Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administration
2.3. Behavioral Tests
2.4. Tissue Preparation
2.5. Immunohistochemistry
2.6. Microscopic Quantification
2.7. Statistical Analysis
3. Results
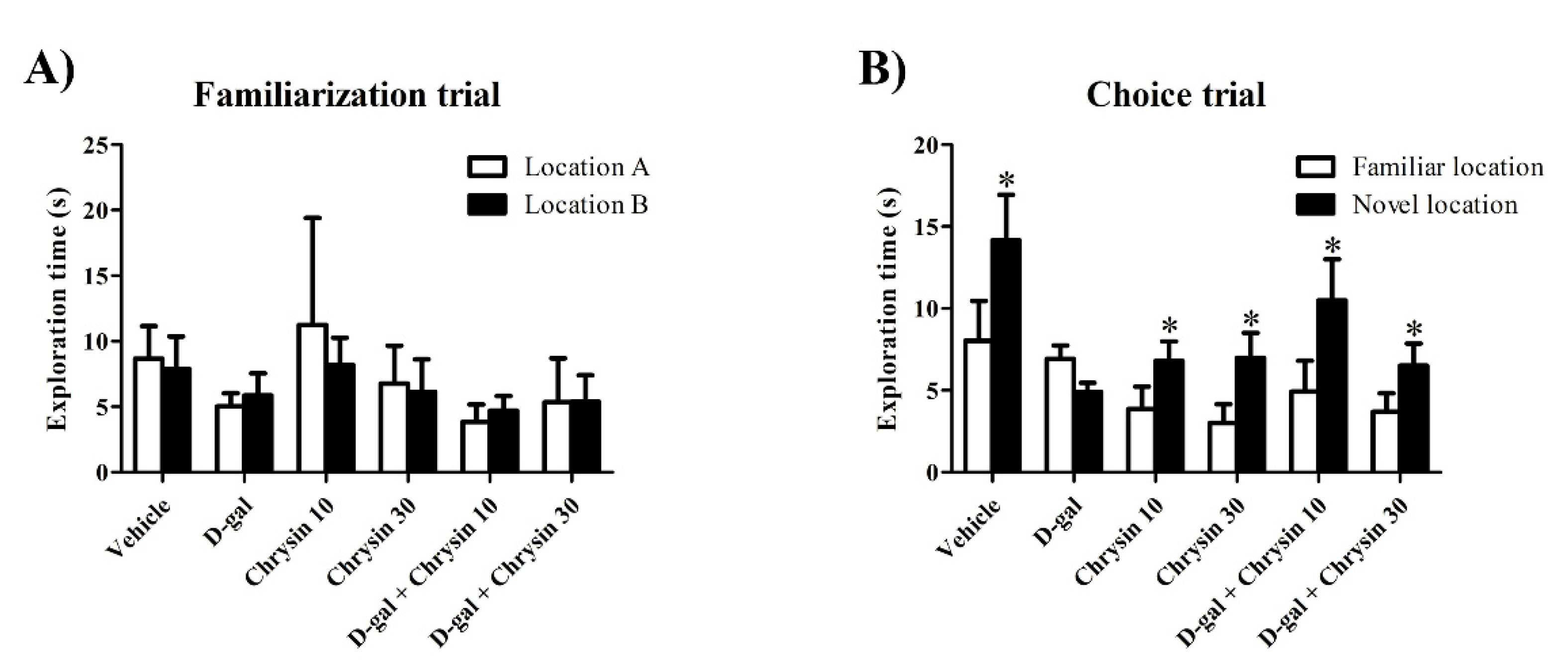
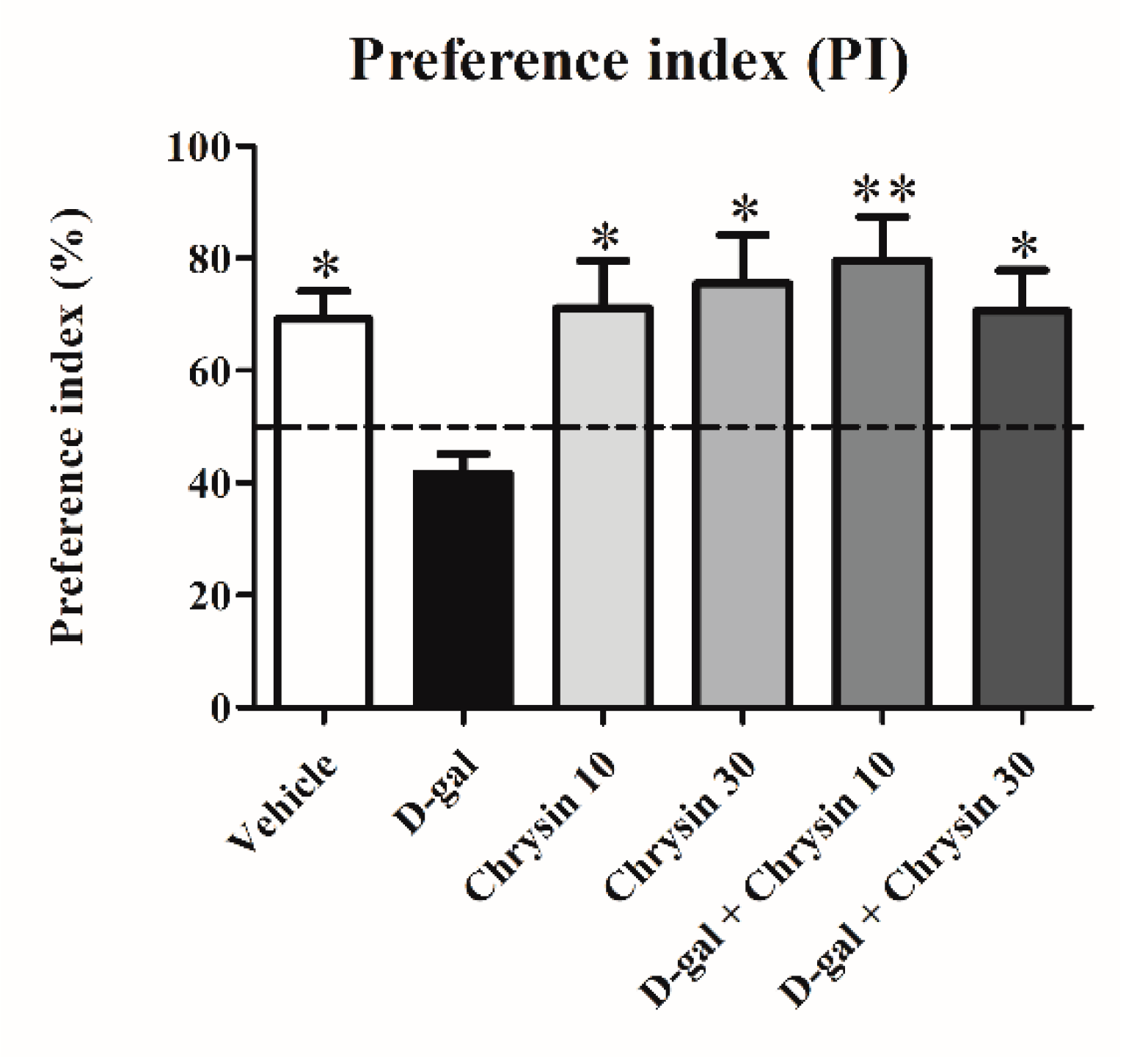
3.1. Effects of D-Gal and Chrysin on NOL Test Results
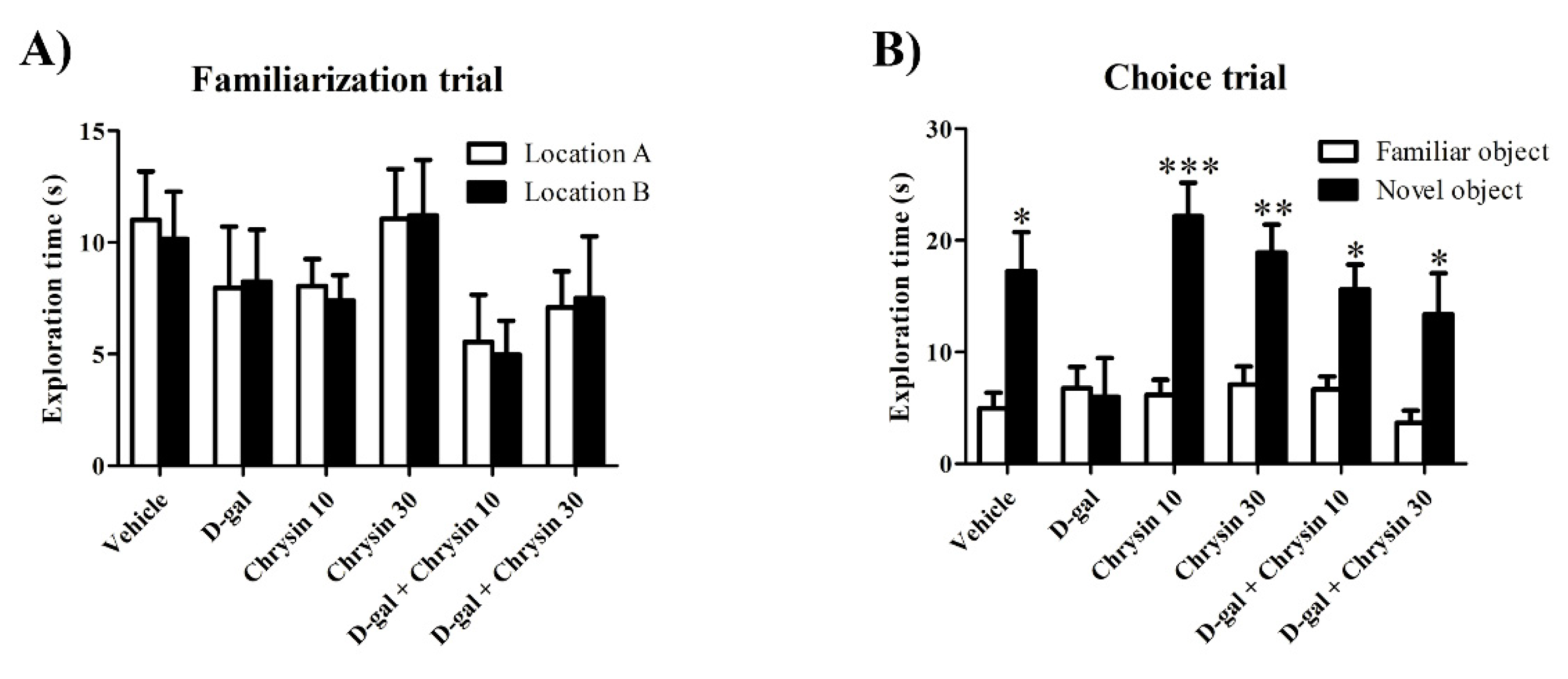
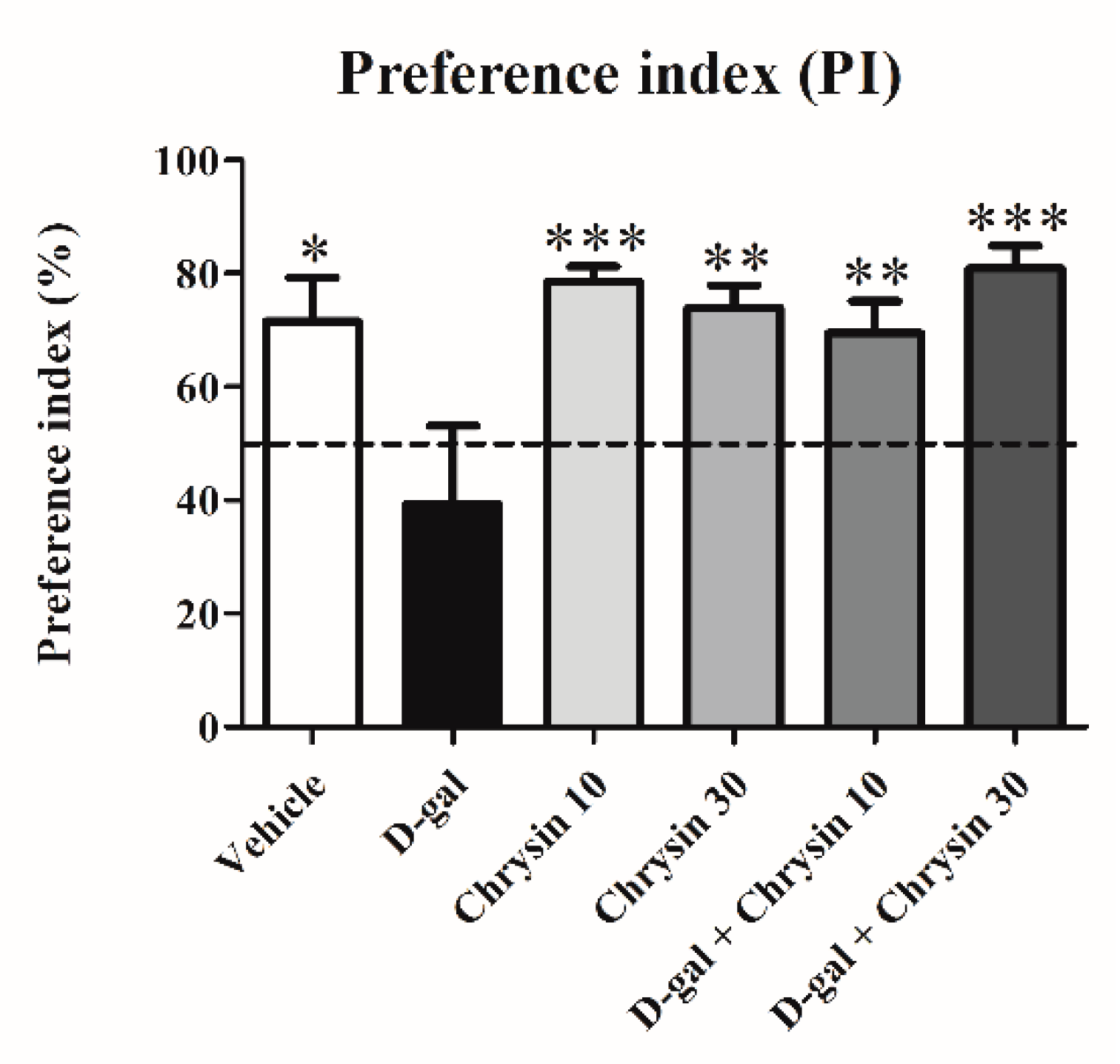
3.2. Effects of D-Gal and Chrysin on NOR Test Results
3.3. Effects of D-Gal and Chrysin on Cell Proliferation in the SGZ
3.4. Effects of D-Gal and Chrysin on Cell Survival in the SGZ
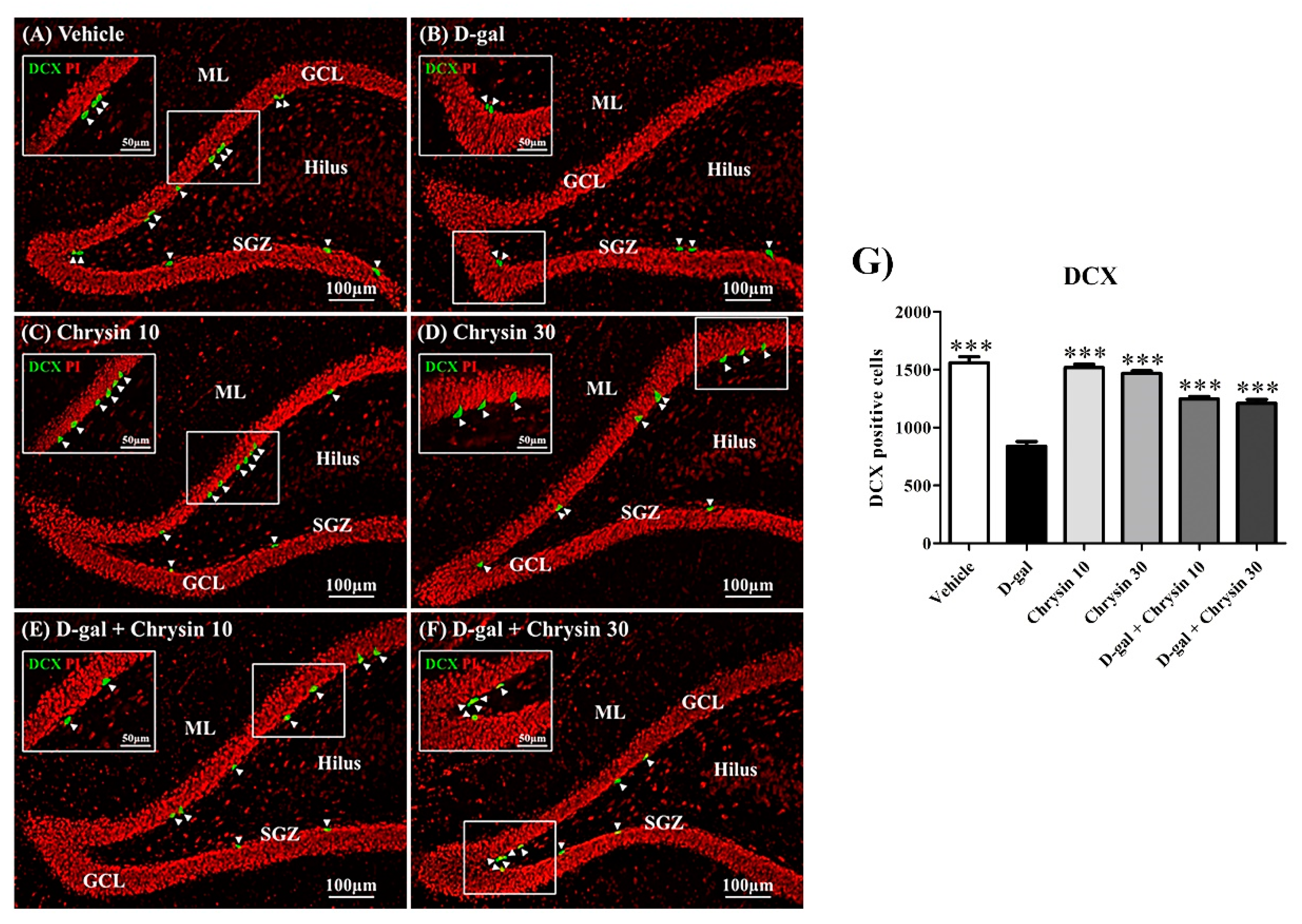
3.5. Effects of D-Gal and Chrysin on Immature Neurons in the SGZ
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braun, S.M.G.; Jessberger, S. Adult neurogenesis: Mechanisms and functional significance. Development 2014, 141, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Kitabatake, Y.; Sailor, K.A.; Ming, G.; Song, H. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg. Clin. N. Am. 2007, 18, 105–113. [Google Scholar] [CrossRef][Green Version]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Khaje-Bishak, Y.; Payahoo, L.; Pourghasem, B.; Asghari Jafarabadi, M. Assessing the Quality of Life in Elderly People and Related Factors in Tabriz, Iran. J. Caring Sci. 2014, 3, 257–263. [Google Scholar]
