Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing and Characteristics of Passiflora setacea Juice
2.2. Subjects and Study Design
2.3. Blood Sampling and Treatment
2.4. Biochemical Parameters and Cytokines Analysis
2.5. Total RNA Extraction
2.6. Microarray Analyses and Bioinformatic Analysis
2.7. Bioinformatic Analyzes
2.8. Statistical Analyses
3. Results
3.1. Volunteers’ Baseline Characteristics
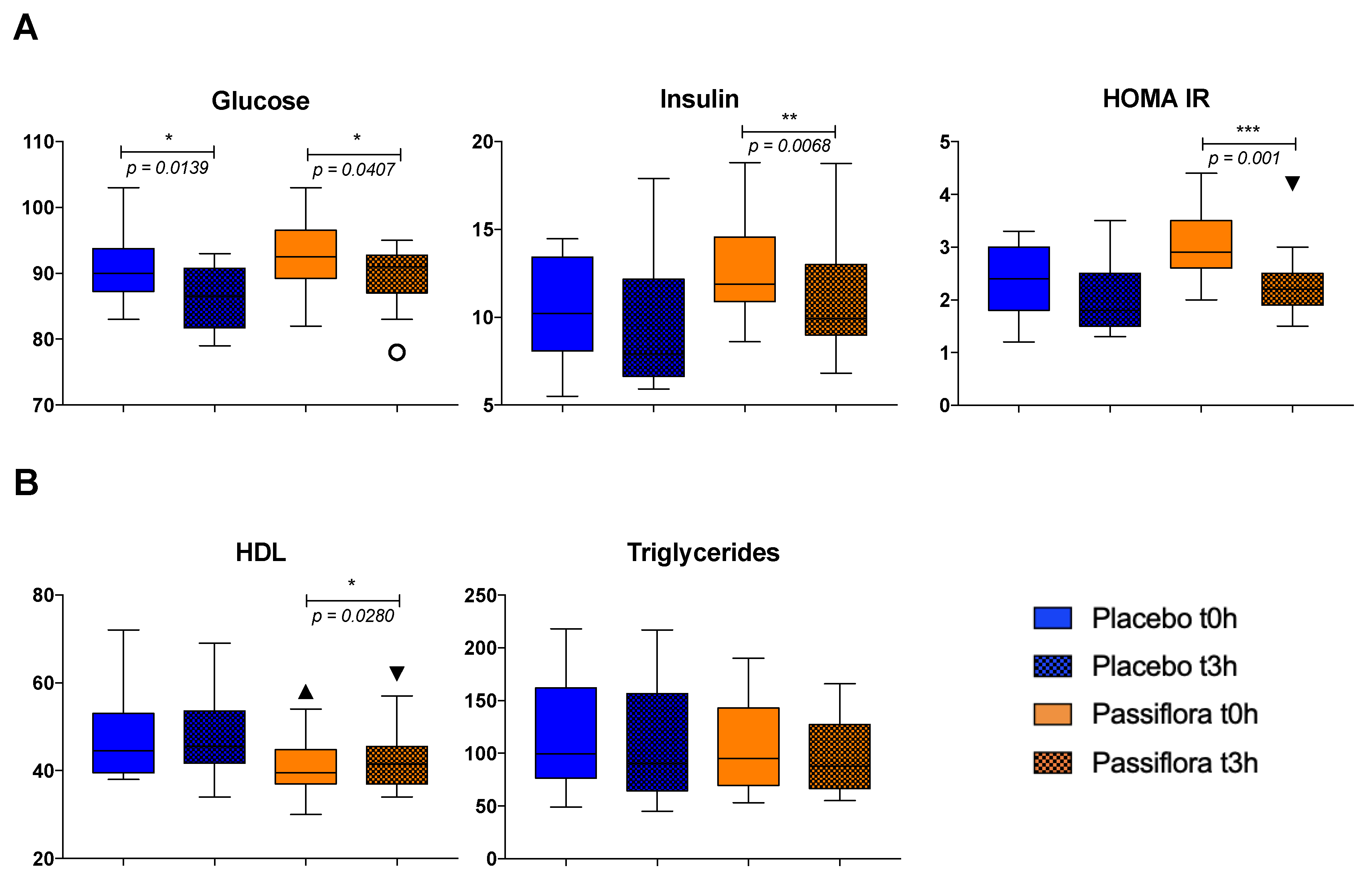
3.2. Effect of Passiflora setacea Juice on Glucose and Lipid Metabolism (Phase 1)
3.3. Effect of Passiflora setacea Juice Intake on Cytokine Serum Levels (Phase 1)
