Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Induction of Diabetes and Pharmacological Treatment
2.4. Interleukin-6 Detection
2.5. Behavioral Tests
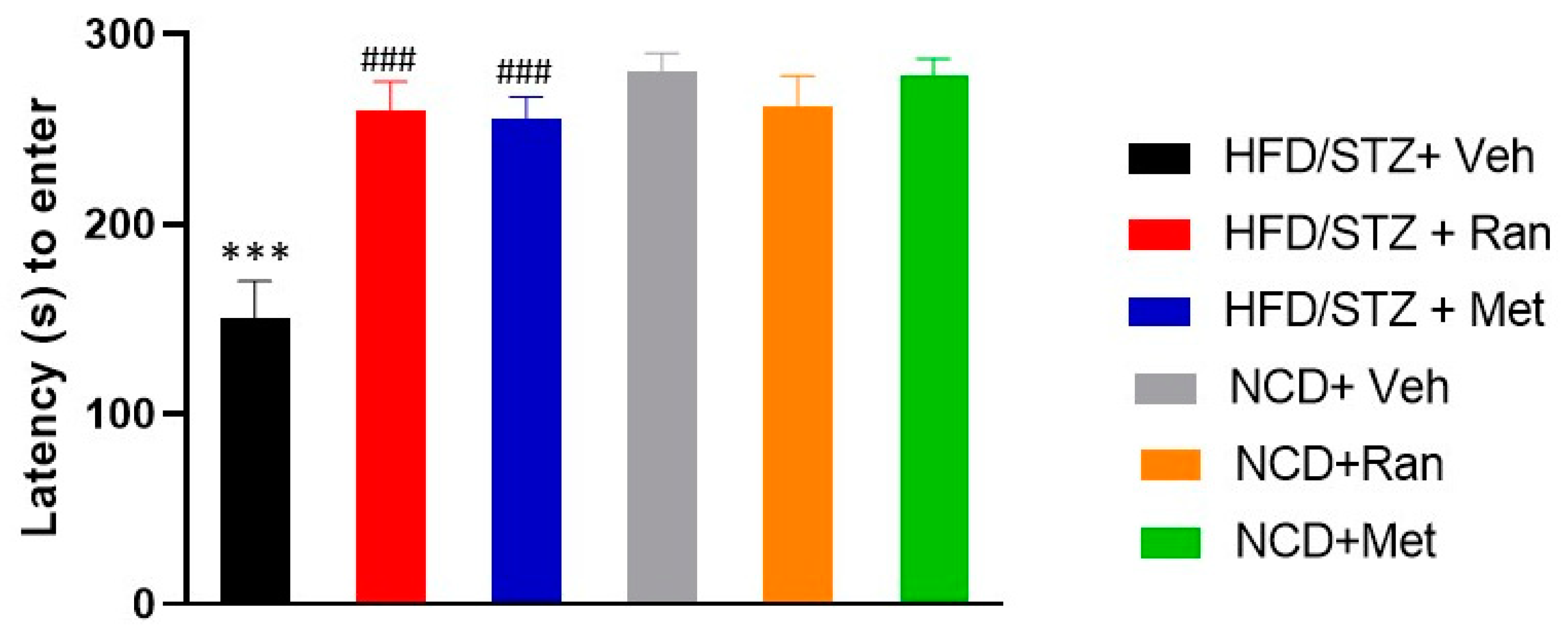
2.6. Passive Avoidance (PA)
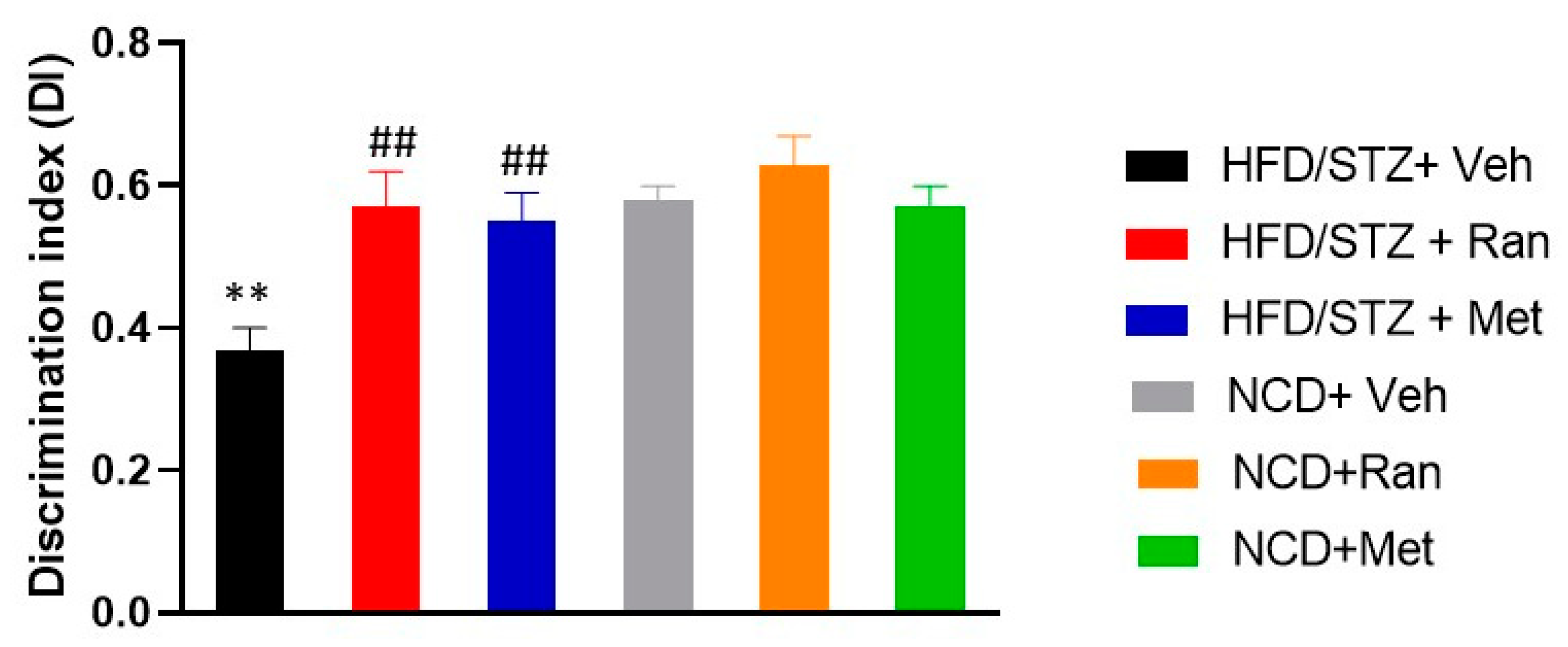
2.7. Novel Object Recognition Test (nORT)
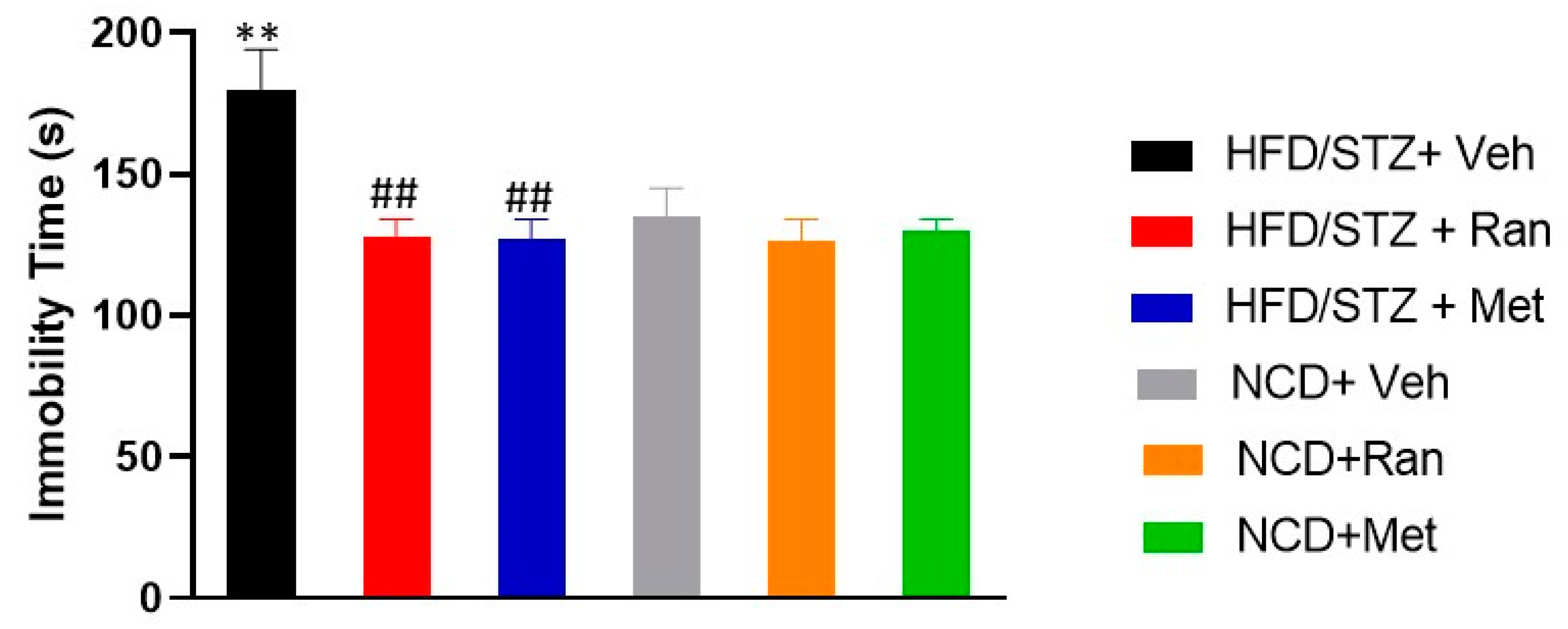
2.8. Forced Swimming Test (FST)
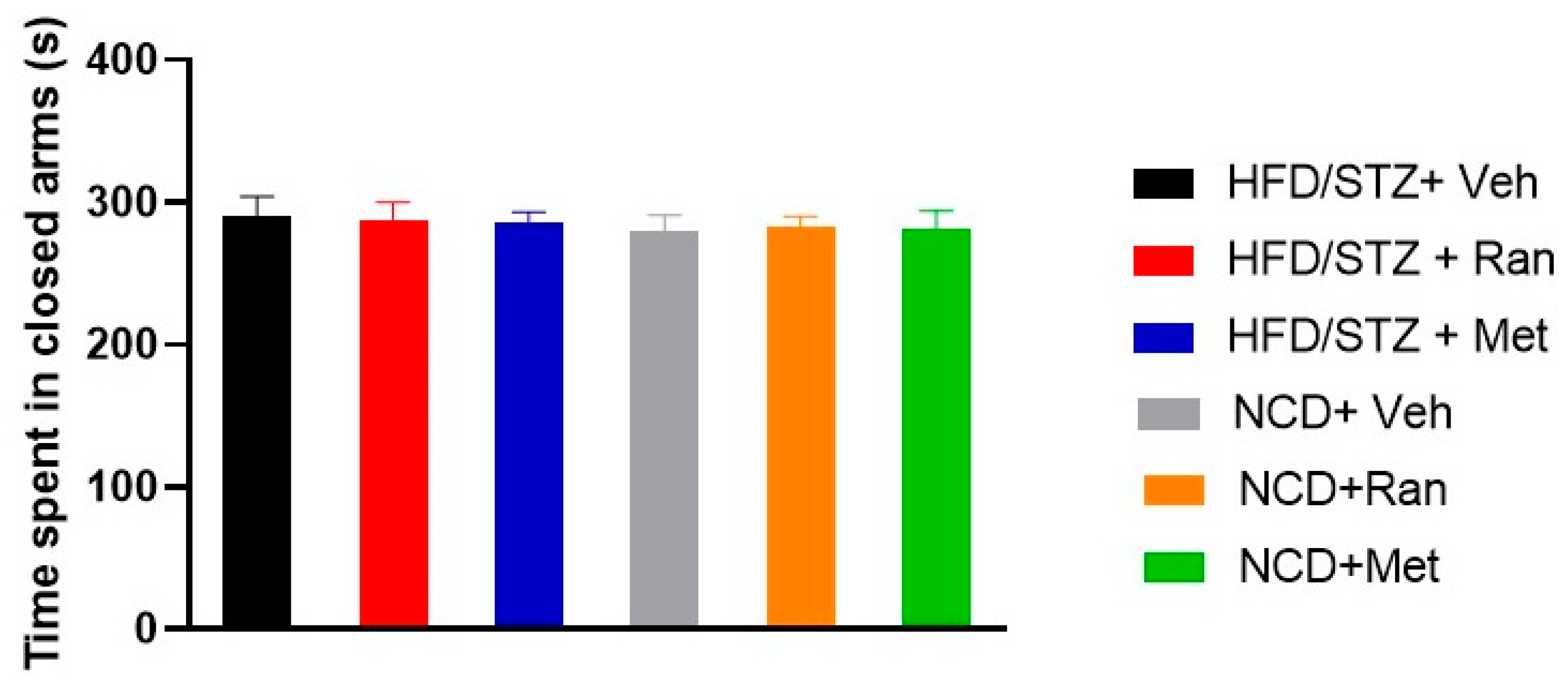
2.9. Elevate Plus Maze (EPM)
2.10. Statistical Analysis