- Wickens, A.P. Ageing and the free radical theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Klempin, F.; Kempermann, G. Adult hippocampal neurogenesis and aging. Eur. Arch. Psychiatr. Clin. Neurosci. 2007, 257, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Dash, D. Amadori glycated proteins: Role in production of autoantibodies in diabetes mellitus and effect of inhibitors on non-enzymatic glycation. Aging Dis. 2013, 4, 50–56. [Google Scholar]
- Hsieh, H.-M.; Wu, W.-M.; Hu, M.-L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem. Toxicol. 2009, 47, 625–632. [Google Scholar] [CrossRef]
- Ali, T.; Badshah, H.; Kim, T.H.; Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J. Pineal Res. 2015, 58, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Ali, T.; Ullah, N.; Kim, M.O. Caffeine prevents d-galactose-induced cognitive deficits, oxidative stress, neuroinflammation and neurodegeneration in the adult rat brain. Neurochem. Int. 2015, 90, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, W.; Lee, C.H.; Shin, B.N.; Nam, S.M.; Choi, J.H.; Won, M.-H.; Yoon, Y.S.; Hwang, I.K. Melatonin improves d-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression: Effects of melatonin ond-galactose-induced hippocampal functions. J. Pineal Res. 2012, 52, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mu, X.; Zeng, J.; Xu, C.; Liu, J.; Zhang, M.; Li, C.; Chen, J.; Li, T.; Wang, Y. Ginsenoside Rg1 Prevents Cognitive Impairment and Hippocampus Senescence in a Rat Model of D-Galactose-Induced Aging. PLoS ONE 2014, 9, e101291. [Google Scholar] [CrossRef] [PubMed]