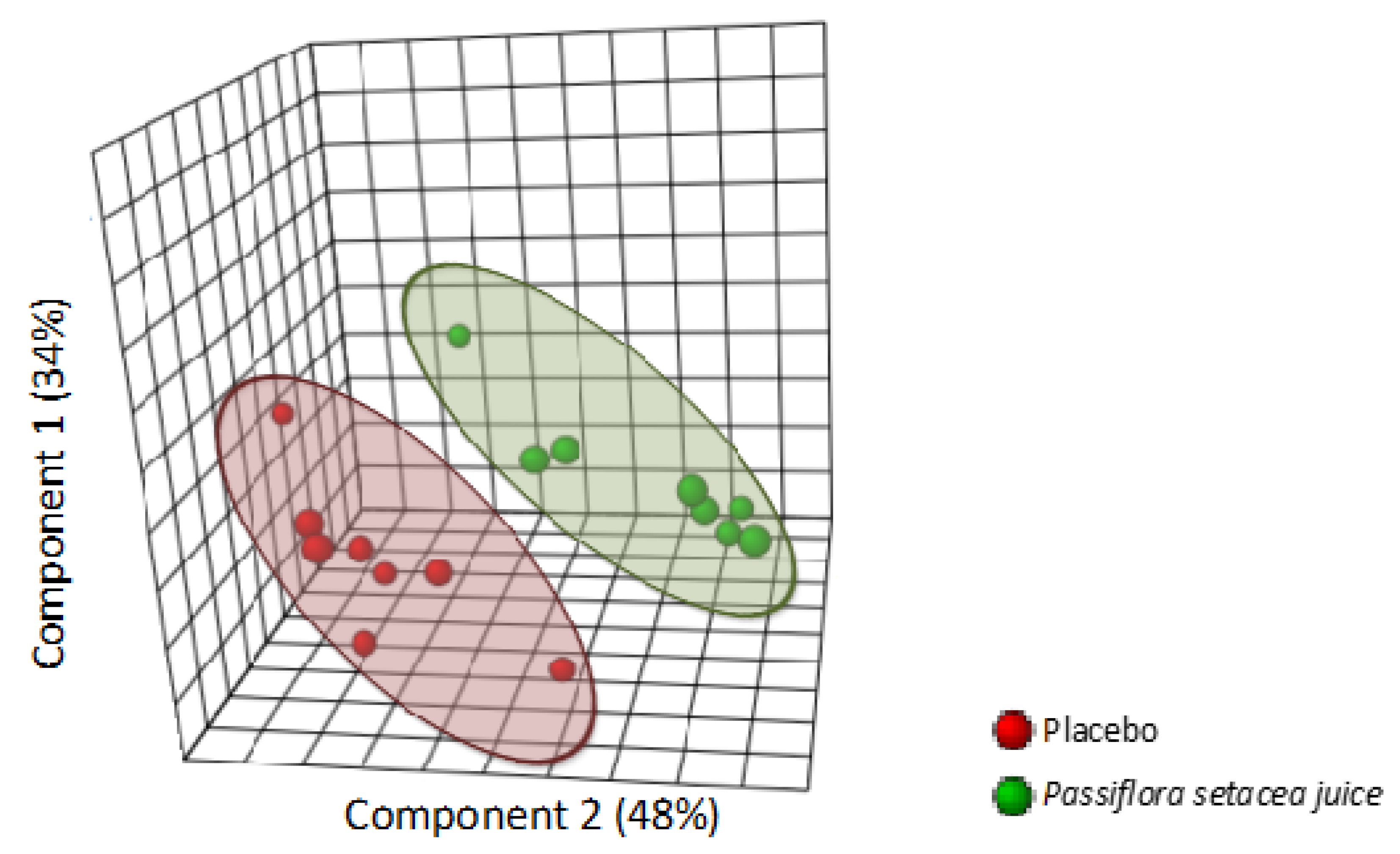
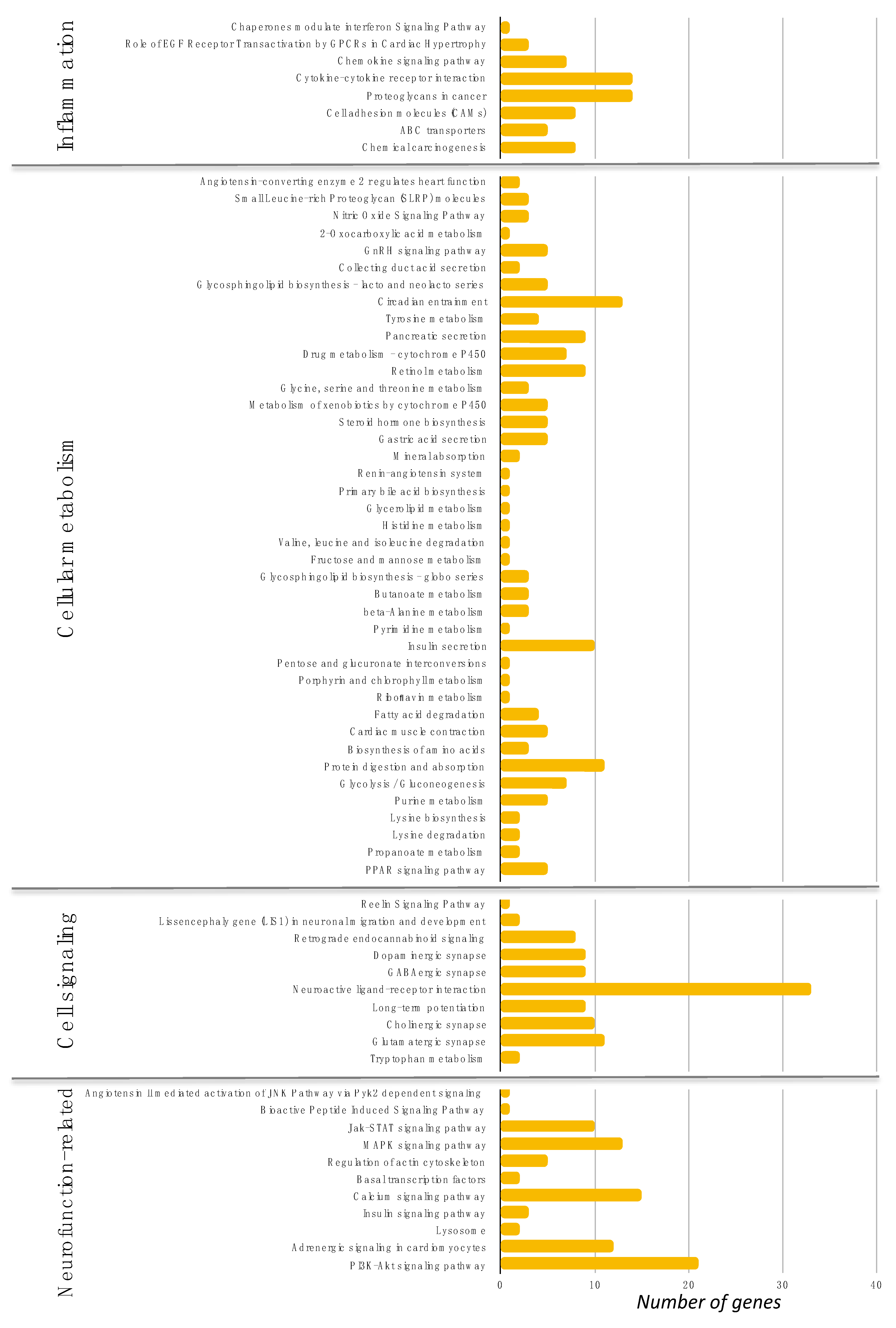
3.4. Passiflora setacea Modulated Gene Expression in Circulating Cells (Phase 2)
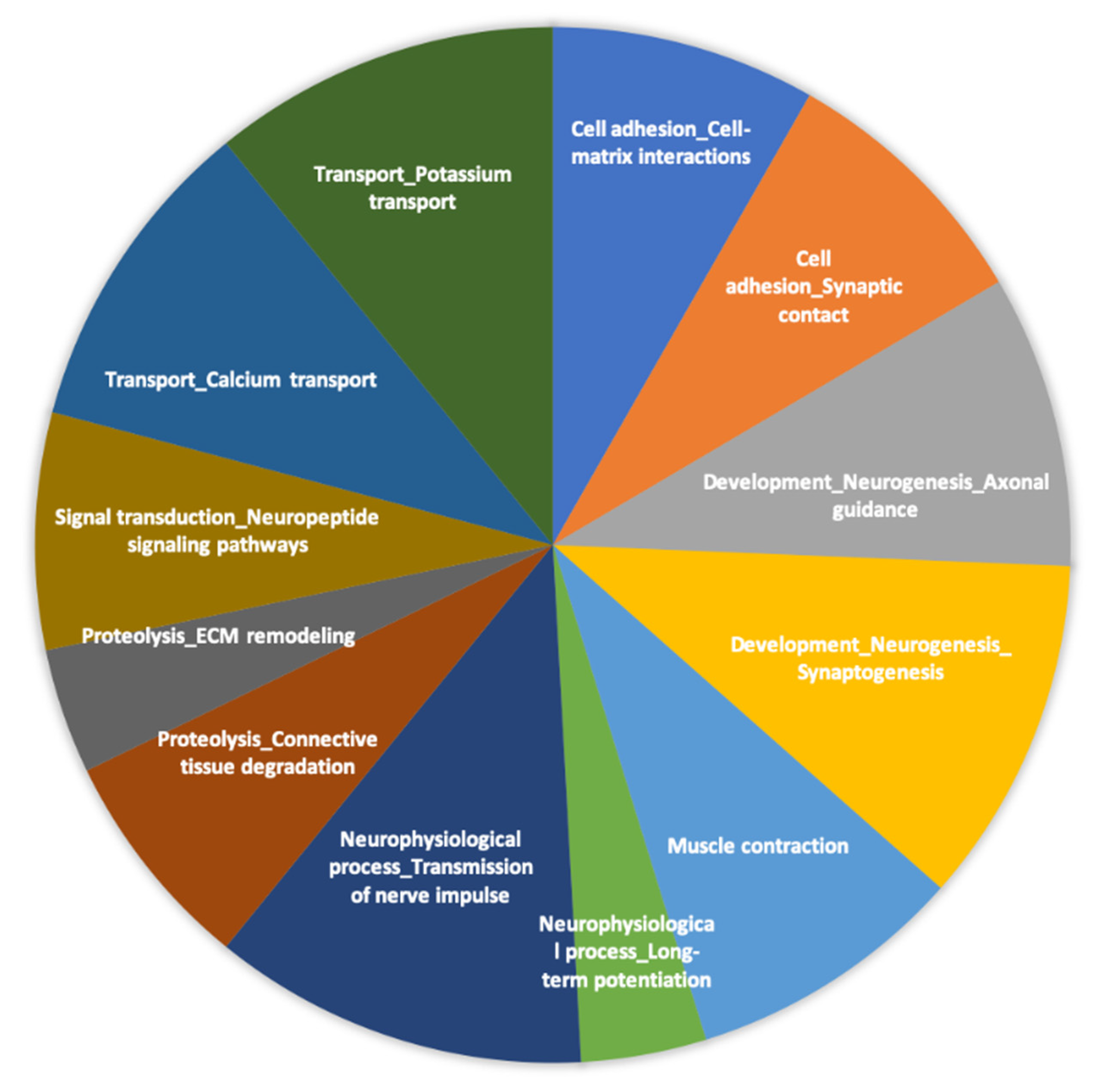
3.5. Protein–Protein Interaction (Phase 2)
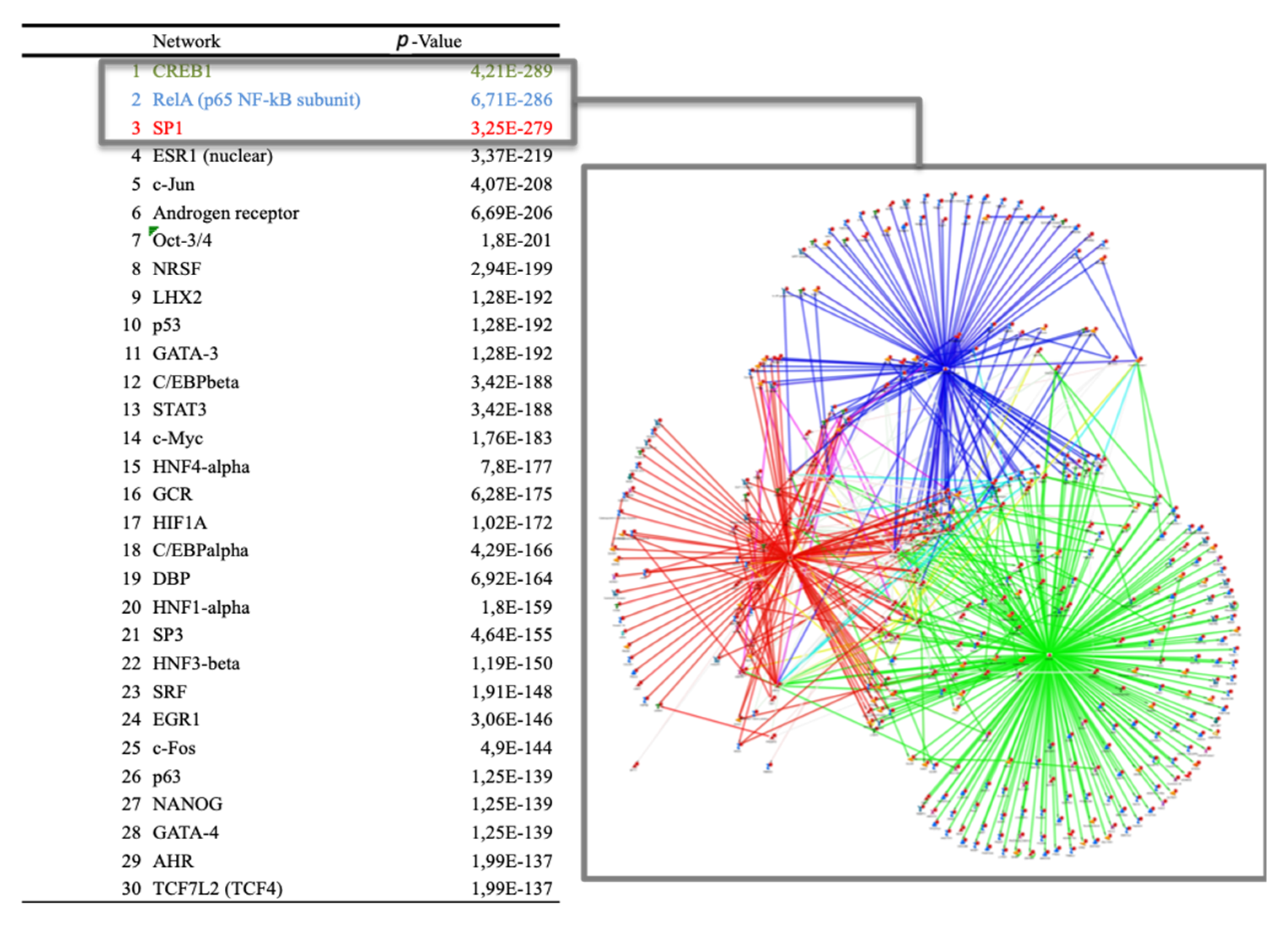
