Glycemic Variability and CNS Inflammation: Reviewing the Connection
Abstract
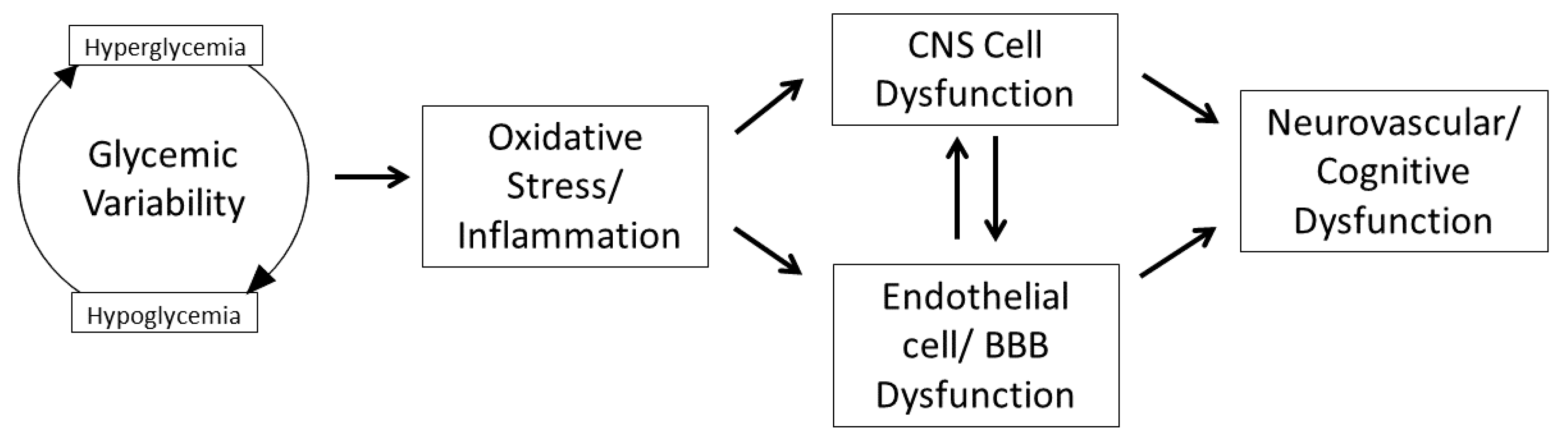
:1. Introduction
2. Glycemic Variability, Oxidative Stress, and Inflammation
3. Impact of GV on Central Nervous System Inflammation
3.1. GV on Endothelium and Blood–Brain Barrier
3.2. GV and Microglia, Neuronal, and Astroglial Cells
3.3. GV and the Human Brain
4. Strategies to Minimize GV
4.1. Therapeutic Interventions to Minimize GV
4.2. Dietary Interventions to Minimize GV
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman: Basingstoke, UK, 2012. [Google Scholar]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikołajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Isaev, N.K.; Stel’mashuk, E.V.; Zorov, D.B. Cellular mechanisms of brain hypoglycemia. Biochemistry 2007, 72, 471–478. [Google Scholar] [CrossRef]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Zhao, S.; Brock, G.; Matsouaka, R.A.; Kline, D.; Joseph, J.J. Visit-to-Visit Glycemic Variability and Risks of Cardiovascular Events and All-Cause Mortality: The ALLHAT Study. Diabetes Care 2019, 42, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.J.; Schwenke, D.C.; Bahn, G.; Reaven, P. Glycemic Variation and Cardiovascular Risk in the Veterans Affairs Diabetes Trial. Diabetes Care 2018, 41, 2187–2194. [Google Scholar] [CrossRef] [Green Version]
- Gross, T.M.; Bode, B.W.; Einhorn, D.; Kayne, D.M.; Reed, J.H.; White, N.H.; Mastrototaro, J.J. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol. Ther. 2000, 2, 49–56. [Google Scholar] [CrossRef]
- Rodbard, D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes Technol. Ther. 2009, 11 (Suppl. 1), S55–S67. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Kovatchev, B.P. Glycemic Variability: How to Measure and Its Clinical Implication for Type 2 Diabetes. Am. J. Med. Sci. 2018, 356, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [PubMed]
- Anonymous. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Bunn, H.F.; Haney, D.N.; Kamin, S.; Gabbay, K.H.; Gallop, P.M. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Investig. 1976, 57, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Kohnert, K.D.; Heinke, P.; Vogt, L.; Zander, E.; Fritzsche, G.; Augstein, P.; Salzsieder, E. Reduced Glucose Variability Is Associated With Improved Quality of Glycemic Control in Patients With Type 2 Diabetes: A 12-Month Observational Study. J. Endocrinol. Metab. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gutierrez, R.; Gonzalez-Gonzalez, J.G.; A Zuñiga-Hernandez, J.; McCoy, R.G. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 2019, 367, l5887. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Jung, H.S.; Hwang-Bo, Y.; Cho, S.W.; Jang, H.C.; Kim, S.Y.; Park, K.S. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2013, 50, 505–510. [Google Scholar] [CrossRef]
- Chan, C.L.; Pyle, L.; Kelsey, M.M.; Newnes, L.; Baumgartner, A.; Zeitler, P.S.; Nadeau, K.J. Alternate glycemic markers reflect glycemic variability in continuous glucose monitoring in youth with prediabetes and type 2 diabetes. Pediatr. Diabetes 2017, 18, 629–636. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Sango, K.; Suzuki, T.; Yanagisawa, H.; Takaku, S.; Hirooka, H.; Tamura, M.; Watabe, K. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J. Neurochem. 2006, 98, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Chung, S.S. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999, 13, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horal, M.; Zhang, Z.; Stanton, R.; Virkamäki, A.; Loeken, M.R. Activation of the hexosamine pathway causes oxidative stress and abnormal embryo gene expression: Involvement in diabetic teratogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kolm-Litty, V.; Sauer, U.; Nerlich, A.; Lehmann, R.; Schleicher, E.D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Investig. 1998, 101, 160–169. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Pragada, R.R.; Nammi, S. Attenuation of Glucose-Induced Myoglobin Glycation and the Formation of Advanced Glycation End Products (AGEs) by (R)-α-Lipoic Acid In Vitro. Biomolecules 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. Chemical modification of proteins by methylglyoxal. Cell. Mol. Biol. 1998, 44, 1139–1145. [Google Scholar]