- Banji, O.J.F.; Banji, D.; Ch, K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem. Toxicol. 2014, 74, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.V.; Mohamed Jaabir, M.S.; Thomas, P.A.; Geraldine, P. Protective role of chrysin against oxidative stress in d-galactose-induced aging in an experimental rat model. Geriatr. Gerontol. Int. 2012, 12, 741–750. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozdemir, I.; Aydin, M.; Beytur, A. Beneficial effects of chrysin on the reproductive system of adult male rats: Chrysin improved male reproductive activity in rats. Andrologia 2012, 44, 181–186. [Google Scholar] [CrossRef]
- Pushpavalli, G.; Kalaiarasi, P.; Veeramani, C.; Pugalendi, K.V. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur. J. Pharmacol. 2010, 631, 36–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef] [PubMed]
- He, X.-L.; Wang, Y.-H.; Bi, M.-G.; Du, G.-H. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 2012, 680, 41–48. [Google Scholar] [CrossRef]
- Rashno, M.; Sarkaki, A.; Farbood, Y.; Rashno, M.; Khorsandi, L.; Naseri, M.K.G.; Dianat, M. Therapeutic effects of chrysin in a rat model of traumatic brain injury: A behavioral, biochemical, and histological study. Life Sci. 2019, 228, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Thangarajan, S.; Ramachandran, S.; Krishnamurthy, P. Chrysin exerts neuroprotective effects against 3-Nitropropionic acid induced behavioral despair—Mitochondrial dysfunction and striatal apoptosis via upregulating Bcl-2 gene and downregulating Bax—Bad genes in male wistar rats. Biomed. Pharmacother. 