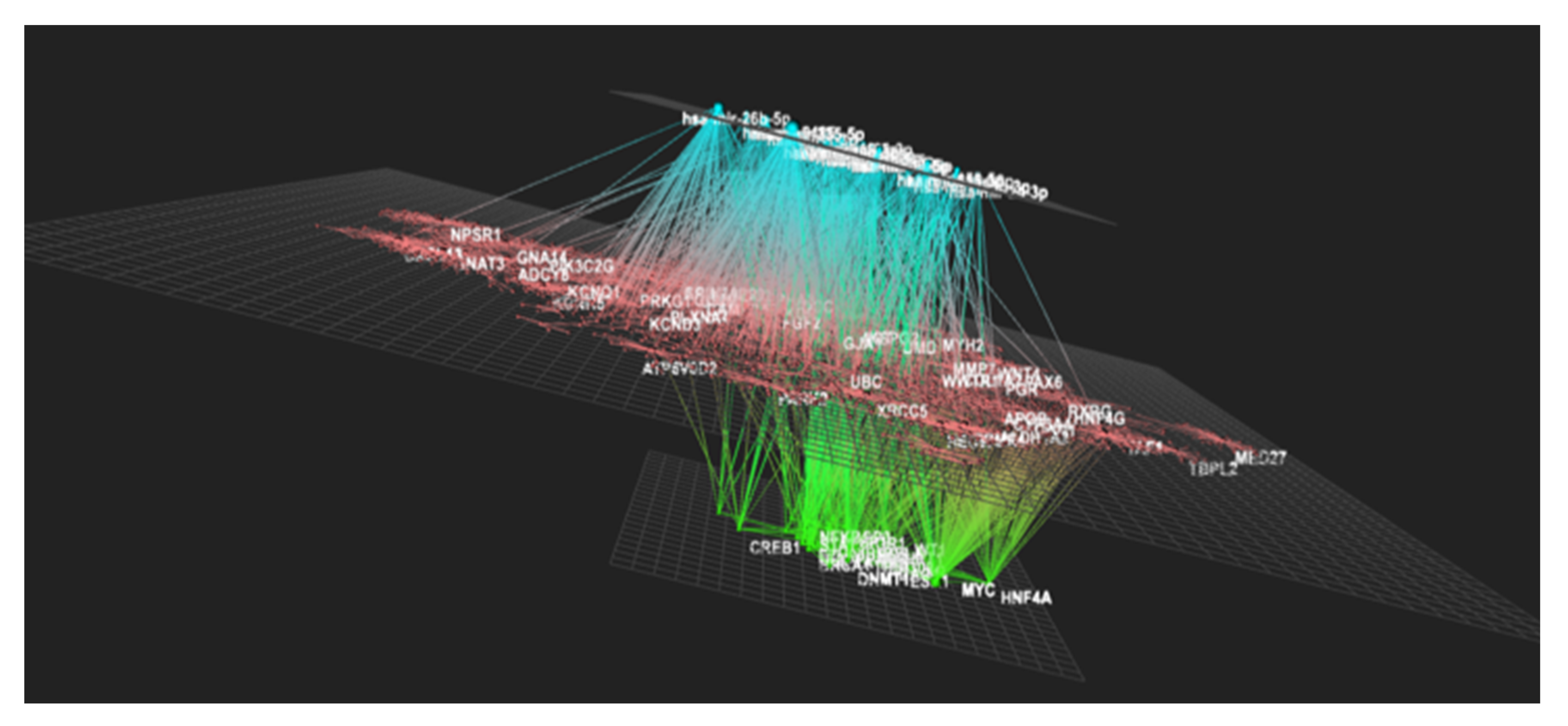
3.6. Transcriptional and Post-Transcriptional Regulators of the Nutrigenomic Effect (Phase 2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO, W.H.O. Noncommunicable Diseases. Available online: http://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 16 September 2018).
