Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals, Reagents, and Antibodies
2.3. Isolation of TA from Loquat Leaf
2.4. Chromatographic Conditions of HPLC
2.5. Animals
2.6. Induction of COPD in Mice with CS and Drug Administration
2.7. Lung Histological Analysis
2.8. Quantitation of Inflammatory Mediators
2.9. Measurement of SOD and MDA
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. HPLC Profile and Content Analysis of TA
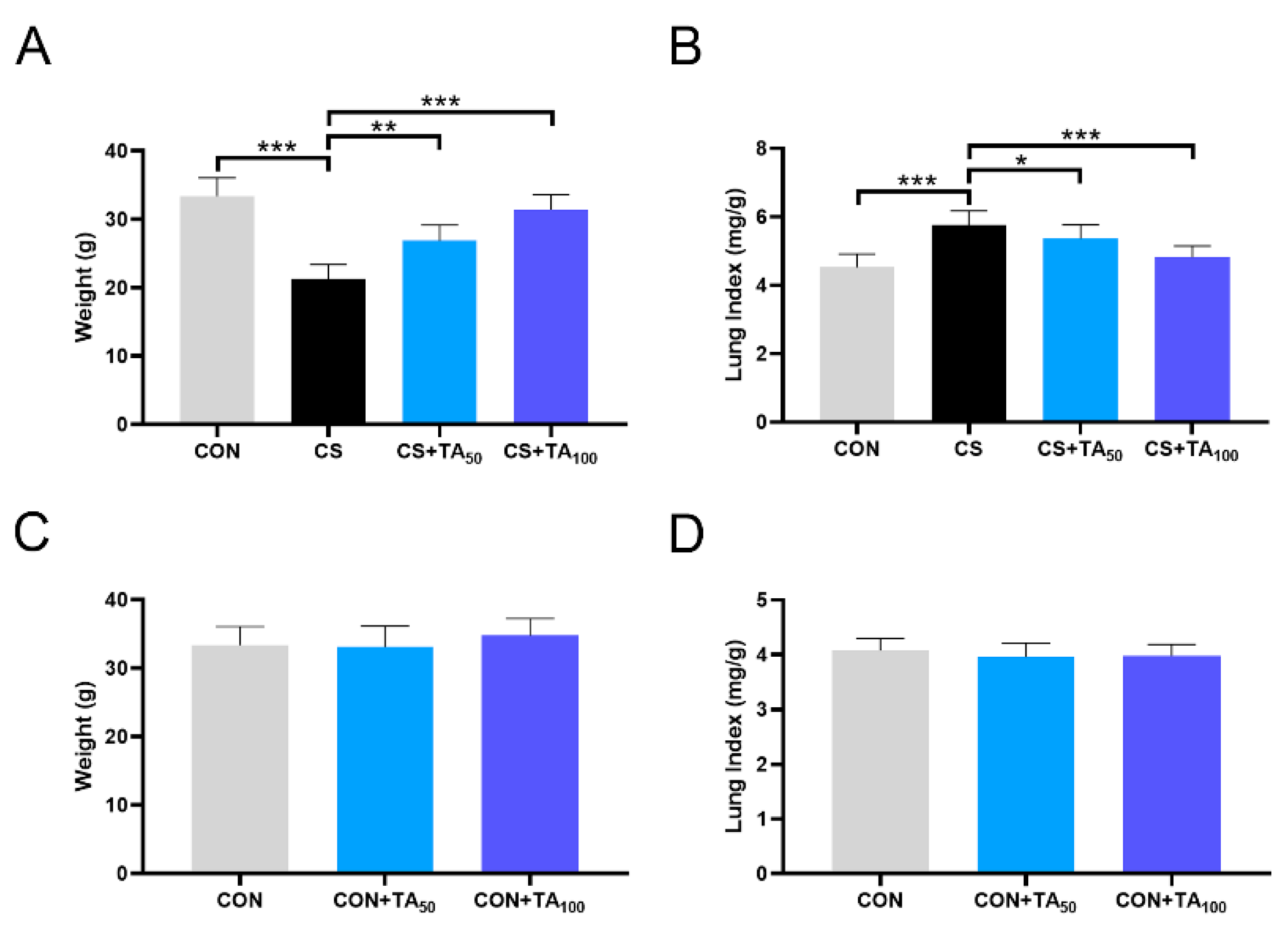
3.2. TA prevents Weight Loss and Pulmonary Swelling in CS-Induced COPD
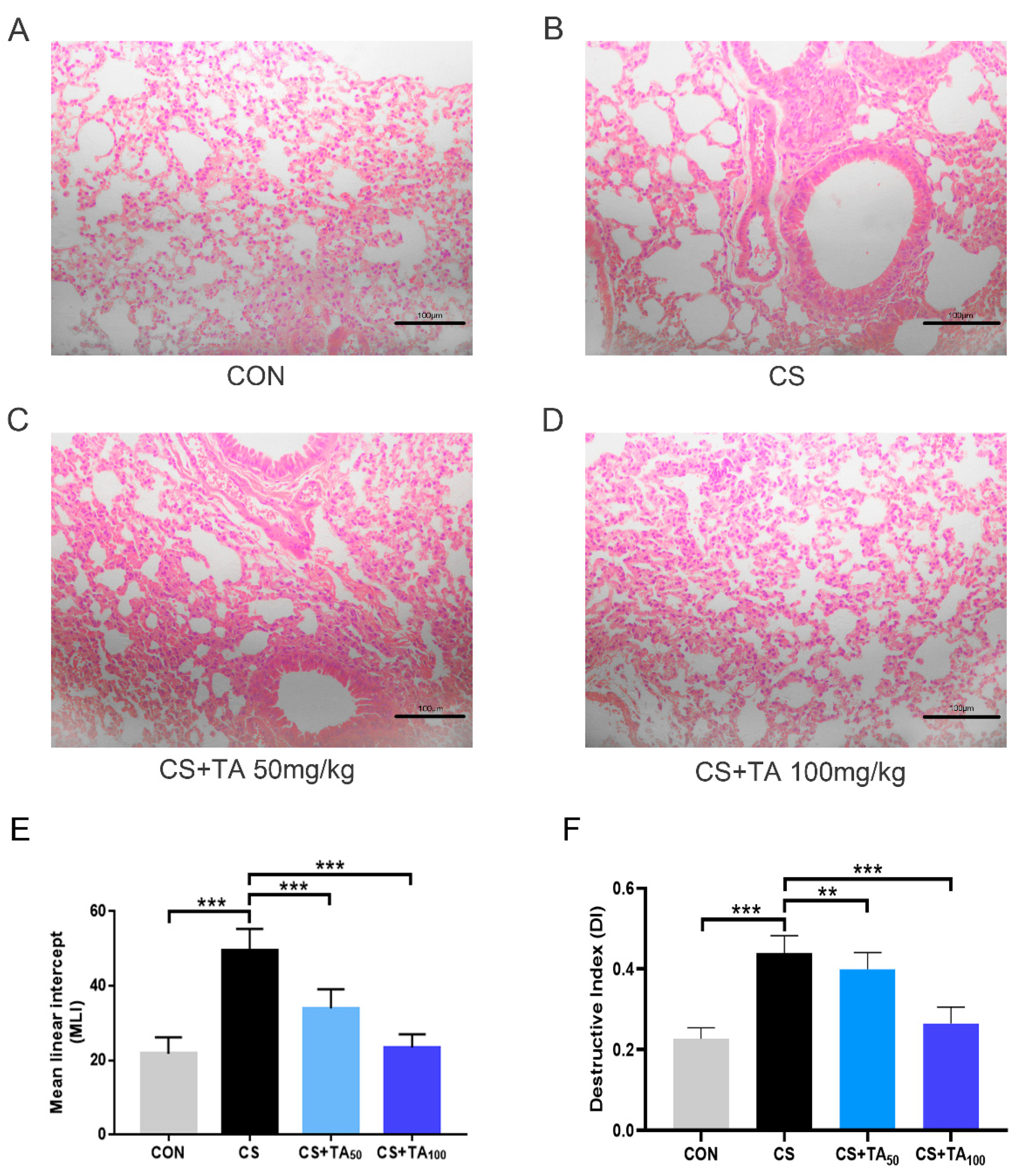
3.3. TA Attenuates CS-Induced Lung Injury
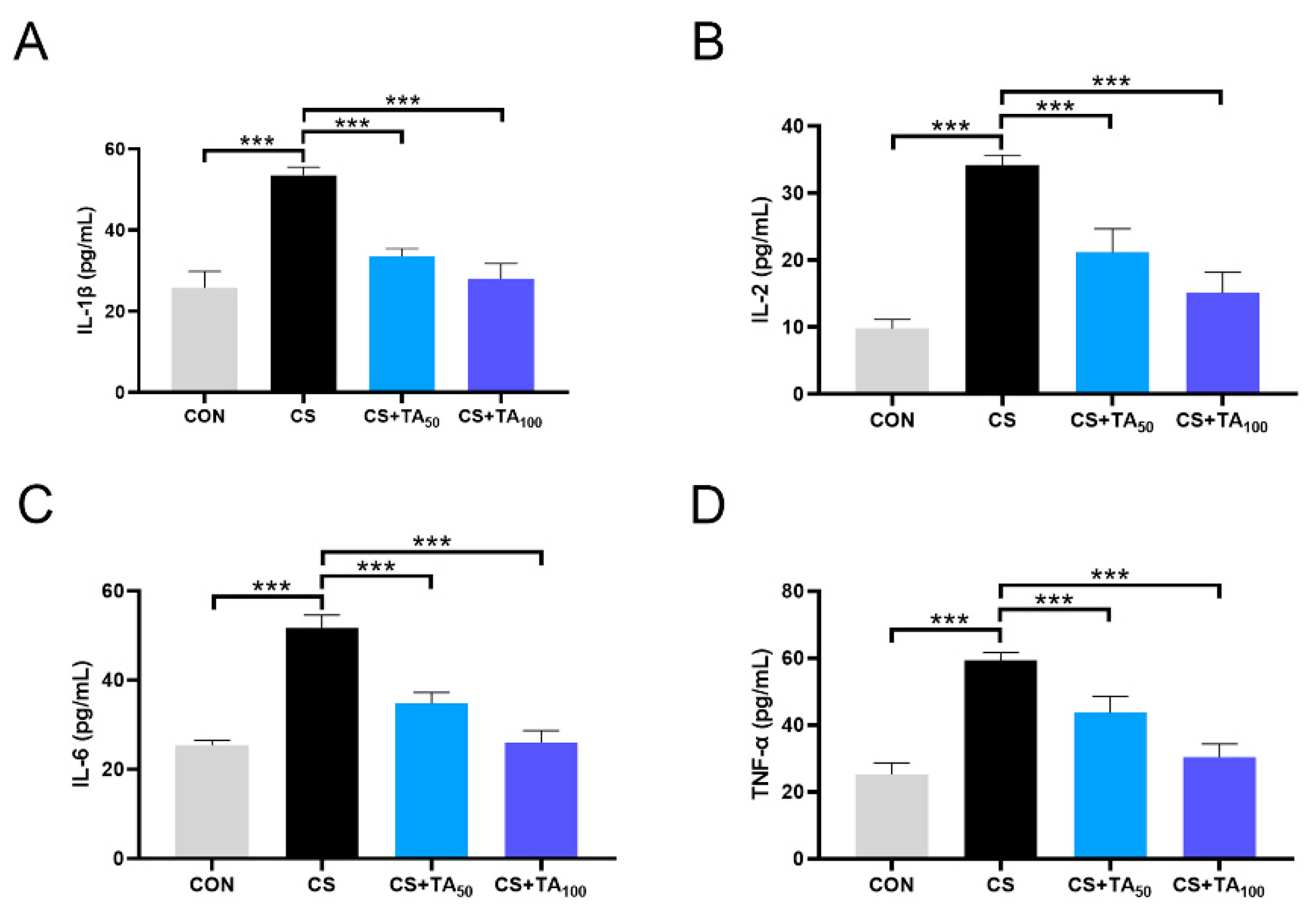
3.4. TA Decreases COPD Inflammatory Cytokine Concentration in CS-exposed mice
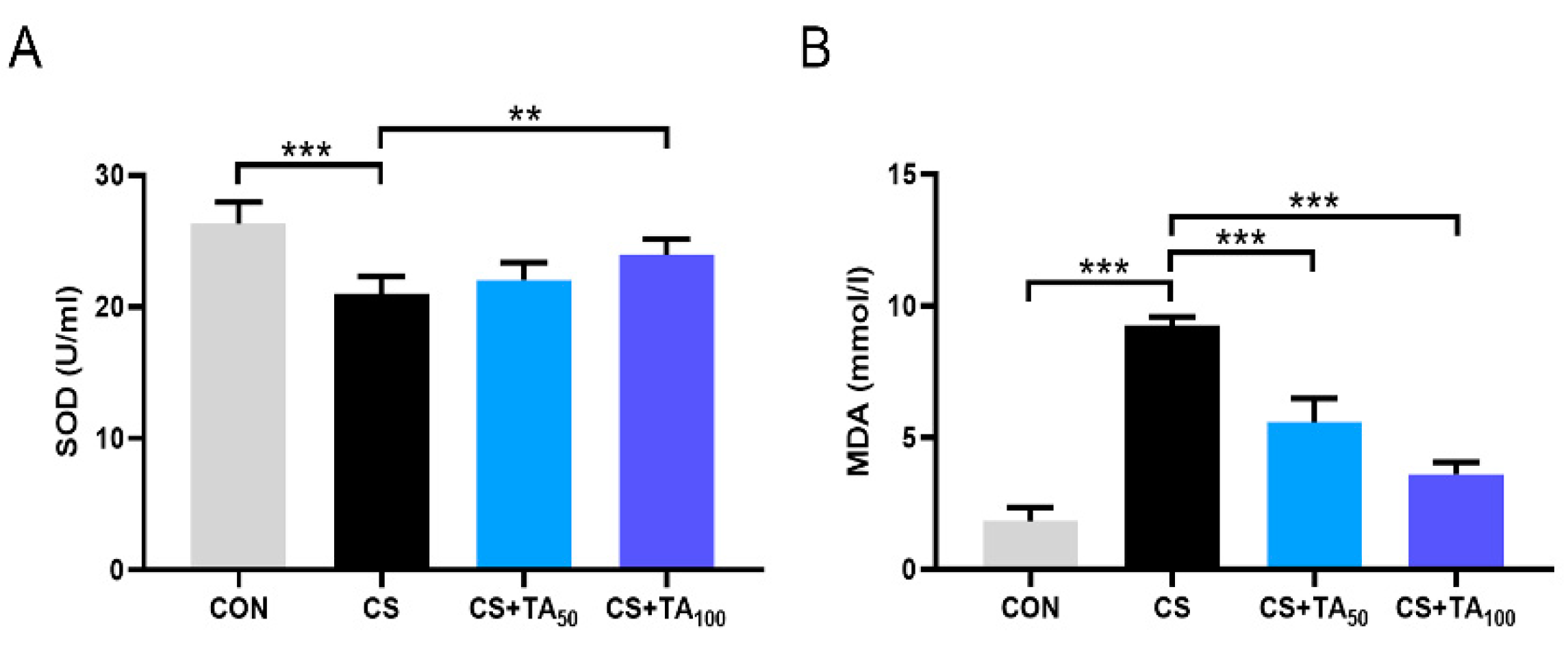
3.5. TA Improves the Oxidative Stress Imbalance of CS-induced COPD
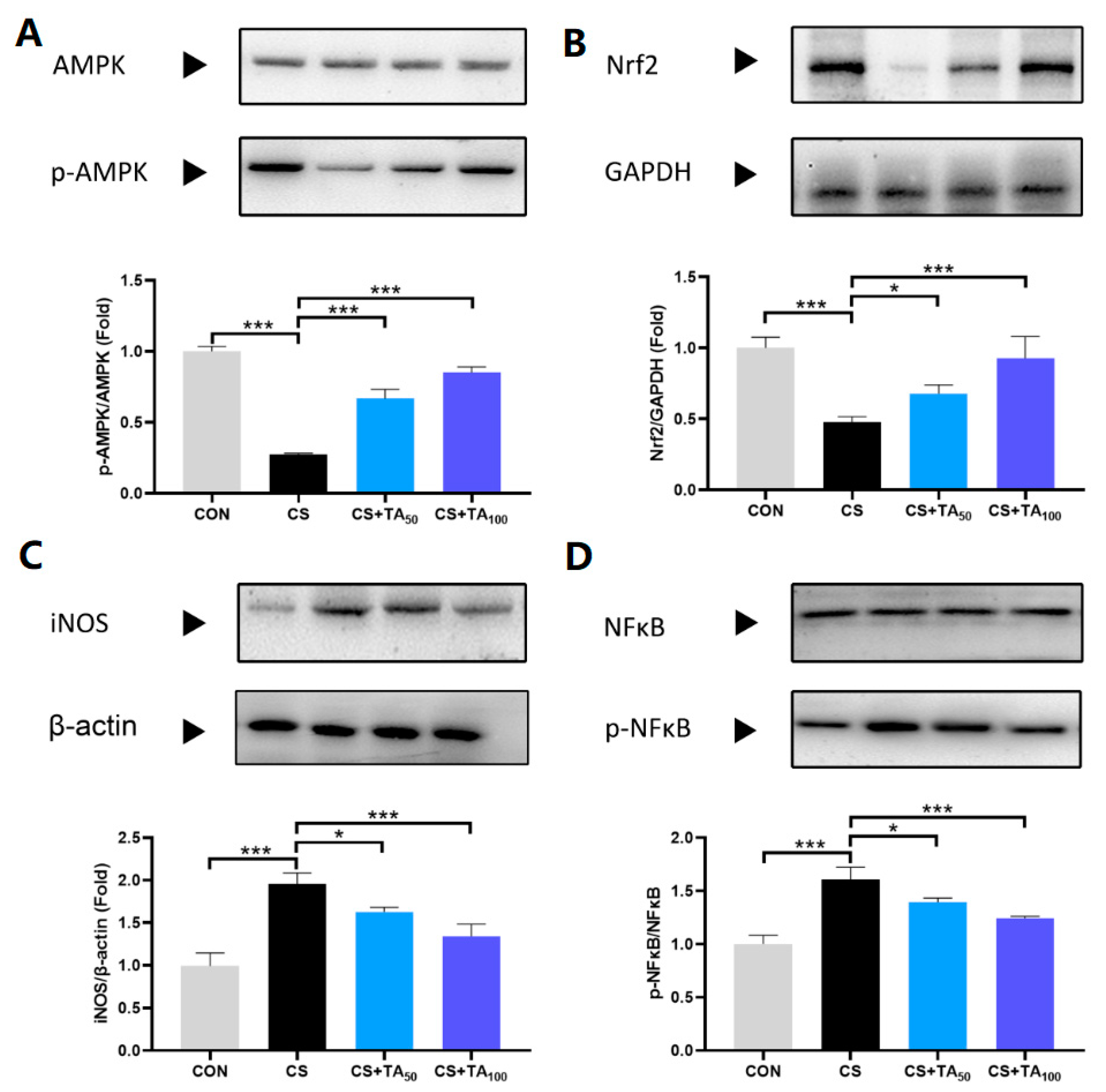
3.6. Expression of AMPK, Nrf2, iNOS, and NFκB in Lung Tissues of COPD Mice
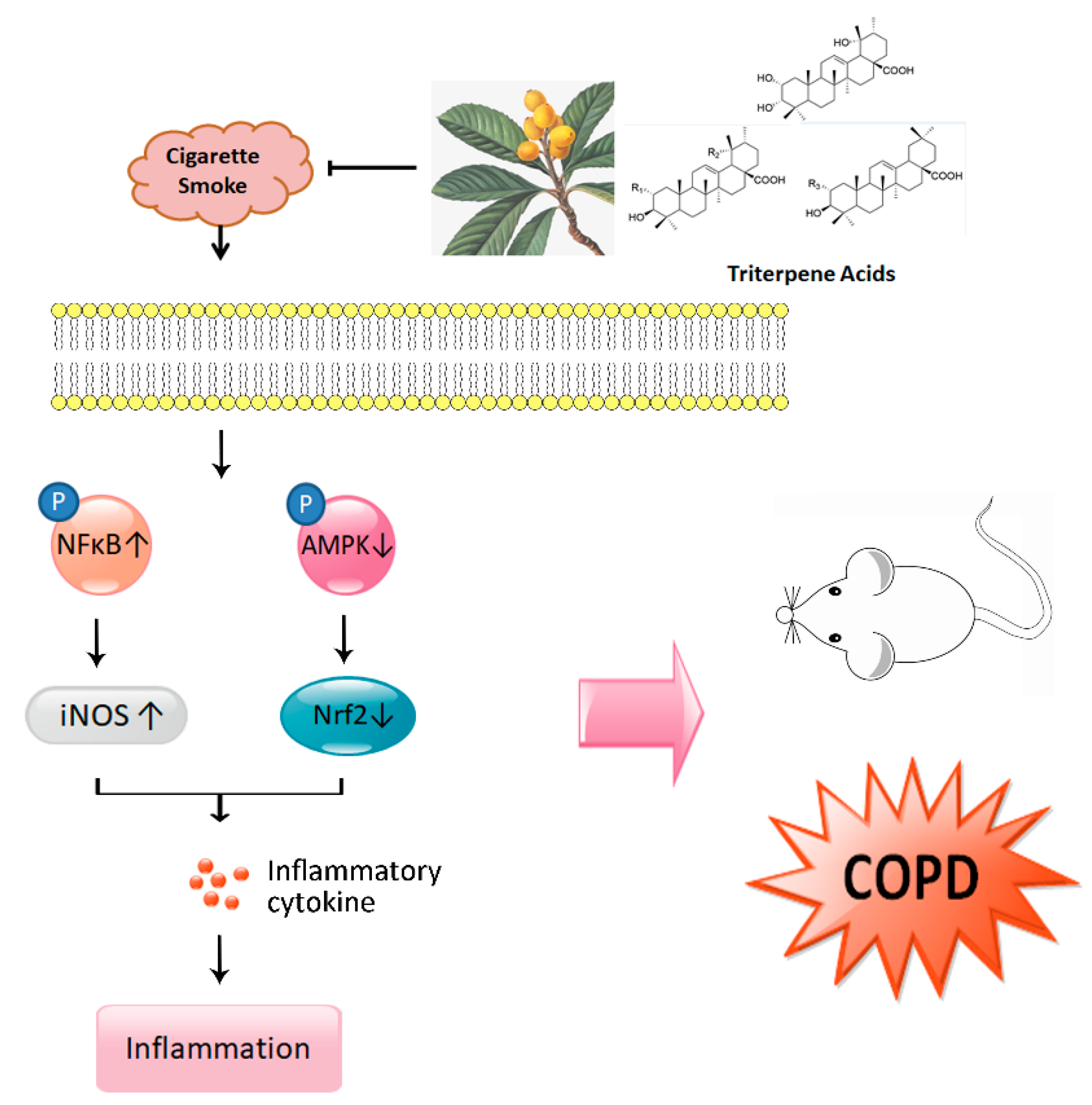
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirza, S.; Clay, R.D.; Koslow, M.A.; Scanlon, P.D. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clin. Proc. 2018, 93, 1488–1502. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Brandsma, C.A.; Van den Berge, M.; Hackett, T.L.; Brusselle, G.; Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2019. [Google Scholar] [CrossRef]
- Ejike, C.O.; Dransfield, M.T.; Hansel, N.N.; Putcha, N.; Raju, S.; Martinez, C.H.; Han, M.K. Chronic Obstructive Pulmonary Disease in America’s Black Population. Am. J. Respir. Crit. Care Med. 2019, 200, 423–430. [Google Scholar] [CrossRef]