2016, 84, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Neuroprotective role of chrysin in attenuating loss of dopaminergic neurons and improving motor, learning and memory functions in rats. Int. J. Health Sci. 2018, 12, 35–43. [Google Scholar]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Lyons, L.; Elbeltagy, M.; Bennett, G.; Wigmore, P. The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS ONE 2011, 6, e21445. [Google Scholar] [CrossRef]
- Aranarochana, A.; Chaisawang, P.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Protective effects of melatonin against valproic acid-induced memory impairments and reductions in adult rat hippocampal neurogenesis. Neuroscience 2019, 406, 580–593. [Google Scholar] [CrossRef]
- Naewla, S.; Sirichoat, A.; Pannangrong, W.; Chaisawang, P.; Wigmore, P.; Welbat, J.U. Hesperidin Alleviates Methotrexate-Induced Memory Deficits via Hippocampal Neurogenesis in Adult Rats. Nutrients 2019, 11, 936. [Google Scholar] [CrossRef]
- Sirichoat, A.; Krutsri, S.; Suwannakot, K.; Aranarochana, A.; Chaisawang, P.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Melatonin protects against methotrexate-induced memory deficit and hippocampal neurogenesis impairment in a rat model. Biochem. Pharmacol. 2019, 163, 225–233. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; de Haan, E.H.F.; Kappelle, L.J.; Postma, A. Varieties of human spatial memory: A meta-analysis on the effects of hippocampal lesions. Brain Res. Rev. 2001, 35, 295–303. [Google Scholar] [CrossRef]
- Pourmemar, E.; Majdi, A.; Haramshahi, M.; Talebi, M.; Karimi, P.; Sadigh-Eteghad, S. Intranasal Cerebrolysin Attenuates Learning and Memory Impairments in D-galactose-Induced Senescence in Mice. Exp. Gerontol. 2017, 87, 16–22. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Clarendon Press: Oxford, UK; Oxford University Press: Oxford, UK, 1978; ISBN 978-0-19-857206-0. [Google Scholar]