- World Health Organization. Depression and Other Common Mental Disorders: Glocal Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Melaku, Y.A.; Renzaho, A.; Gill, T.K.; Taylor, A.W.; Dal Grande, E.; de Courten, B.; Baye, E.; Gonzalez-Chica, D.; Hyppönen, E.; Shi, Z.; et al. Burden and trend of diet-related non-communicable diseases in Australia and comparison with 34 OECD countries, 1990–2015: Findings from the Global Burden of Disease Study 2015. Eur. J. Nutr. 2018, 58, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, C.M.; Dhana, K.; Schoufour, J.D.; Ikram, M.A.; Kavousi, M.; Franco, O.H. Impact of physical activity on the association of overweight and obesity with cardiovascular disease: The Rotterdam Study. Eur. J. Prev. Cardiol. 2017, 24, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Carneiro, F.S.; Matsumoto, T.; Tostes, R.C. Bonus Effects of Antidiabetic Drugs: Possible Beneficial Effects on Endothelial Dysfunction, Vascular Inflammation and Atherosclerosis. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Raguzzini, A.; V Villano, D.; Cesqui, E.; Toti, E.; Catasta, G.; Serafini, M. High Fat Meal Increase of IL-17 is Prevented by Ingestion of Fruit Juice Drink in Healthy Overweight Subjects. Curr. Pharm. Des. 2012, 18, 85–90. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Dubray, C.; Mazur, A.; Morand, C. Hesperidin Displays Relevant Role in the Nutrigenomic Effect of Orange Juice on Blood Leukocytes in Human Volunteers: A Randomized Controlled Cross-Over Study. PLoS ONE 2011, 6, e26669. [Google Scholar] [CrossRef]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Denny, A.; Buttriss, J. Plant. Foods and Health: Focus on Plant Bioactives: Synthesis Report no. 4; European Food Information Resource Consortium (EuroFIR): Norwich, UK, 2007; ISBN 978-0-907667-62-9. [Google Scholar]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Turati, F.; Rossi, M.; Pelucchi, C.; Levi, F.; La Vecchia, C. Fruit and vegetables and cancer risk: A review of southern European studies. Br. J. Nutr. 2015, 113, S102–S110. [Google Scholar] [CrossRef]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.-A.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial Variability Across Individuals in the Vascular and Nutrigenomic Response to an Acute Intake of Curcumin: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2018, 62, 1700418. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Rose, T.; Fiebich, B.; Kammler, T.; Hoffmann, C.; Weiss, G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother. Res. 2011, 25, 838–843. [Google Scholar] [CrossRef]
- Bomtempo, L.L.; Costa, A.M.; Lima, H.; Engeseth, N.; Gloria, M.B.A. Bioactive amines in Passiflora are affected by species and fruit development. Food Res. Int. 2016, 89, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- De Carvalho, M.V.O.; de Oliveira, L.; Costa, A.M. Effect of training system and climate conditions on phytochemicals of Passiflora setacea, a wild Passiflora from Brazilian savannah. Food Chem. 2018, 266, 350–358. [Google Scholar] [CrossRef]
- De Santana, F.C.; Shinagawa, F.B.; Araujo, E.; Costa, A.M.; Mancini-Filho, J. Chemical Composition and Antioxidant Capacity of Brazilian Passiflora Seed Oils. J. Food Sci. 2015, 80, C2647–C2654. [Google Scholar] [CrossRef]
- Santana, F.C. de Evaluation of bioactive compounds present in Passiflora spp. seed and its influence on oxidative stress and inflammation in a high fat-fed mice. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Wang, C.; Xu, F.-Q.; Shang, J.-H.; Xiao, H.; Fan, W.-W.; Dong, F.-W.; Hu, J.-M.; Zhou, J. Cycloartane triterpenoid saponins from water soluble of Passiflora edulis Sims and their antidepressant-like effects. J. Ethnopharmacol. 2013, 148, 812–817. [Google Scholar] [CrossRef]
- Ku, S.-K.; Kwak, S.; Bae, J.-S. Orientin Inhibits High Glucose-Induced Vascular Inflammation In Vitro and In Vivo. Inflammation 2014, 37, 2164–2173. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vasc. Pharmacol. 2014, 62, 3–14. [Google Scholar] [CrossRef]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.; Kim, M. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Soulimani, R.; Younos, C.; Jarmouni, S.; Bousta, D.; Misslin, R.; Mortier, F. Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J. Ethnopharmacol. 1997, 57, 11–20. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.M.; Wirth, M.D.; Shivappa, N.; Wingard, E.E.; Fayad, R.; Wilcox, S.; Frongillo, E.A.; Hébert, J.R. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutr. Res. 2015, 35, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef]
- Da Rocha, P.E.C.P. Medidas e Avaliação em Ciências do Esporte, 4th ed.; Sprint: Rio de Janeiro, Brazil, 2000; ISBN 85-85031-87-5. [Google Scholar]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Zapata-Bustos, R.; Gómez-Espinoza, G.; Salazar-Olivo, L.A. Isoorientin Reverts TNF-α-Induced Insulin Resistance in Adipocytes Activating the Insulin Signaling Pathway. Endocrinology 2012, 153, 5222–5230. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 1–15. [Google Scholar] [CrossRef]