- Howard, G.; Wagenknecht, L.E.; Burke, G.L.; Diez-Roux, A.; Evans, G.W.; McGovern, P.; Nieto, F.J.; Tell, G.S. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA 1998, 279, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Y.; Wang, Y.; Sun, C.; Zhong, Y.; Liao, J.; Wang, G. Fine particulate matter (PM2.5) aggravates apoptosis of cigarette-inflamed bronchial epithelium in vivo and vitro. Environ. Pollut. 2019, 248, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, X.; Yue, L.; Cui, W.; Zhou, W.; Gao, J.; Yao, H. Molecular pathogenesis in chronic obstructive pulmonary disease and therapeutic potential by targeting AMP-activated protein kinase. J. Cell. Physiol. 2018, 233, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Wang, B.; Chen, H.; Ho, K.F.; Cao, J.; Hai, G.; Jalaludin, B.; Herbert, C.; Thomas, P.S.; Saad, S.; et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L424–L430. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, Z.; Zhang, P.; Qu, J.; Zheng, C.; Mo, X.; Zhou, W.; Xu, L.; Yao, H.; Gao, J. Nrf2 attenuates inflammatory response in COPD/emphysema: Crosstalk with Wnt3a/beta-catenin and AMPK pathways. J. Cell. Mol. Med. 2018, 22, 3514–3525. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Z.; Yao, C.; Bai, J.; Yang, H.; Ma, P.; Fan, Y.; Li, S.; Yuan, J.; Lin, M.; et al. Amurensin H, a Derivative From Resveratrol, Ameliorates Lipopolysaccharide/Cigarette Smoke-Induced Airway Inflammation by Blocking the Syk/NF-kappaB Pathway. Front. Pharmacol. 2019, 10, 1157. [Google Scholar] [CrossRef]