- Turriziani, P.; Oliveri, M.; Salerno, S.; Costanzo, F.; Koch, G.; Caltagirone, C.; Carlesimo, G.A. Recognition memory and prefrontal cortex: Dissociating recollection and familiarity processes using rTMS. Behav. Neurol. 2008, 19, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.D.; Saksida, L.M.; Bussey, T.J. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008, 32, 1055–1070. [Google Scholar] [CrossRef]
- Clarke, J.R.; Cammarota, M.; Gruart, A.; Izquierdo, I.; Delgado-García, J.M. Plastic modifications induced by object recognition memory processing. Proc. Natl. Acad. Sci. USA 2010, 107, 2652–2657. [Google Scholar] [CrossRef]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial prefrontal cortex role in recognition memory in rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef]
- Vedagiri, A.; Sumathi, T. Chrysin, a natural flavonoid attenuates cognitive dysfunction and neuronal loss associated with Amyloid β(25-35)—Induced oxidative stress: An experimental model of Alzheimer’s disease. Inter. J. Pharmacogn. Phytochem. Res. 2015, 7, 224–236. [Google Scholar]
- Samaila Chiroma, M.; Taufik Hidayat Baharuldin, M.; Mat Taib, C.N.; Amom, Z.; Jagadeesan, S.; Adenan, M.; Mohd Moklas, M.A. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: Behavioral and ultrastructural approaches. Biomed. Pharmacother. 2019, 109, 853–864. [Google Scholar] [CrossRef]