- Sun, L.; Chen, W.; Meng, Y.; Yang, X.; Yuan, L.; Guo, Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem. 2016, 208, 51–60. [Google Scholar] [CrossRef]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (−)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-K.; Cho, S.-J.; Jung, U.; Ryu, R.; Choi, M.-S. Phlorizin Supplementation Attenuates Obesity, Inflammation, and Hyperglycemia in Diet-Induced Obese Mice Fed a High-Fat Diet. Nutrients 2016, 8, 92. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Williamson, G. Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: A randomised, controlled, single-blind, cross-over intervention. Br. J. Nutr. 2016, 116, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Lima-Cabello, E.; Biel-Glesson, S.; Fernandez-Navarro, J.; Calleja, M.; Roca, M.; Espejo-Calvo, J.; Gil-Extremera, B.; Soria-Florido, M.; de la Torre, R.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Metabolic Syndrome and Endothelial Functional Risk Biomarkers in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2018, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, A.; Souza, S.; Teixeira, A.; Pontilho, P.; Souza, J.; Luzia, L.; Rondó, P. Effect of Chocolate and Yerba Mate Phenolic Compounds on Inflammatory and Oxidative Biomarkers in HIV/AIDS Individuals. Nutrients 2016, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive Oil Polyphenols Enhance High-Density Lipoprotein Function in Humans: A Randomized Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.V.; Di Battista, J.A.; Martel-Pelletier, J.; Jolicoeur, F.C.; He, Y.; Zhang, M.; Mineau, F.; Pelletier, J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998, 160, 3513–3521. [Google Scholar]
- Kharwar, N.K.; Prasad, K.N.; Singh, K.; Paliwal, V.K.; Modi, D.R. Polymorphisms of IL-17 and ICAM-1 and their expression in Guillain–Barré syndrome. Int. J. Neurosci. 2017, 127, 680–687. [Google Scholar] [CrossRef]
- Bosteen, M.H.; Tritsaris, K.; Hansen, A.J.; Dissing, S. IL-17A potentiates TNFα-induced secretion from human endothelial cells and alters barrier functions controlling neutrophils rights of passage. Pflügers Arch. Eur. J. Physiol. 2014, 466, 961–972. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, L.; Fukuda, K.; Ouyang, S.; Chen, X.; Wang, C.; Zhang, C.; Martin, B.; Gu, C.; Qin, L.; et al. The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo. Sci. Signal. 2017, 10, eaaf8823. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimizu, N.; Shimoda, H. Passionflower Extract Induces High-amplitude Rhythms without Phase Shifts in the Expression of Several Circadian Clock Genes in Vitro and in Vivo. Int. J. Biomed. Sci. 2017, 13, 84–92. [Google Scholar] [PubMed]
- Kubo, T.; Fujino, Y.; Nakamura, T.; Kunimoto, M.; Tabata, H.; Tsuchiya, T.; Kadowaki, K.; Odoi, H.; Oyama, I.; Matsuda, S. An Industry-Based Cohort Study of the Association between Weight Gain and Hypertension Risk Among Rotating Shift Workers. J. Occup. Environ. Med. 2013, 55, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Oyama, I.; Kubo, T.; Fujino, Y.; Kadowaki, K.; Kunimoto, M.; Shirane, K.; Tabata, H.; Sabanai, K.; Nakamura, T.; Matsuda, S. Retrospective cohort study of the risk of impaired glucose tolerance among shift workers. Scand. J. Work. Health 2012, 38, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Matsumoto, C.; Shibano, M.; Fujimori, K. Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients 2018, 10, 130. [Google Scholar] [CrossRef]
- Weber, C.; Fraemohs, L.; Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 2007, 7, 467–477. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.M.; Gowhari Shabgah, A.; Pishgouyi, S.; Tavakol Afshari, J.; Zeidabadi, H.; Mohammadi, M. The Serum Levels of CCL2 and CCL16 Expression in Patients with Irritable Bowel Syndrome. Rep. Biochem. Mol. Biol. 2019, 8, 9–14. [Google Scholar]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. New Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Fevang, B.; Yndestad, A.; Damås, J.K.; Halvorsen, B.; Holm, A.M.; Beiske, K.; Aukrust, P.; Frøland, S.S. Chemokines and common variable immunodeficiency; possible contribution of CCL19, CCL21 and CCR7 to immune dysregulation. Clin. Exp. Immunol. 2009, 158, 237–245. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Lin, X.; Yan, X.; Xia, Y.; Zhang, L.; Cao, J. IL-36 induces cytokine IL-6 and chemokine CXCL8 expression in human lung tissue cells: Implications for pulmonary inflammatory responses. Cytokine 2017, 99, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871747-8. [Google Scholar]
