P.; Onopiuk, B.M.; Dabrowska, E. Protective effect of increased zinc supply against oxidative damage of sublingual gland in chronic exposure to cadmium: Experimental study on rats. Oxidative Med. Cell. Longev. 2018, 2018, 3732842. [Google Scholar] [CrossRef] [PubMed]
- Mizari, N.; Hirbod-Mobarakeh, A.; Shahinpour, S.; Ghalichi-Tabriz, M.; Beigy, M.; Yamini, A.; Dehpour, A.R. Effect of subchronic zinc toxicity on rat salivary glands and serum composition. Toxicol. Ind. Health 2012, 28, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Marten, S.M.; Walther, W.; Vital, M.; Pieper, D.H.; Dotsch, A. Impact of dental cement on the peri-implant biofilm-microbial comparison of two different cements in an in vivo observational study. Clin. Implant. Dent. Relat. Res. 2018, 20, 806–813. [Google Scholar] [CrossRef]
- Quaranta, A.; Lim, Z.W.; Tang, J.; Perrotti, V.; Leichter, J. The impact of residual subgingival cement on biological complications around dental implants: A systematic review. Implant Dent. 2017, 26, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Zahra, S.F.; Razdan, R. Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J. Biomater. Sci. Polym. Ed. 2018, 29, 74–91. [Google Scholar] [CrossRef]
- Kussovski, V.; Mantareva, V.; Durmus, M.; Angelov, I. Quaternized Zn(II) phthalocyanines for photodynamic strategy against resistant periodontal bacteria. Z. Fur Nat. C J. Biosci. 2018, 73, 221–228. [Google Scholar] [CrossRef]
- Lauritano, D.; Candotto, V.; Bignozzi, C.A.; Pazzi, D.; Carinci, F.; Cura, F.; Tagliabue, A.; Tettamanti, L. Zinc plus octenidine: A new formulation for treating periodontal pathogens. A single blind study. J. Biol. Regul. Homeost. Agents 2018, 32, 231–236. [Google Scholar]
- Mou, J.; Liu, Z.; Liu, J.; Lu, J.; Zhu, W.; Pei, D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: Preparation, characterization and evaluation. Drug Deliv. 2019, 26, 179–187. [Google Scholar] [CrossRef]
- Ke, M.R.; Eastel, J.M.; Ngai, K.L.; Cheung, Y.Y.; Chan, P.K.; Hui, M.; Ng, D.K.; Lo, P.C. Photodynamic inactivation of bacteria and viruses using two monosubstituted zinc(II) phthalocyanines. Eur. J. Med. Chem. 2014, 84, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. (Bethesda, Md.) 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Remichkova, M.; Mukova, L.; Nikolaeva-Glomb, L.; Nikolova, N.; Doumanova, L.; Mantareva, V.; Angelov, I.; Kussovski, V.; Galabov, A.S. Virus inactivation under the photodynamic effect of phthalocyanine Zinc(II) complexes. Z. Fur Nat. C J. Biosci. 2017, 72, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Guan, G. A case series: Zinc deficiency as a potential contributor to oral dysgeusia. Mod. Approaches Dent. Oral Health Care 2018, 2. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.F.; Zhu, W.Q.; Cheng, K.; Chen, Q.X.; Xu, Y.; Pang, Y.; Liu, G.J.; Ge, J.B. Elevated glycated hemoglobin levels may increase the risk of atrial fibrillation in patients with diabetes mellitus. Int. J. Clin. Exp. Med. 2015, 8, 3271–3280. [Google Scholar]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Ciubotariu, D.; Ghiciuc, C.M.; Lupusoru, C.E. Zinc involvement in opioid addiction and analgesia--should zinc supplementation be recommended for opioid-treated persons? Subst. Abus. Treat. Prev. Policy 2015, 10, 29. [Google Scholar] [CrossRef]
- Richards, C.D.; Burke, R. Local and systemic effects of targeted zinc redistribution in Drosophila neuronal and gastrointestinal tissues. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2015, 28, 967–974. [Google Scholar] [CrossRef]