- Wu, B.; Chen, Y.; Huang, J.; Ning, Y.; Bian, Q.; Shan, Y.; Cai, W.; Zhang, X.; Shen, Z. Icariin improves cognitive deficits and activates quiescent neural stem cells in aging rats. J. Ethnopharmacol. 2012, 142, 746–753. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Urruticoechea, A.; Smith, I.E.; Dowsett, M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005, 23, 7212–7220. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Stumpff, J. Measuring Microtubule Thickness: An Exercise in Cooperativity. Dev. Cell 2012, 23, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Kosaka, J.; Mori, T.; Takamori, Y.; Yamada, H. Doublecortin expression continues into adulthood in horizontal cells in the rat retina. Neurosci. Lett. 2008, 442, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, G.; Aigner, L. Doublecortin expression in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Nam, S.M.; Seo, M.; Seo, J.-S.; Rhim, H.; Nahm, S.-S.; Cho, I.-H.; Chang, B.-J.; Kim, H.-J.; Choi, S.-H.; Nah, S.-Y. Ascorbic Acid Mitigates D-galactose-Induced Brain Aging by Increasing Hippocampal Neurogenesis and Improving Memory Function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, L.; Xiao, J.; Wang, C.; Jiang, W.; Zhang, R.; Hao, J. Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2014, 15, 20913–20926. [Google Scholar] [CrossRef]


| Groups | Distance Moved (cm) | Velocity (cm/s) |
|---|---|---|
| Vehicle | 3018 ± 593.3 | 1.680 ± 0.3302 |
| D-gal | 3001 ± 300.6 | 1.667 ± 0.1668 |
| Chrysin 10 | 2404 ± 1337.0 | 1.337 ± 0.2804 |
| Chrysin 30 | 2045 ± 505.3 | 1.138 ± 0.1009 |