- Miele, C.; Paturzo, F.; Teperino, R.; Sakane, F.; Fiory, F.; Oriente, F.; Ungaro, P.; Valentino, R.; Beguinot, F.; Formisano, P. Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J. Biol. Chem. 2007, 282, 31835–31843. [Google Scholar] [CrossRef] [Green Version]
- Koya, D.; King, G.L. Protein kinase C activation and the development of diabetic complications. Diabetes 1998, 47, 859–866. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Sharma, M.L.; Gulati, G.; Chhabra, A.; Kaushik, R.; Sharma, P.; Kaur, G. Effect of starvation and insulin-induced hypoglycemia on oxidative stress scavenger system and electron transport chain complexes from rat brain, liver, and kidney. Mol. Chem. Neuropathol. 1998, 34, 157–168. [Google Scholar] [CrossRef]
- Cardoso, S.; Santos, M.S.; Seiça, R.; Moreira, P.I. Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim. Biophys. Acta 2010, 1802, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Santos, R.X.; Correia, S.C.; Carvalho, C.; Santos, M.S.; Baldeiras, I.; Oliveira, C.R.; Moreira, P.I. Insulin-induced recurrent hypoglycemia exacerbates diabetic brain mitochondrial dysfunction and oxidative imbalance. Neurobiol. Dis. 2013, 49, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Gallagher, J.R.; Dinkova-Kostova, A.T.; Hayes, J.D.; Sharkey, J.; Ashford, M.L.J.; McCrimmon, R.J. Nrf2-Mediated Neuroprotection Against Recurrent Hypoglycemia Is Insufficient to Prevent Cognitive Impairment in a Rodent Model of Type 1 Diabetes. Diabetes 2016, 65, 3151–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Graumann, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef] [Green Version]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic variability in diabetes: Clinical and therapeutic implications. Lancet Diabetes Endocrinol. 2019, 7, 221–230. [Google Scholar] [CrossRef] [Green Version]
- The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995, 44, 968–983. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Brownlee, M. Should minimal blood glucose variability become the gold standard of glycemic control? J. Diabetes Its Complicat. 2005, 19, 178–181. [Google Scholar] [CrossRef]
- Hirsch, I.B. Glycemic variability: It’s not just about A1C anymore! Diabetes Technol. Ther. 2005, 7, 780–783. [Google Scholar] [CrossRef]
- Wentholt, I.M.E.; Kulik, W.; Michels, R.P.J.; Hoekstra, J.B.L.; Devries, J.H. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia 2008, 51, 183. [Google Scholar] [CrossRef] [Green Version]
- Siegelaar, S.E.; Barwari, T.; Kulik, W.; Hoekstra, J.B.; Devries, J.H. No relevant relationship between glucose variability and oxidative stress in well-regulated type 2 diabetes patients. J. Diabetes Sci. Technol. 2011, 5, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohata, Y.; Ohara, M.; Nagaike, H.; Fujikawa, T.; Osaka, N.; Goto, S.; Fukase, A.; Kushima, H.; Hiromura, M.; Terasaki, M.; et al. Association of Hemoglobin A1c, 1,5-Anhydro-d-Glucitol and Glycated Albumin with Oxidative Stress in Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study. Diabetes Ther. 2020, 11, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Ohara, M.; Nagaike, H.; Goto, S.; Fukase, A.; Tanabe, Y.; Tomoyasu, M.; Yamamoto, T.; Hayashi, T.; Fukui, T.; Hirano, T. Improvements of ambient hyperglycemia and glycemic variability are associated with reduction in oxidative stress for patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 139, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalysnyk, L.; Hernandez-Medina, M.; Krishnarajah, G. Glycaemic variability and complications in patients with diabetes mellitus: Evidence from a systematic review of the literature. Diabetes Obes. Metab. 2010, 12, 288–298. [Google Scholar] [CrossRef]