- Kennedy-Feitosa, E.; Cattani-Cavalieri, I.; Barroso, M.V.; Romana-Souza, B.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol promotes lung repair in mice following cigarette smoke-induced emphysema. Phytomedicine 2019, 55, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Ryu, H.W.; Lee, S.; Shin, I.S.; Choi, J.H.; Lee, J.W.; Lee, J.; Kim, M.O.; Lee, H.J.; Ahn, K.S.; et al. Lignans Isolated From Flower Buds of Magnolia fargesii Attenuate Airway Inflammation Induced by Cigarette Smoke in vitro and in vivo. Front. Pharmacol. 2018, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Kim, C.; Seo, C.S.; Ko, J.W.; Cho, Y.K.; Kim, J.C.; Kim, J.S.; Shin, I.S. So-Cheong-Ryoung-Tang Attenuates Pulmonary Inflammation Induced by Cigarette Smoke in Bronchial Epithelial Cells and Experimental Mice. Front. Pharmacol. 2018, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Qian, X.; van der Velden, J.L.; Chia, S.B.; McMillan, D.H.; Flemer, S.; Hoffman, S.M.; Lahue, K.G.; Schneider, R.W.; Nolin, J.D.; et al. Glutathione S-transferase pi modulates NF-kappaB activation and pro-inflammatory responses in lung epithelial cells. Redox Biol. 2016, 8, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tian, Y.; Li, J.; Zhang, L.; Wu, M.; Zhu, L.; Liu, S. Effect of Bufei Yishen Granules Combined with Electroacupuncture in Rats with Chronic Obstructive Pulmonary Disease via the Regulation of TLR-4/NF-kappaB Signaling. Evid. Based Complement. Altern. Med. 2019, 2019, 6708645. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological Activities of Extracts from Loquat (Eriobotrya japonica Lindl.): A Review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef]