3. Results
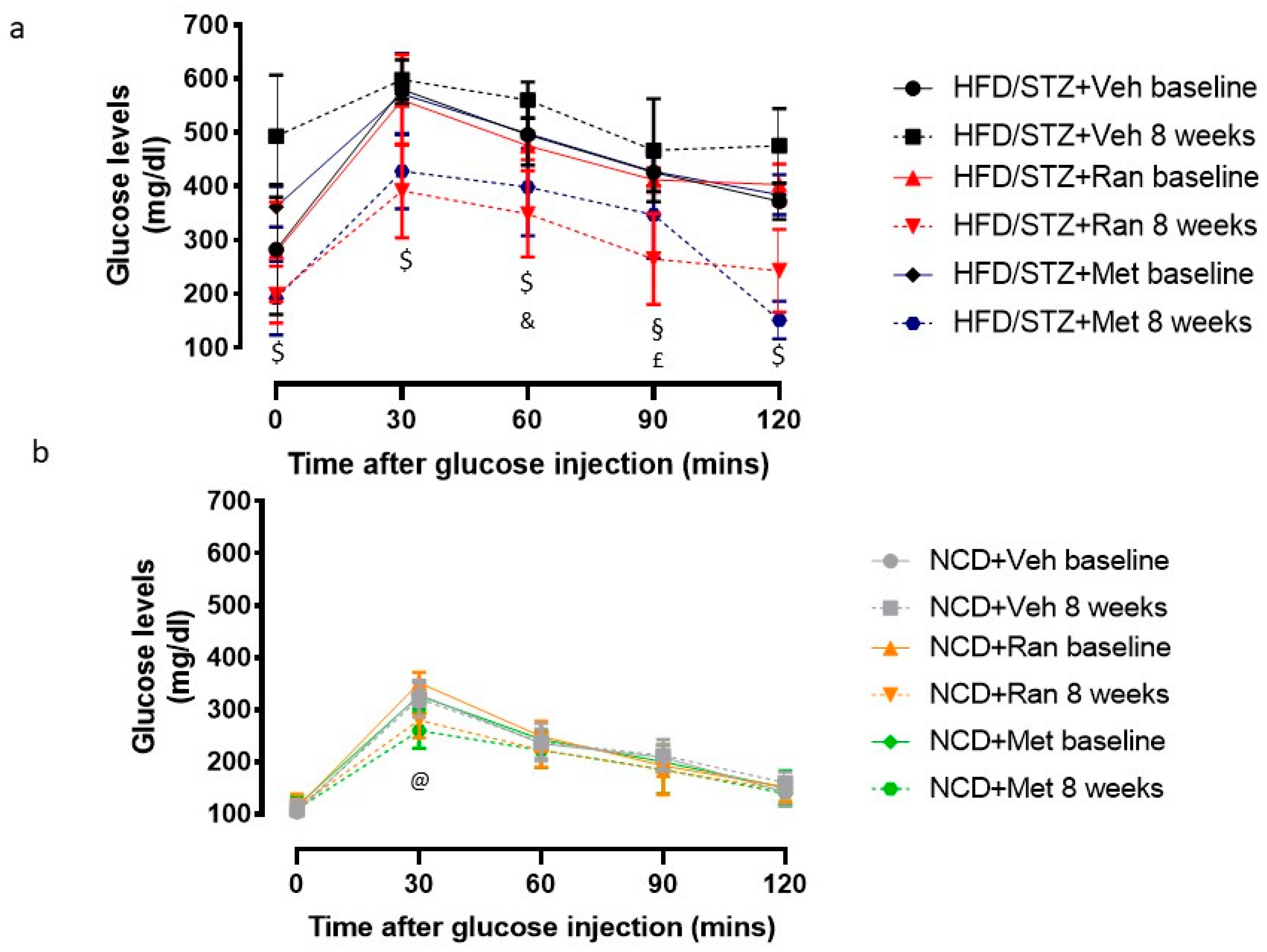
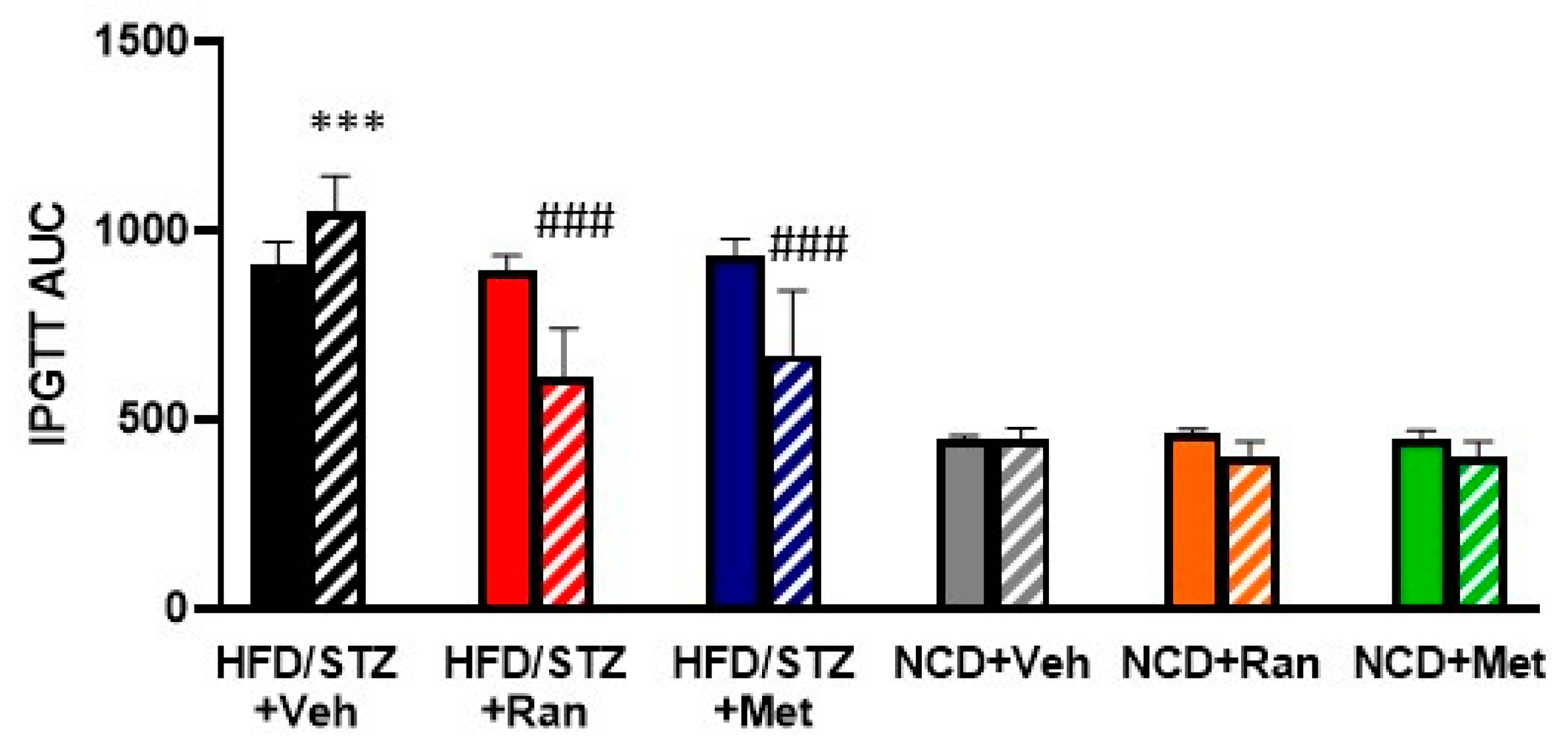
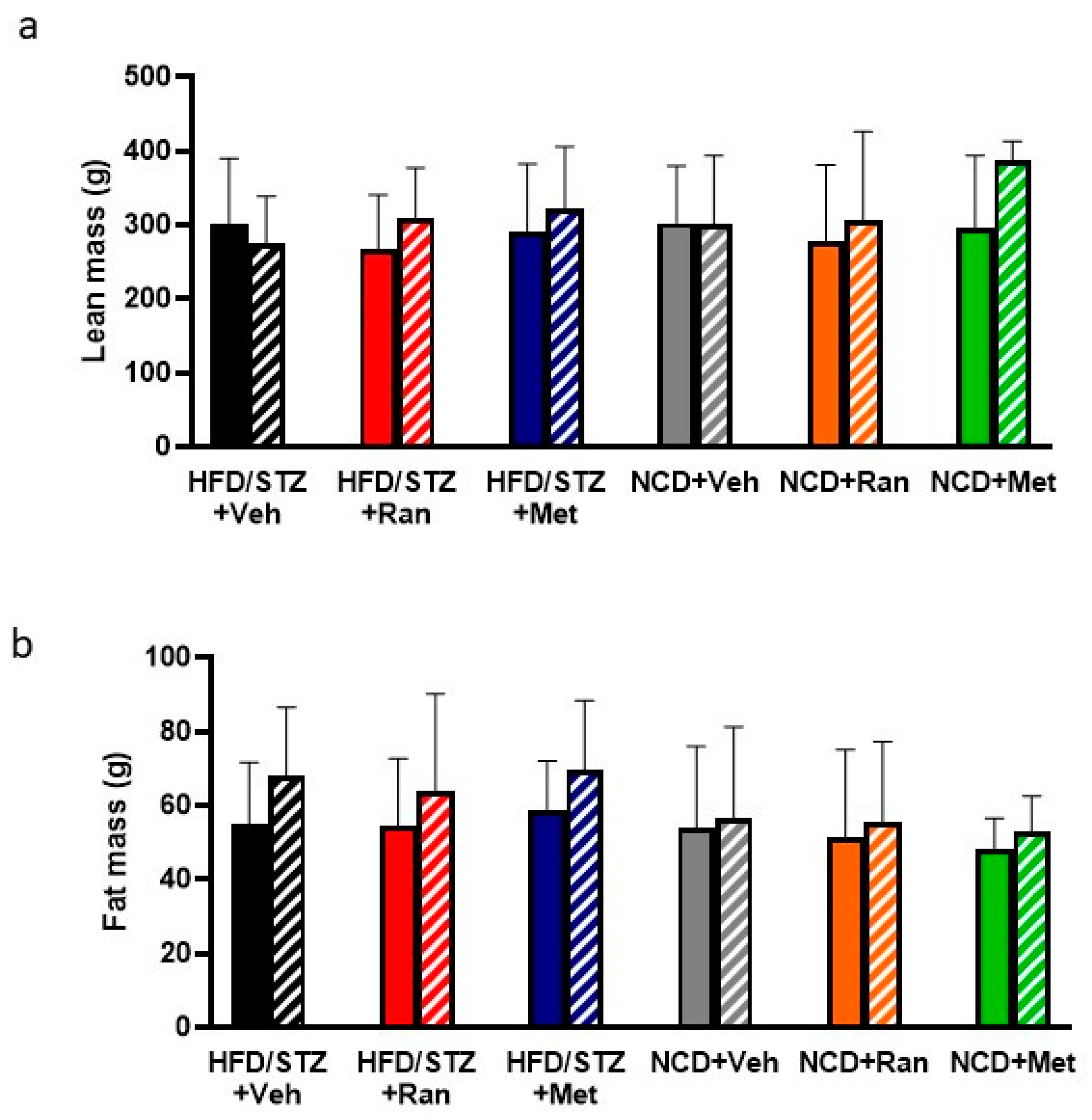
3.1. Blood Glucose Levels and Body Composition
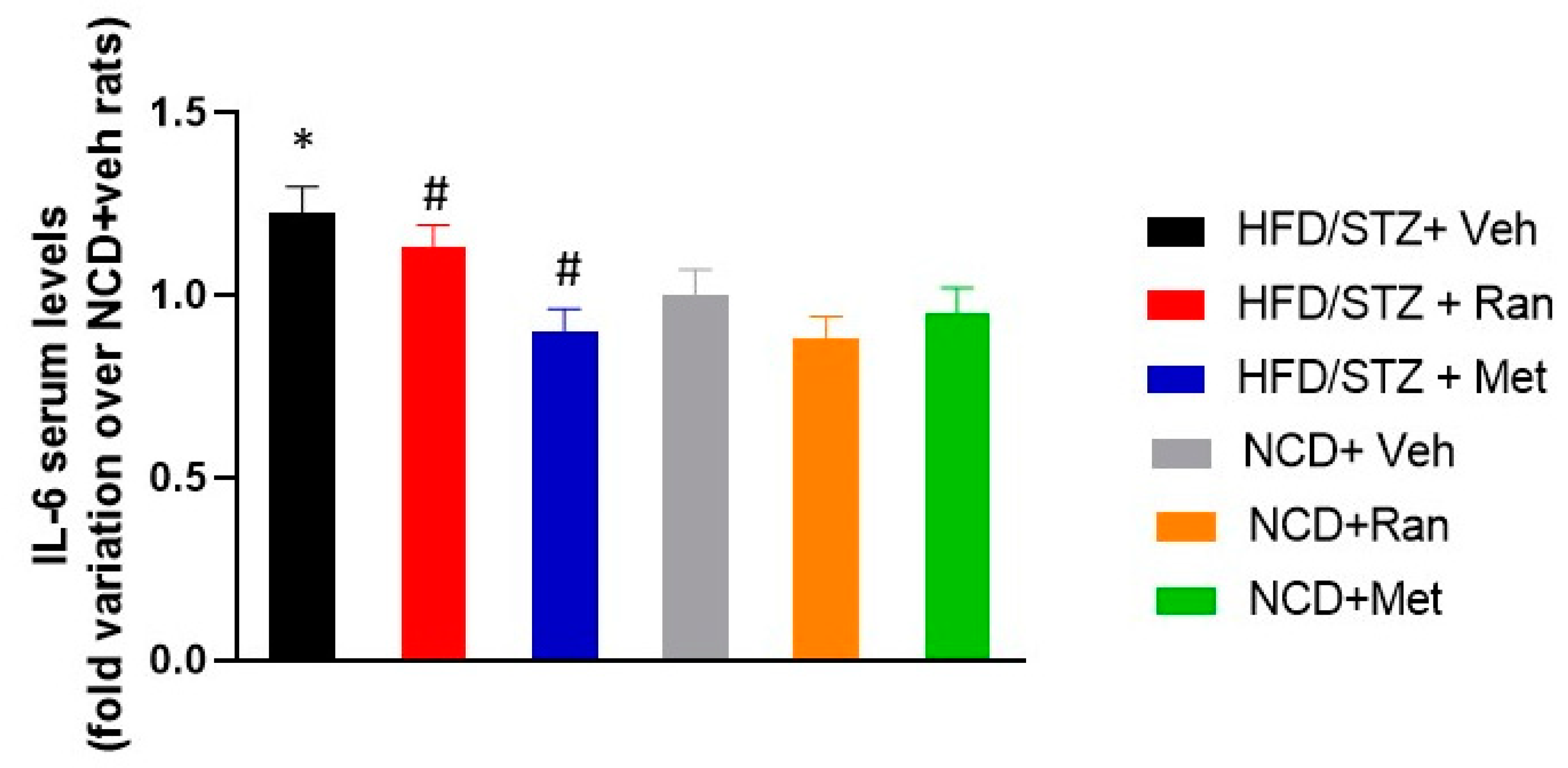
3.2. IL-6 Serum Levels
3.3. Passive Avoidance (PA)
3.4. Novel Object Recognition Test (nORT)
3.5. Forced Swimming Test (FST) and Elevated Plus Maze (EPM)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dharmadhikari, G.; Stolz, K.; Hauke, M.; Morgan, N.G.; Varki, A.; De