- Siegelaar, S.E.; Holleman, F.; Hoekstra, J.B.L.; Devries, J.H. Glucose variability; does it matter? Endocr. Rev. 2010, 31, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [Green Version]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.L.; Sherwin, R.S. Blood glucose and the brain in diabetes: Between a rock and a hard place? Curr. Diabetes Rep. 2002, 2, 101–102. [Google Scholar] [CrossRef]
- Hayden, M.R. Type 2 Diabetes Mellitus Increases the Risk of Late-Onset Alzheimer’s Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Machida, T.; Takata, F.; Matsumoto, J.; Miyamura, T.; Hirata, R.; Kimura, I.; Kataoka, Y.; Dohgu, S.; Yamauchi, A. Contribution of thrombin-reactive brain pericytes to blood-brain barrier dysfunction in an in vivo mouse model of obesity-associated diabetes and an in vitro rat model. PLoS ONE 2017, 12, e0177447. [Google Scholar] [CrossRef] [Green Version]
- Yatomi, Y.; Tanaka, R.; Shimada, Y.; Yamashiro, K.; Liu, M.; Mitome-Mishima, Y.; Miyamoto, N.; Ueno, Y.; Urabe, T.; Hattori, N. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience 2015, 289, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, Z.; Lin, X.; Wu, X.; Chen, X.; Bai, X.; Zhuang, Y.; Yang, Y.; Zhang, J. Overexpression of miR-146a Might Regulate Polarization Transitions of BV-2 Cells Induced by High Glucose and Glucose Fluctuations. Front. Endocrinol. 2019, 10, 719. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, B.T.; Lundeen, T.F.; Norwood, K.M.; Brooks, H.L.; Egleton, R.D. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 2007, 50, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, B.T.; Lundeen, T.F.; Norwood, K.M.; Brooks, H.L.; Egleton, R.D. Blood–Brain Barrier Disruption and Neurovascular Unit Dysfunction in Diabetic Mice: Protection with the Mitochondrial Carbonic Anhydrase Inhibitor Topiramate. J. Pharmacol. Exp. Ther. 2016, 359, 452. [Google Scholar]
- Halvorson, B.D.; Whitehead, S.N.; McGuire, J.J.; Wiseman, R.W.; Frisbee, J.C. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R149–R159. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; RMaloney, E.; Aw, T.Y. High glucose, glucose fluctuation and carbonyl stress enhance brain microvascular endothelial barrier dysfunction: Implications for diabetic cerebral microvasculature. Redox Biol. 2015, 5, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Sajja, R.K.; Cucullo, L. Altered glycaemia differentially modulates efflux transporter expression and activity in hCMEC/D3 cell line. Neurosci. Lett. 2015, 598, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Osta, A.; Brasacchio, D.; Yao, D.; Pocai, A.; Jones, P.L.; Roeder, R.G.; Cooper, M.E.; Brownlee, M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 2008, 205, 2409–2417. [Google Scholar] [CrossRef]
- Schisano, B.; Tripathi, G.; McGee, K.; McTernan, P.G.; Ceriello, A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia 2011, 54, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Risso, A.; Mercuri, F.; Quagliaro, L.; Damante, G.; Ceriello, A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E924–E930. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Appel, N.M.; Hokari, M.; Oki, J.; Holman, G.D.; Maher, F.; Koehler-Stec, E.M.; Vannucci, S.J.; Smith, Q.R. Blood—Brain Barrier Glucose Transporter: Effects of Hypo-and Hyperglycemia Revisited. J. Neurochem. 1999, 72, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.K.; Kang, Y.S.; Boado, R.J.; Pardridge, W.M. Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 1995, 44, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Duelli, R.; Maurer, M.H.; Staudt, R.; Heiland, S.; Duembgen, L.; Kuschinsky, W. Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res. 2000, 858, 338–347. [Google Scholar] [CrossRef]