- Shii, T.; Tanaka, T.; Watarumi, S.; Matsuo, Y.; Miyata, Y.; Tamaya, K.; Tamaru, S.; Tanaka, K.; Matsui, T.; Kouno, I. Polyphenol composition of a functional fermented tea obtained by tea-rolling processing of green tea and loquat leaves. J. Agric. Food Chem. 2011, 59, 7253–7260. [Google Scholar] [CrossRef]
- Chen, B.; Long, P.; Sun, Y.; Meng, Q.; Liu, X.; Cui, H.; Lv, Q.; Zhang, L. The chemical profiling of loquat leaf extract by HPLC-DAD-ESI-MS and its effects on hyperlipidemia and hyperglycemia in rats induced by a high-fat and fructose diet. Food Funct. 2017, 8, 687–694. [Google Scholar] [CrossRef]
- Ge, J.F.; Wang, T.Y.; Zhao, B.; Lv, X.W.; Jin, Y.; Peng, L.; Yu, S.C.; Li, J. Anti-inflammatory effect of triterpenoic Acids of Eriobotrya japonica (Thunb.) Lindl. Leaf on rat model of chronic bronchitis. Am. J. Chin. Med. 2009, 37, 309–321. [Google Scholar] [CrossRef]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Taguchi, Y.; Akazawa, H.; Ukiya, M.; Kimura, Y.; Suzuki, T.; Nishino, H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol. Pharm. Bull. 2005, 28, 1995–1999. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Meng, X.M.; Jiang, G.L.; Li, H.; Cao, Q.; Yu, S.C.; Lv, X.W.; Cheng, W.M. Effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. Leaf and MAPK signal transduction pathway on inducible nitric oxide synthase expression in alveolar macrophage of chronic bronchitis rats. Am. J. Chin. Med. 2009, 37, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Wang, R.; Wu, Q.; Li, Y.H.; Yu, S.C.; Cheng, W.M.; Wang, Y.Y. Effect