| D-gal + chrysin 10 | 2607 ± 180.8 | 1.449 ± 0.1807 |
| D-gal + chrysin 30 | 1688 ± 324.8 | 0.939 ± 0.1851 |
| Groups | Distance Moved (cm) | Velocity (cm/s) |
|---|---|---|
| Vehicle | 2273 ± 295.5 | 1.264 ± 0.1638 |
| D-gal | 1800 ± 365.5 | 1.002 ± 0.2036 |
| Chrysin 10 | 2495 ± 312.9 | 1.389 ± 0.1741 |
| Chrysin 30 | 1590 ± 260.5 | 0.883 ± 0.1452 |
| D-gal + chrysin 10 | 2248 ± 469.6 | 1.250 ± 0.2600 |
| D-gal + chrysin 30 | 1718 ± 469.2 | 0.957 ± 0.2609 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajit, R.; Sritawan, N.; Suwannakot, K.; Naewla, S.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats. Nutrients 2020, 12, 1100. https://doi.org/10.3390/nu12041100
Prajit R, Sritawan N, Suwannakot K, Naewla S, Aranarochana A, Sirichoat A, Pannangrong W, Wigmore P, Welbat JU. Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats. Nutrients. 2020; 12(4):1100. https://doi.org/10.3390/nu12041100
Chicago/Turabian StylePrajit, Ram, Nataya Sritawan, Kornrawee Suwannakot, Salinee Naewla, Anusara Aranarochana, Apiwat Sirichoat, Wanassanan Pannangrong, Peter Wigmore, and Jariya Umka Welbat. 2020. "Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats" Nutrients 12, no. 4: 1100. https://doi.org/10.3390/nu12041100
APA StylePrajit, R., Sritawan, N., Suwannakot, K., Naewla, S., Aranarochana, A., Sirichoat, A., Pannangrong, W., Wigmore, P., & Welbat, J. U. (2020). Chrysin Protects against Memory and Hippocampal Neurogenesis Depletion in D-Galactose-Induced Aging in Rats. Nutrients, 12(4), 1100. https://doi.org/10.3390/nu12041100