- Hwang, J.J.; Jiang, L.; Hamza, M.; Rangel, E.S.; Dai, F.; Belfort-DeAguiar, R.; Parikh, L.; Koo, B.B.; Rothman, D.L.; Mason, G.; et al. Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2017, 2, e95913. [Google Scholar] [CrossRef] [Green Version]
- Seaquist, E.R.; Tkáč, I.; Damberg, G.; Thomas, W.; Gruetter, R. Brain glucose concentrations in poorly controlled diabetes mellitus as measured by high-field magnetic resonance spectroscopy. Metabolism 2005, 54, 1008–1013. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.J.; Jiang, L.; Rangel, E.S.; Fan, X.; Ding, Y.; Lam, W.; Leventhal, J.; Dai, F.; Rothman, D.L.; Mason, G.F.; et al. Glycemic Variability and Brain Glucose Levels in Type 1 Diabetes. Diabetes 2019, 68, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Esposito, K.; Ihnat, M.; Thorpe, J.; Giugliano, D. Effect of acute hyperglycaemia, long-term glycaemic control and insulin on endothelial dysfunction and inflammation in Type 1 diabetic patients with different characteristics. Diabet. Med. 2010, 27, 911–917. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Novials, A.; Ortega, E.; La Sala, L.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Esposito, K.; Giugliano, D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012, 61, 2993–2997. [Google Scholar] [CrossRef] [Green Version]
- Costantino, S.; Paneni, F.; Battista, R.; Castello, L.; Capretti, G.; Chiandotto, S.; Tanese, L.; Russo, G.; Pitocco, D.; Lanza, G.A.; et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017, 66, 2472–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiorino, M.I.; Casciano, O.; Della Volpe, E.; Bellastella, G.; Giugliano, D.; Esposito, K. Reducing glucose variability with continuous subcutaneous insulin infusion increases endothelial progenitor cells in type 1 diabetes: An observational study. Endocrine 2016, 52, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Gross, C.T. Microglia in development: Linking brain wiring to brain environment. Neuron Glia Biol. 2011, 7, 77–83. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef] [Green Version]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- van Gool, W.A.; van de Beek, D.; Eikelenboom, P. Systemic infection and delirium: When cytokines and acetylcholine collide. Lancet 2010, 375, 773–775. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- McNally, L.; Bhagwagar, Z.; Hannestad, J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008, 13, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, T.; Bazov, I.; Watanabe, H.; Hauser, K.F.; Bakalkin, G. Transcriptional control of maladaptive and protective responses in alcoholics: A role of the NF-κB system. Brain Behav. Immun. 2011, 25 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wu, S.; Hong, Z.; Chen, X.; Shan, X.; Fischbach, S.; Xiao, X. Chronic hyperglycemia regulates microglia polarization through ERK5. Aging 2019, 11, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-F.; Liu, C.-K.; Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.C.; Higgins, S.; Werther, G.A.; Cameron, F. Effects of fluctuating glucose levels on neuronal cells in vitro. Neurochem. Res. 2012, 37, 1768–1782. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; De Assis, A.M.; Gonçalves, C.-A.; Souza, D.O. Fluctuations in glucose levels induce glial toxicity with glutamatergic, oxidative and inflammatory implications. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Deng, J.; Chen, L.; Ding, K.; Wang, Y. Acute glucose fluctuation induces inflammation and neurons apoptosis in hippocampal tissues of diabetic rats. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Wang, H.; Deng, J.; Chen, L.; Ding, K.; Wang, Y. The mechanisms of glycemic variability accelerate diabetic central neuropathy and diabetic peripheral neuropathy in diabetic rats. Biochem. Biophys. Res. Commun. 2019, 510, 35–41. [Google Scholar]