of triterpene acids of Eriobotrya japonica (Thunb.) Lindl. Leaf on inflammatory cytokine and mediator induction from alveolar macrophages of chronic bronchitic rats. Inflamm. Res. 2007, 56, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, J.; Li, W.L.; Zhang, H.Q. Studies on the triterpenes from loquat leaf (Eriobotrya japonica). Zhong Yao Cai 2008, 31, 1351–1354. [Google Scholar] [PubMed]
- Wu, Y.; Jian, T.; Lv, H.; Ding, X.; Zuo, Y.; Ren, B.; Chen, J.; Li, W. Antitussive and expectorant properties of growing and fallen leaves of loquat (Eriobotrya japonica). Rev. Bras. Farmacogn. 2018, 28, 239–242. [Google Scholar] [CrossRef]
- Yu, X.; Seow, H.J.; Wang, H.; Anthony, D.; Bozinovski, S.; Lin, L.; Ye, J.M.; Vlahos, R. Matrine reduces cigarette smoke-induced airway neutrophilic inflammation by enhancing neutrophil apoptosis. Clin. Sci. (Lond.) 2019, 133, 551–564. [Google Scholar] [CrossRef]
- Fan, L.; Chen, R.; Li, L.; Liang, Z.; Yu, X.; Huang, K.; Yin, S.; Wu, L.; Chen, Y.; Xu, Y.; et al. Protective Effect of Jianpiyifei II Granule against Chronic Obstructive Pulmonary Disease via NF-kappaB Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 4265790. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Shiner, R.J.; Angus, G.E.; Kim, W.D.; Wang, N.S.; King, M.; Ghezzo, H.; Cosio, M.G. Destructive index: A measurement of lung parenchymal destruction in smokers. Am. Rev. Respir. Dis. 1985, 131, 764–769. [Google Scholar]
- Rab, A.; Rowe, S.M.; Raju, S.V.; Bebok, Z.; Matalon, S.; Collawn, J.F. Cigarette smoke and CFTR: Implications in the pathogenesis of COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L530–L541. [Google Scholar] [CrossRef]