Koning, E.; Kelm, S.; Maedler, K. Siglec-7 restores β-cell function and survival and reduces inflammation in pancreatic islets from patients with diabetes. Sci. Rep. 2017, 7, 45319. [Google Scholar] [CrossRef]
- Cannon, C.P. Mixed Dyslipidemia, Metabolic Syndrome, Diabetes Mellitus, and Cardiovascular Disease: Clinical Implications. Am. J. Cardiol. 2008, 102, 5L–9L. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Zhu, L.; Sha, L.; Yang, S.; Wei, J.; Ji, L.; Tang, X.; Mao, K.; Cao, L.; et al. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer’s disease: Possible mechanisms of IDE in T2D and AD. Biosci. Rep. 2018, 38, BSR20170862. [Google Scholar] [CrossRef]
- Geijselaers, S.L.C.; Sep, S.J.S.; Stehouwer, C.D.A.; Biessels, G.J. Glucose regulation, cognition, and brain MRI in type 2 diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015, 3, 75–89. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef]
- Biessels, G.J.; Whitmer, R. A Cognitive dysfunction in diabetes: How to implement emerging guidelines. Diabetologia 2020, 63, 3–9. [Google Scholar] [CrossRef]
- Biessels, G.J.; Verhagen, C.; Janssen, J.; Van den Berg, E.; Zinmann, B.; Rosenstock, J.; George, J.T.; Passera, A.; Schnaidt, S.; Johansen, O.E.; et al. Effect of Linagliptin on Cognitive Performance in Patients With Type 2 Diabetes and Cardiorenal Comorbidities: The CARMELINA Randomized Trial. Diabetes Care 2019, 42, 1930–1938. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Fraser, H. Inhibition of the late sodium current as a potential cardioprotective principle: Effects of the late sodium current inhibitor ranolazine. Hearth 2006, 92, iv6–iv14. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Schwarz, K.Q.; Rosero, S.; McNitt, S.; Robinson, J.L. Ranolazine Shortens Repolarization in Patients with Sustained Inward Sodium Current Due To Type-3 Long QT Syndrome J Cardiovasc Electrophysiol. 2008, 19, 1289–1293.