- Minami, T.; Ito, Y.; Yamada, M.; Furuta, R.; Minagawa, F.; Kamata, K.; Kameda, A.; Terauchi, Y. The effect of long-term past glycemic control on executive function among patients with type 2 diabetes mellitus. Diabetol. Int. 2020, 11, 114–120. [Google Scholar] [CrossRef]
- Kim, C.; Sohn, J.-H.; Jang, M.U.; Kim, S.-H.; Choi, M.-G.; Ryu, O.-H.; Lee, S.; Choi, H.-C. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: A cross-sectional study. PLoS ONE 2015, 10, e0132118. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Abduljalil, A.; Manor, B.D.; Peng, C.-K.; Novak, V. Multi-scale glycemic variability: A link to gray matter atrophy and cognitive decline in type 2 diabetes. PLoS ONE 2014, 9, e86284. [Google Scholar] [CrossRef] [PubMed]
- Akrivos, J.; Ravona-Springer, R.; Schmeidler, J.; Leroith, D.; Heymann, A.; Preiss, R.; Hoffman, H.; Koifman, K.; Silverman, J.M.; Beeri, M.S. Glycemic control, inflammation, and cognitive function in older patients with type 2 diabetes. Int. J. Geriatr. Psychiatry 2015, 30, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.-C.; Yang, C.-P.; Tseng, S.-T.; Li, C.-I.; Liu, C.-S.; Lin, W.-Y.; Hwang, K.-L.; Yang, S.-Y.; Chiang, J.-H.; Lin, C. Visit-to-Visit Variations in Fasting Plasma Glucose and HbA(1c) Associated With an Increased Risk of Alzheimer Disease: Taiwan Diabetes Study. Diabetes Care 2017, 40, 1210–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Luo, Y.; Chen, Y.-C.; Chen, H.; Ma, J.; Yin, X. Glucose Fluctuations Are Linked to Disrupted Brain Functional Architecture and Cognitive Impairment. J. Alzheimer’s Dis. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.M.; Sharrett, A.R.; Mosley, T.H.; Ballew, S.H.; Deal, J.A.; Selvin, E. Glucose Peaks and the Risk of Dementia and 20-Year Cognitive Decline. Diabetes Care 2017, 40, 879–886. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Ryder, A.G.; Li, S.; Liu, W.; Zhu, X. Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: A meta-analysis. J. Diabetes Investig. 2018, 9, 1342–1353. [Google Scholar] [CrossRef]
- Chaytor, N.S.; Barbosa-Leiker, C.; Ryan, C.M.; Germine, L.T.; Hirsch, I.B.; Weinstock, R.S. Clinically significant cognitive impairment in older adults with type 1 diabetes. J. Diabetes Its Complicat. 2019, 33, 91–97. [Google Scholar] [CrossRef]
- Knight, M.F.; Perfect, M.M. Glycemic control influences on academic performance in youth with Type 1 diabetes. Sch. Psychol. 2019, 34, 646. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, Y.; Li, D.; Wu, M.-Y.; Tang, M.-L.; Wang, J.; Chen, K. Association between visit-to-visit variability of HbA 1c and cognitive decline: A pooled analysis of two prospective population-based cohorts. Diabetologia 2020, 63, 85–94. [Google Scholar] [CrossRef]
- Bancks, M.; Carnethon, M.R.; Jacobs, D.R.; Launer, L.J.; Reis, J.; Schreiner, P.J.; Shah, R.V.; Sidney, S.; Yaffe, K.; Yano, Y.; et al. Fasting Glucose Variability in Young Adulthood and Cognitive Function in Middle Age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care 2018, 41, 2579–2585. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Iwahashi, N.; Kirigaya, J.; Kataoka, S.; Minamimoto, Y.; Gohbara, M.; Abe, T.; Okada, K.; Matsuzawa, Y.; Konishi, M.; et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc. Diabetol. 2018, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Darier, R.; Montaudon, M.; Beauvieux, M.-C.; Coffin-Boutreux, C.; Coste, P.; Douard, H.; Ouattara, A.; Catargi, B. Glycemic Variability Is a Powerful Independent Predictive Factor of Midterm Major Adverse Cardiac Events in Patients With Diabetes With Acute Coronary Syndrome. Diabetes Care 2019, 42, 674–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara-Lemarroy, C.; González-Moreno, E.; Garza-Villarreal, E.; Treviño-Herrera, A.; Tena-Montiel, R.; Muruet, W.; Rivera, J.G. Glycemic Variability and Functional Outcome after Acute Ischemic Stroke (P1.191); AAN Enterprises: Minneapolis, MN, USA, 2016. [Google Scholar]