- Vij, N.; Chandramani-Shivalingappa, P.; Van Westphal, C.; Hole, R.; Bodas, M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell. Physiol. 2018, 314, C73–C87. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [Google Scholar] [CrossRef]
- Golpe, R.; Martin-Robles, I.; Sanjuan-Lopez, P.; Perez-de-Llano, L.; Gonzalez-Juanatey, C.; Lopez-Campos, J.L.; Arellano-Orden, E. Differences in systemic inflammation between cigarette and biomass smoke-induced COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2639–2646. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell. Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Li, Y.Y.; Huang, C.; Li, J.; Yao, H.W. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget 2017, 8, 22513–22523. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.J.; Cho, Y.S.; Huh, J.W.; Oh, Y.M.; Lee, S.D. Role of AMP-Activated Protein Kinase (AMPK) in Smoking-Induced Lung Inflammation and Emphysema. Tuberc. Respir. Dis. 2015, 78, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in LPS-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/Nrf2-dependent mechanism. Cell. Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tan, B.; Zhang, H.; Guo, Y.; Tu, Y.; Qiu, F.; Yang, A. Effects of Sodium Houttuyfonate on Pulmonary Inflammation in COPD Model Rats. Inflammation 2017, 40, 2109–2117. [Google Scholar] [CrossRef]
- Pang, M.; Liu, H.Y.; Li, T.; Wang, D.; Hu, X.Y.; Zhang, X.R.; Yu, B.F.; Guo, R.; Wang, H.L. Recombinant club cell protein 16 (CC16) ameliorates cigarette smokeinduced lung inflammation in a murine disease model of COPD. Mol. Med. Rep. 2018, 18, 2198–2206. [Google Scholar]