- Goodson, J.M.; Shi, P.; Razzaque, M.S. Dietary phosphorus enhances inflammatory response: A study of human gingivitis. J. Steroid Biochem. Mol. Biol. 2019, 188, 166–171. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Rahman, S.; Ojeh, N.; Grant, W.B.; Haq, A.; Razzaque, M.S. Oral manifestations of magnesium and vitamin D inadequacy. J. Steroid Biochem. Mol. Biol. 2020, 194, 105400. [Google Scholar] [CrossRef]
- Erem, S.; Razzaque, M.S. Dietary phosphate toxicity: An emerging global health concern. Histochem. Cell Biol. 2018, 150, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Can excessive dietary phosphate intake influence oral diseases? Adv. Hum. Biol. 2020. [Google Scholar] [CrossRef]
- Goodson, J.M.; Shi, P.; Mumena, C.H.; Haq, A.; Razzaque, M.S. Dietary phosphorus burden increases cariogenesis independent of vitamin D uptake. J. Steroid Biochem. Mol. Biol. 2017, 167, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Murererehe, J.; Ineza, M.C.; Harelimana, E.I.; Nsabimana, U.; Uwambaye, P.; Gatarayiha, A.; Haq, A.; Razzaque, M.S. Effects of vitamin D status on oral health. J. Steroid Biochem. Mol. Biol. 2018, 175, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef]
| Categories of Foods | Types of Foods (per 100 gm) | Milligrams (mg) per Serving |
|---|---|---|
| Cereals and Whole Grains | Brown/black rice | 2.2–5.9 |
| Black sesame | 7.7 | |
| Rye | 5.0 | |
| Oats | 4.2 | |
| Quinoa | 1.0 | |
| Meat and Poultry | Chicken | 1.6 |
| Lamb | 4.6 | |
| Liver | 4.0 | |
| Beef | 8.4 | |
| Seafood | Oysters | 78.6 |
| Crab | 5.4 | |
| Lobster | 3.5 | |
| Fruits | Dates | 0.4 |
| Pomegranates | 0.3 | |
| Berries | 0.6 | |
| Avocadoes | 0.6 | |
| Vegetables | Soy foods | 0.9–4.8 |
| Mushrooms | 1.0 | |
| Cabbage | 0.2 | |
| Spinach | 0.7 | |
| Broccoli | 0.4 | |
| Garlic | 1.1 | |
| Milk and Dairy | Whole milk | 0.3 |
| Yogurt | 0.5 | |
| Cheese | 3.2 | |
| Legumes | Peas | 0.8 |
| Lentils | 4.7 | |
| Beans | 0.2–5.4 | |
| Peanuts | 2.1 | |
| Chickpeas | 3.4 | |
| Edamame | 1.3 | |
| Nuts and Seeds | Nuts/Cashews | 5.7 |
| Sunflower seeds | 5.3 | |
| Almonds | 3.0 | |
| Pumpkin seeds | 7.6–10.3 | |
| Pecans | 4.5 | |
| Chia seeds | 4.5 |
| Age | Zinc Recommended Daily Intakes in mg/day | |
|---|---|---|
| Males | 9–13 years | 8 |
| 14–18 years | 11 | |
| 19–30 years | 11 | |
| 31–50 years | 11 | |
| 51–70 years | 11 | |
| >70 years | 11 | |
| Females | 9–13 years | 8 |
| 14–18 years | 9 | |
| 19–30 years | 8 | |
| 31–50 years | 8 | |
| 51–70 years | 8 | |
| >70 years | 8 | |
| Females - Pregnant | < 18 years | 13 |
| 19–30 years | 11 | |
| 31–50 years | 11 | |
| Females - Lactating | < 18 years | 13 |
| 19–30 years | 11 | |
| 31–50 years | 11 | |
| Children | 1–3 years | 3 |
| 4–8 years | 5 | |
| Infants | 0–6 months | 2 |
| 7–12 months | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. https://doi.org/10.3390/nu12040949
Uwitonze AM, Ojeh N, Murererehe J, Atfi A, Razzaque MS. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients. 2020; 12(4):949. https://doi.org/10.3390/nu12040949
Chicago/Turabian StyleUwitonze, Anne Marie, Nkemcho Ojeh, Julienne Murererehe, Azeddine Atfi, and Mohammed S. Razzaque. 2020. "Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health" Nutrients 12, no. 4: 949. https://doi.org/10.3390/nu12040949
APA StyleUwitonze, A. M., Ojeh, N., Murererehe, J., Atfi, A., & Razzaque, M. S. (2020). Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients, 12(4), 949. https://doi.org/10.3390/nu12040949