- Timmis, A.D.; Chaitman, B.R.; Crager, M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur. Heart J. 2006, 27, 42–48. [Google Scholar] [CrossRef]
- Morrow, D.A.; Scirica, B.M.; Chaitman, B.R.; McGuire, D.K.; Murphy, S.A.; Prokopczuk, E.K.; McCabe, C.H.; Braunwald, E. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009, 119, 2032–2039. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Yang, M.; Ning, Y.; Kahlig, K.M.; Krause, M.; Rajamani, S.; Belardinelli, L. Blockade of na+ channels in pancreatic α-cells has antidiabetic effects. Diabetes 2014, 63, 3545–3556. [Google Scholar] [CrossRef]
- Ning, Y.; Zhen, W.; Fu, Z.; Jiang, J.; Liu, D.; Belardinelli, L.; Dhalla, A.K. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J. Pharmacol. Exp. Ther. 2011, 337, 50–58. [Google Scholar] [CrossRef]
- Aldasoro, M.; Guerra-Ojeda, S.; Aguirre-Rueda, D.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Iradi, A.; Aldasoro, C.; Jorda, A.; Obrador, E.; et al. Effects of ranolazine on astrocytes and neurons in primary culture. PLoS ONE 2016, 11, e0150619. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Tallarico, M.; Iannone, M.; Fedosova, E.; Nesci, V.; De Sarro, G.; Sarkisova, K.; Russo, E. Cognitive impairment in the WAG/Rij rat absence model is secondary to absence seizures and depressive-like behavior. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2019, 94, 109652. [Google Scholar] [CrossRef]
- Citraro, R.; Gallelli, L.; Leo, A.; De Fazio, P.; Gallelli, P.; Russo, E.; De Sarro, G. Effects of chronic sodium alendronate on depression and anxiety in a menopausal experimental model. Pharmacol. Biochem. Behav. 2015, 129, 65–71. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Mourouzis, I.; Mantzouratou, P.; Galanopoulos, G.; Kostakou, E.; Dhalla, A.K.; Belardinelli, L.; Pantos, C. The beneficial effects of ranolazine on cardiac function after myocardial infarction are greater in diabetic than in nondiabetic rats. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 457–469. [Google Scholar] [CrossRef]
- Katakam, P.V.G.; Ujhelyi, M.R.; Hoenig, M.; Miller, A.W. Metformin Improves Vascular Function in Insulin-Resistant Rats. Hypertension 2000, 35, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Zahid, S. Alterations in adult hippocampal neurogenesis, aberrant protein s-nitrosylation, and associated spatial memory loss in streptozotocin-induced diabetes mellitus type 2 mice. Iran. J. Basic Med. Sci. 2017, 20, 1159–1165. [Google Scholar]
- Palleria, C.; Leo, A.; Andreozzi, F.; Citraro, R.; Iannone, M.; Spiga, R.; Sesti, G.; Constanti, A.; De Sarro, G.; Arturi, F.; et al. Liraglutide prevents cognitive decline in a rat model of streptozotocin-induced diabetes independently from its peripheral metabolic effects. Behav. Brain Res. 2017, 321, 157–169. [Google Scholar] [CrossRef]
- Blumenfeld, H.; Klein, J.P.J.P.; Schridde, U.; Vestal, M.; Rice, T.; Khera, D.S.D.S.; Bashyal, C.; Giblin, K.; Paul-Laughinghouse, C.; Wang, F.; et al. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 2008, 49, 400–409. [Google Scholar] [CrossRef]
- Leo, A.; De Caro, C.; Nesci, V.; Palma, E.; Tallarico, M.; Iannone, M.; Constanti, A.; De Sarro, G.; Russo, E.; Citraro, R. Antiepileptogenic effects of Ethosuximide and Levetiracetam in WAG/Rij rats are only temporary. Pharmacol. Reports 2019, 71, 833–838. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Amodio, N.; De Sarro, C.; Gallo Cantafio, M.E.; Constanti, A.; De Sarro, G.; Russo, E. Fingolimod Exerts only Temporary Antiepileptogenic Effects but Longer-Lasting Positive Effects on Behavior in the WAG/Rij Rat Absence Epilepsy Model. Neurotherapeutics 2017, 14, 1134–1147. [Google Scholar] [CrossRef]
- Zovkic, I.B.; Sweatt, J.D. Epigenetic mechanisms in learned fear: Implications for PTSD. Neuropsychopharmacology 2013, 38, 77–93. [Google Scholar] [CrossRef]