- Yuan, J.; Liu, R.; Ma, Y.; Zhang, Z.; Xie, Z. Curcumin Attenuates Airway Inflammation and Airway Remolding by Inhibiting NF-kappaB Signaling and COX-2 in Cigarette Smoke-Induced COPD Mice. Inflammation 2018, 41, 1804–1814. [Google Scholar] [CrossRef]
- Shin, N.R.; Ko, J.W.; Park, S.H.; Cho, Y.K.; Oh, S.R.; Ahn, K.S.; Ryu, J.M.; Kim, J.C.; Seo, C.S.; Shin, I.S. Protective effect of HwangRyunHaeDok-Tang water extract against chronic obstructive pulmonary disease induced by cigarette smoke and lipopolysaccharide in a mouse model. J. Ethnopharmacol. 2017, 200, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ryu, H.W.; Park, S.Y.; Park, H.A.; Kwon, O.K.; Yuk, H.J.; Shrestha, K.K.; Park, M.; Kim, J.H.; Lee, S.; et al. Protective effects of neem (Azadirachta indica A. Juss.) leaf extract against cigarette smoke- and lipopolysaccharide-induced pulmonary inflammation. Int. J. Mol. Med. 2017, 40, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jung, Y.; Kim, Y.; Jho, E.H.; Yoon, Y. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci. Rep. 2017, 7, 41612. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.G.; Lee, T.W.; Xu, H.; Yip, J.H.; Li, M.; Mok, T.S.; Yim, A.P. Increased inducible nitric oxide synthase in lung carcinoma of smokers. Cancer 2008, 112, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Kim, C.; Seo, C.S.; Ko, J.W.; Cho, Y.K.; Shin, I.S.; Kim, J.S. Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IkappaBalpha/NF-kappaB Signaling. Molecules 2018, 23, 2489. [Google Scholar] [CrossRef]