- Swee, M.; Wilson, C.L.; Wang, Y.; McGuire, J.K.; Parks, W.C. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J. Leukoc. Biol. 2008, 83, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chen, C.; Meng, T.; Zhang, W.; Zhou, Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-β pathway on matrix metalloproteinase 7. Exp. Biol. Med. 2016, 241, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E.N.; Ferrara, N.; Bauer, E.A.; Amento, E.P. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J. Cell. Physiol. 1992, 153, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Xinyue, Z.; Shengyan, X.; Yanhui, W.; Yangxinzi, X.; Galia, P.; Yu, S.; Yao, C.; Guanjie, L.; Pei, L. Efficacy of Ciji Hua’ai Baosheng formula on the expressions of vascular endothelial growth factor, kinase insert domain-containing receptor and basic fibroblast growth factor in mouse models of H 22 hepatocellular carcinoma. J. Tradit. Chin. Med. 2017, 37, 88–95. [Google Scholar] [CrossRef]
- Monfoulet, L.-E.; Mercier, S.; Bayle, D.; Tamaian, R.; Barber-Chamoux, N.; Morand, C.; Milenkovic, D. Curcumin modulates endothelial permeability and monocyte transendothelial migration by affecting endothelial cell dynamics. Free Radic. Biol. Med. 2017, 112, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Giebeler, N.; Zigrino, P. A Disintegrin and Metalloprotease (ADAM): Historical Overview of Their Functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef]
- Hooper, N.M.; Lendeckel, U. (Eds.) The Adam family of proteases; Proteases in biology and disease; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-0-387-25149-3. [Google Scholar]
- Masaki, M.; Kurisaki, T.; Shirakawa, K.; Sehara-Fujisawa, A. Role of Meltrin α (ADAM12) in Obesity Induced by High- Fat Diet. Endocrinology 2005, 146, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.A.; Maksimovic, J.; Wadeson, J.; Fahri, F.T.; Webster, T.; Leyton, C.; McDonagh, M.B.; White, J.D. Knockdown of a disintegrin A metalloprotease 12 (ADAM12) during adipogenesis reduces cell numbers, delays differentiation, and increases lipid accumulation in 3T3-L1 cells. Mol. Biol. Cell 2018, 29, 1839–1855. [Google Scholar] [CrossRef] [PubMed]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Graae, A.-S.; Grarup, N.; Ribel-Madsen, R.; Lystbæk, S.H.; Boesgaard, T.; Staiger, H.; Fritsche, A.; Wellner, N.; Sulek, K.; Kjolby, M.; et al. ADAMTS9 Regulates Skeletal Muscle Insulin Sensitivity Through Extracellular Matrix Alterations. Diabetes 2019, 68, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.J.; Pérusse, L.; Sarzynski, M.A.; Fornage, M.; Sidney, S.; Sternfeld, B.; Rice, T.; Terry, J.G.; Jacobs, D.R.; Katzmarzyk, P.; et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int. J. Obes. 2016, 40, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Sze, J.; Marin, T.; Arevalo, J.M.G.; Doll, R.; Ma, R.; Cole, S.W. A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-κB Signaling. Biol. Psychiatry 2008, 64, 266–272. [Google Scholar] [CrossRef]
- Heyes, S.; Pratt, W.S.; Rees, E.; Dahimene, S.; Ferron, L.; Owen, M.J.; Dolphin, A.C. Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog. Neurobiol. 2015, 134, 36–54. [Google Scholar] [CrossRef]
- McDonald, K.C.; Saunders, K.E.; Geddes, J.R. Sleep problems and suicide associated with mood instability in the Adult Psychiatric Morbidity Survey, 2007. Aust. New Zealand J. Psychiatry 2017, 51, 822–828. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Carnethon, M.R.; Matthews, K.A.; McIntyre, R.S.; Miller, G.E.; Raghuveer, G.; Stoney, C.M.; Wasiak, H.; McCrindle, B.W. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 965–986. [Google Scholar] [CrossRef]
- Mozhui, K.; Karlsson, R.M.; Kash, T.L.; Ihne, J.; Norcross, M.; Patel, S.; Farrell, M.R.; Hill, E.E.; Graybeal, C.; Martin, K.P.; et al. Strain Differences in Stress Responsivity Are Associated with Divergent Amygdala Gene Expression and Glutamate-Mediated Neuronal Excitability. J. Neurosci. 2010, 30, 5357–5367. [Google Scholar] [CrossRef]
- Ryan, J.; Carrière, I.; Ritchie, K.; Ancelin, M.-L. Involvement of GPR50 polymorphisms in depression: independent replication in a prospective elderly cohort. Brain Behav. 2015, 5, e00313. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, D.A.; Sidibe, A.; Saer, B.R.C.; Li, J.; Hand, L.E.; Ivanova, E.A.; Darras, V.M.; Dam, J.; Jockers, R.; Luckman, S.M.; et al. A Role for the Melatonin-Related Receptor GPR50 in Leptin Signaling, Adaptive Thermogenesis, and Torpor. Curr. Biol. 2012, 22, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, R.; Long, W.; Guo, H.; Shi, W.; Yuan, S.; Xu, G.; Zhang, T. CREB1 functional polymorphisms modulating promoter transcriptional activity are associated with type 2 diabetes mellitus risk in Chinese population. Gene 2018, 665, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Samblas, M.; Milagro, F.I.; Riezu-Boj, J.I.; Crujeiras, A.B.; Martinez, J.A.; Project, M. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol. Int. 2018, 35, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Zubenko, G.S.; Hughes, H.B.; Jordan, R.M.; Lyons-Weiler, J.; Cohen, B.M. Differential hippocampal gene expression and pathway analysis in an etiology-based mouse model of major depressive disorder. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2014, 165, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, R.; Qin, X.; Wang, L.; Xiao, J.; Song, Y.; Sheng, X.; Guo, M.; Ji, X. Sp1 Plays an Important Role in Vascular Calcification Both In Vivo and In Vitro. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Wu, C.-C.; Hsu, H.-Y.; Chuang, H.-Y.; Huang, S.-Y.; Tsai, C.-H.; Chang, Y.; Tsao, G.; Chen, C.-L.; Chen, J.-Y. EGCG Inhibits Proliferation, Invasiveness and Tumor Growth by Up-Regulation of Adhesion Molecules, Suppression of Gelatinases Activity, and Induction of Apoptosis in Nasopharyngeal Carcinoma Cells. Int. J. Mol. Sci. 2015, 16, 2530–2558. [Google Scholar] [CrossRef]
- Milenkovic, D.; Jude, B.; Morand, C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Berghe, W.V.; Morand, C.; Claude, S.; van de Sandt, A.; Gorressen, S.; Monfoulet, L.-E.; Chirumamilla, C.S.; Declerck, K.; Szic, K.S.v.; et al. A systems biology network analysis of nutri(epi)genomic changes in endothelial cells exposed to epicatechin metabolites. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Heyn, J.; Ledderose, C.; Hinske, L.C.; Limbeck, E.; Möhnle, P.; Lindner, H.A.; Kreth, S. Adenosine A2A Receptor Upregulation in Human PMNs Is Controlled by miRNA-214, miRNA-15, and miRNA-16. Shock 2012, 37, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Magalhães, F.C.; Roque, F.R.; Phillips, M.I.; Oliveira, E.M. Exercise Training Prevents the Microvascular Rarefaction in Hypertension Balancing Angiogenic and Apoptotic Factors: Role of MicroRNAs-16, -21, and -126. Hypertension 2012, 59, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Z.-M.; Guo, X.-M.; Su, D.-F.; Liu, X. An updated role of microRNA-124 in central nervous system disorders: a review. Front. Cell. Neurosci. 2015, 9, 193. [Google Scholar] [CrossRef]

| Parameter | Mean ± SD (Range) |
|---|---|
| Age, years | 48.66 ± 6.82 (41–62) |
| BMI, kg/m2 | 28.18 ± 2.08 (25.0–30.8) |
| Waist circumference, cm | 96.83 ± 6.49 (88–112) |
| Fasting glucose, mg/dL | 90.83 ± 5.54 (83-103) |
| Basal insulin, μUI/mL | 11.14 ± 3.58 (5.5–17.7) |
| HOMA IR | 2.49 ± 0.77 (1.2–3.7) |
| HOMA BETA | 152.8 ± 71.58 (73–304) |
| Triglycerides, mg/dL | 116.58 ± 57.55 (49–218) |
| Total cholesterol, mg/dL | 188.17 ± 33.40 (115–232) |
| HDL cholesterol, mg/dL | 47.91 ± 10.67 (38–72) |
| LDL cholesterol, mg/dL | 117.00 ± 27.09 (60–156) |
| Apoliprotein A, mg/dL | 133.67 ± 20.95 (107–172) |
| Apoliprotein B, mg/dL | 101.77 ± 24.80 (49–133) |
| Creatinin, mg/dL | 0.97 ± 0.14 (0.7–1.2) |
| TGO, U/L | 20.67 ± 2.87 (14–25) |
| TGP, U/L | 25.41 ± 6.97 (11–35) |
| hsa-mir-335-5p | MIMAT0000765 | 228 |
| hsa-mir-26b-5p | MIMAT0000083 | 193 |
| hsa-mir-16-5p | MIMAT0000069 | 161 |
| hsa-mir-92a-3p | MIMAT0000092 | 161 |
| hsa-mir-124-3p | MIMAT0000422 | 152 |
| hsa-mir-155-5p | MIMAT0000093 | 116 |
| hsa-mir-93-5p | MIMAT0000646 | 116 |
| hsa-mir-1-3p | MIMAT0000416 | 114 |
| hsa-mir-17-5p | MIMAT0000070 | 113 |
| hsa-mir-615-3p | MIMAT0003283 | 112 |
| hsa-let-7b-5p | MIMAT0000063 | 104 |
| hsa-mir-106b-5p | MIMAT0000680 | 101 |
| hsa-mir-20a-5p | MIMAT0000075 | 99 |
| hsa-mir-218-5p | MIMAT0000275 | 99 |
| hsa-mir-1-1 | MI0000651 | 98 |
| hsa-mir-484 | MIMAT0002174 | 90 |
| hsa-mir-193b-3p | MIMAT0002819 | 89 |
| hsa-mir-15b-5p | MIMAT0000417 | 88 |
| hsa-mir-15a-5p | MIMAT0000068 | 81 |
| hsa-mir-20b-5p | MIMAT0000076 | 80 |
| hsa-mir-21-5p | MIMAT0001413 | 80 |
| hsa-mir-30a-5p | MIMAT0000087 | 79 |
| hsa-mir-186-5p | MIMAT0000456 | 78 |
| hsa-mir-519d-3p | MIMAT0002853 | 77 |
| hsa-mir-24-3p | MIMAT0000080 | 76 |
| hsa-mir-320a | MIMAT0000510 | 75 |
| hsa-mir-8485 | MIMAT0033692 | 75 |
| hsa-mir-192-5p | MIMAT0000222 | 74 |
| hsa-mir-195-5p | MIMAT0000461 | 73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, I.d.A.E.; Milenkovic, D.; Borges, T.K.d.S.; Rosa, A.J.d.M.; Morand, C.; Oliveira, L.d.L.d.; Costa, A.M. Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients 2020, 12, 1104. https://doi.org/10.3390/nu12041104
Duarte IdAE, Milenkovic D, Borges TKdS, Rosa AJdM, Morand C, Oliveira LdLd, Costa AM. Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients. 2020; 12(4):1104. https://doi.org/10.3390/nu12041104
Chicago/Turabian StyleDuarte, Isabella de Araújo Esteves, Dragan Milenkovic, Tatiana Karla dos Santos Borges, Artur Jordão de Magalhães Rosa, Christine Morand, Livia de Lacerda de Oliveira, and Ana Maria Costa. 2020. "Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans" Nutrients 12, no. 4: 1104. https://doi.org/10.3390/nu12041104
APA StyleDuarte, I. d. A. E., Milenkovic, D., Borges, T. K. d. S., Rosa, A. J. d. M., Morand, C., Oliveira, L. d. L. d., & Costa, A. M. (2020). Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients, 12(4), 1104. https://doi.org/10.3390/nu12041104