- Citraro, R.; Leo, A.; Franco, V.; Marchiselli, R.; Perucca, E.; De Sarro, G.; Russo, E. Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive-like behavior. Epilepsia 2017, 58, 231–238. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Bartolini, L.; Casamenti, F.; Pepeu, G. Aniracetam restores object recognition impaired by age, scopolamine, and nucleus basalis lesions. Pharmacol. Biochem. Behav. 1996, 53, 277–283. [Google Scholar] [CrossRef]
- Rispoli, V.; Ragusa, S.; Nisticò, R.; Marra, R.; Russo, E.; Leo, A.; Felicitá, V.; Rotiroti, D.; Nistico, R.; Marra, R.; et al. Huperzine a restores cortico-hippocampal functional connectivity after bilateral AMPA lesion of the nucleus basalis of meynert. J. Alzheimers Dis. 2013, 35, 833–846. [Google Scholar] [CrossRef]
- Bogdanova, O.V.; Kanekar, S.; D’Anci, K.E.; Renshaw, P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013, 118, 227–239. [Google Scholar] [CrossRef]
- Citraro, R.; Leo, A.; De Fazio, P.; De Sarro, G.; Russo, E. Antidepressants but not antipsychotics have antiepileptogenic effects with limited effects on comorbid depressive-like behaviour in the WAG/Rij rat model of absence epilepsy. Br. J. Pharmacol. 2015, 172, 3177–3188. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Scirica, B.M.; Morrow, D.A.; Hod, H.; Murphy, S.A.; Belardinelli, L.; Hedgepeth, C.M.; Molhoek, P.; Verheugt, F.W.A.; Gersh, B.J.; McCabe, C.H.; et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non-ST-segment-elevation acute coronary syndrome. Circulation 2007, 116, 1647–1652. [Google Scholar] [CrossRef]
- Chisholm, J.W.; Goldfine, A.B.; Dhalla, A.K.; Braunwald, E.; Morrow, D.A.; Karwatowska-Prokopczuk, E.; Belardinelli, L. Effect of ranolazine on A1C and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care 2010, 33, 1163–1168. [Google Scholar] [CrossRef]
- Terruzzi, I.; Montesano, A.; Pamela, S.; Vacante, F.; Benedini, S.; Luzzi, L. Ranolazine promotes muscle differentiation and reduces oxidative stress in C2C12 skeletal muscle cells. Endocrine 2017, 58, 33–45. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Campbell, J.M.; Stephenson, M.D.; de Courten, B.; Chapman, I.; Bellman, S.M.; Aromataris, E. Metformin and Alzheimer’s disease, dementia and cognitive impairment: A systematic review protocol. JBI database Syst. Rev. Implement. reports 2017, 15, 2055–2059. [Google Scholar] [CrossRef]
- De Oliveira, W.H.; De Santana Nunes, A.K.; De França, M.E.R.; Dos Santos, L.A.; Lós, D.B.; Rocha, S.W.S.; De Sousa Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Morshedi, M.; Valenlia, K.B.; Hosseinifard, E.S.; Shahabi, P.; Abbasi, M.M.; Ghorbani, M.; Barzegari, A.; Sadigh-Eteghad, S.; Saghafi-Asl, M. Beneficial psychological effects of novel psychobiotics in diabetic rats: The interaction among the gut, blood, and amygdala. J. Nutr. Biochem. 2018, 57, 145–152. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassano, V.; Leo, A.; Tallarico, M.; Nesci, V.; Cimellaro, A.; Fiorentino, T.V.; Citraro, R.; Hribal, M.L.; De Sarro, G.; Perticone, F.; et al. Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model. Nutrients 2020, 12, 382. https://doi.org/10.3390/nu12020382
Cassano V, Leo A, Tallarico M, Nesci V, Cimellaro A, Fiorentino TV, Citraro R, Hribal ML, De Sarro G, Perticone F, et al. Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model. Nutrients. 2020; 12(2):382. https://doi.org/10.3390/nu12020382
Chicago/Turabian StyleCassano, Velia, Antonio Leo, Martina Tallarico, Valentina Nesci, Antonio Cimellaro, Teresa Vanessa Fiorentino, Rita Citraro, Marta Letizia Hribal, Giovambattista De Sarro, Francesco Perticone, and et al. 2020. "Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model" Nutrients 12, no. 2: 382. https://doi.org/10.3390/nu12020382
APA StyleCassano, V., Leo, A., Tallarico, M., Nesci, V., Cimellaro, A., Fiorentino, T. V., Citraro, R., Hribal, M. L., De Sarro, G., Perticone, F., Sesti, G., Russo, E., & Sciacqua, A. (2020). Metabolic and Cognitive Effects of Ranolazine in Type 2 Diabetes Mellitus: Data from an in vivo Model. Nutrients, 12(2), 382. https://doi.org/10.3390/nu12020382