- Nakazawa, H.; Chang, K.; Shinozaki, S.; Yasukawa, T.; Ishimaru, K.; Yasuhara, S.; Yu, Y.M.; Martyn, J.A.; Tompkins, R.G.; Shimokado, K.; et al. iNOS as a Driver of Inflammation and Apoptosis in Mouse Skeletal Muscle after Burn Injury: Possible Involvement of Sirt1 S-Nitrosylation-Mediated Acetylation of p65 NF-kappaB and p53. PLoS ONE 2017, 12, e0170391. [Google Scholar] [CrossRef]
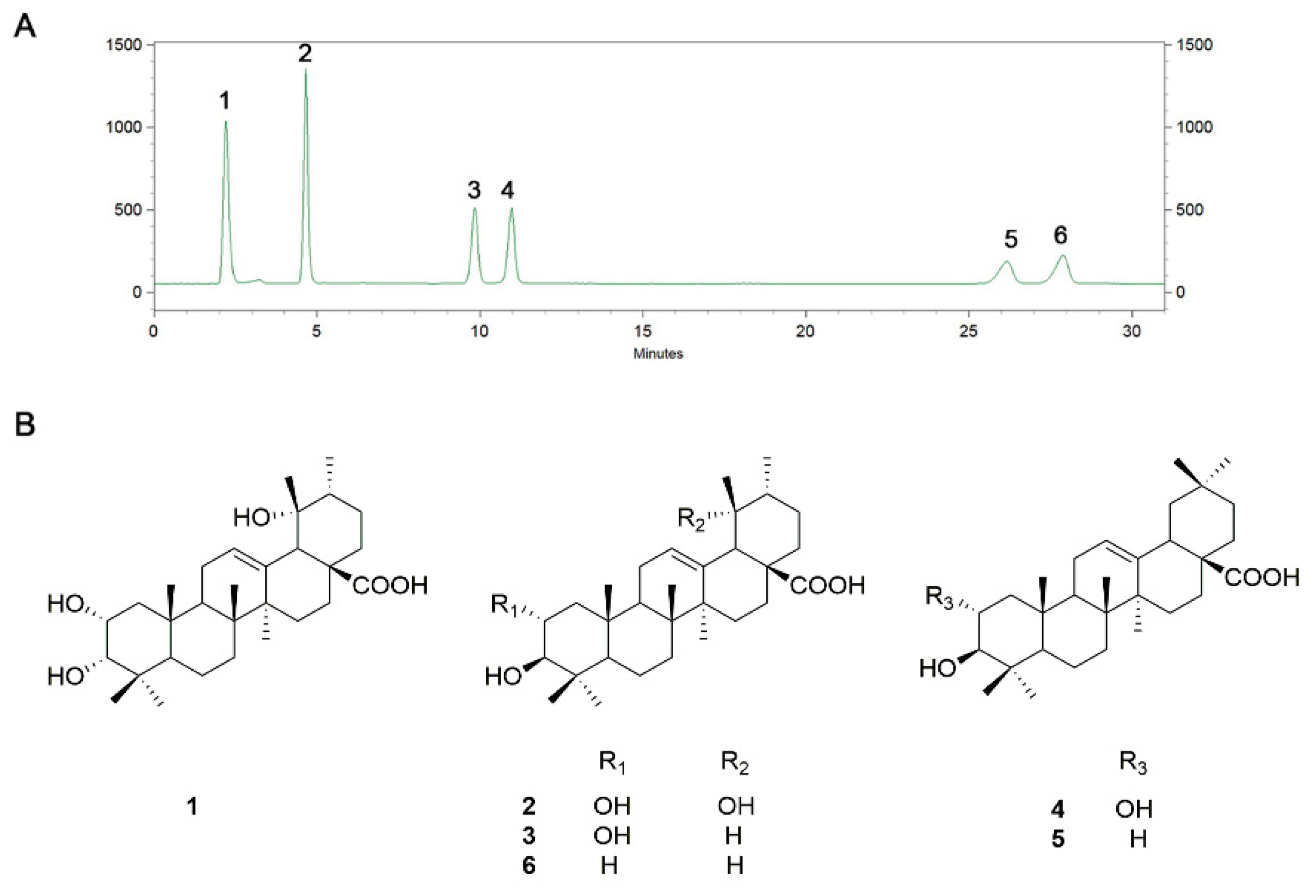
| Peak Number | Compound Name | Concentration (mg/g) |
|---|---|---|
| 1 | Euscaphic acid | 105.69 ± 2.10 |
| 2 | Tormentic acid | 155.43 ± 0.07 |
| 3 | Corosolic acid | 129.84 ± 6.26 |
| 4 | Maslinic acid | 135.24 ± 7.33 |
| 5 | Oleanolic acid | 65.44 ± 1.87 |
| 6 | Ursolic acid | 80.70 ± 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, T.; Ding, X.; Li, J.; Wu, Y.; Ren, B.; Li, J.; Lv, H.; Chen, J.; Li, W. Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients 2020, 12, 657. https://doi.org/10.3390/nu12030657
Jian T, Ding X, Li J, Wu Y, Ren B, Li J, Lv H, Chen J, Li W. Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients. 2020; 12(3):657. https://doi.org/10.3390/nu12030657
Chicago/Turabian StyleJian, Tunyu, Xiaoqin Ding, Jiawei Li, Yuexian Wu, Bingru Ren, Jing Li, Han Lv, Jian Chen, and Weilin Li. 2020. "Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways" Nutrients 12, no. 3: 657. https://doi.org/10.3390/nu12030657
APA StyleJian, T., Ding, X., Li, J., Wu, Y., Ren, B., Li, J., Lv, H., Chen, J., & Li, W. (2020). Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients, 12(3), 657. https://doi.org/10.3390/nu12030657