- Lim, J.-S.; Kim, C.; Oh, M.S.; Lee, J.-H.; Jung, S.; Sohn, J.-H.; Lee, S.-H.; Kim, Y.J.; Kim, Y.; Suh, S.W.; et al. Effects of glycemic variability and hyperglycemia in acute ischemic stroke on post-stroke cognitive impairments. J. Diabetes Its Complicat. 2018, 32, 682–687. [Google Scholar] [CrossRef]
- Wada, S.; Yoshimura, S.; Inoue, M.; Matsuki, T.; Arihiro, S.; Koga, M.; Kitazono, T.; Makino, H.; Hosoda, K.; Ihara, M.; et al. Outcome Prediction in Acute Stroke Patients by Continuous Glucose Monitoring. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, K.; Peng, M.; Velasco, C.; Schaefer, E.; Diaz-Arrastia, R.; Frankel, H. Glucose variability negatively impacts long-term functional outcome in patients with traumatic brain injury. J. Crit. Care 2012, 27, 125–131. [Google Scholar] [CrossRef]
- Robbins, N.M.; Swanson, R.A. Opposing effects of glucose on stroke and reperfusion injury: Acidosis, oxidative stress, and energy metabolism. Stroke 2014, 45, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- ClinicalTrials.gov. U.S. National Library of Medicine. Available online: https://www.clinicaltrials.gov. (accessed on 19 December 2020).
- Ceriello, A. Glycemic variability, persistent oxidative stress, and diabetic complications. Medicographa 2017, 39, 233–236. [Google Scholar]
- Ceriello, A. Glucose variability and diabetic complications: Is it time to treat? Diabetes Care 2020, 43, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- El-Laboudi, A.H.; Godsland, I.F.; Johnston, D.G.; Oliver, N. Measures of Glycemic Variability in Type 1 Diabetes and the Effect of Real-Time Continuous Glucose Monitoring. Diabetes Technol. Ther. 2016, 18, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Antuna, R.; Geelhoed-Duijvestijn, P.; Kröger, J.; Weitgasser, R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: A multicentre, non-masked, randomised controlled trial. Lancet 2016, 388, 2254–2263. [Google Scholar] [CrossRef]
- Bruttomesso, D.; Crazzolara, D.; Maran, A.; Costa, S.; Pos, M.D.; Girelli, A.; Lepore, G.; Aragona, M.; Iori, E.; Valentini, U.; et al. In Type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet. Med. 2008, 25, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Yao, M.-Y.; Ma, J.-X.; Xue, P.; Li, Y.-K. Continuous subcutaneous insulin infusion combined with liraglutide reduced glycemic variability and oxidative stress in type 2 diabetes mellitus: A study based on the flash glucose monitoring system. Endocr. J. 2019, 66, 871–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, M.R.; Barbieri, M.; Marfella, R.; Paolisso, G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: Role of dipeptidyl peptidase-IV inhibition. Diabetes Care 2012, 35, 2076–2082. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Giugliano, D.; Nappo, F.; Marfella, R. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004, 110, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kodani, N.; Saisho, Y.; Tanaka, K.; Kawai, T.; Itoh, H. Effects of mitiglinide, a short-acting insulin secretagogue, on daily glycemic variability and oxidative stress markers in Japanese patients with type 2 diabetes mellitus. Clin. Drug Investig. 2013, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Sheard, N.F.; Clark, N.G.; Brand-Miller, J.C.; Franz, M.J.; Pi-Sunyer, F.X.; Mayer-Davis, E.; Kulkarni, K.; Geil, P. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: A statement by the american diabetes association. Diabetes Care 2004, 27, 2266–2271. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Huang, Y.-Y.; Chen, H.-Y.; Hsieh, S.-H.; Sun, J.-H.; Chen, S.-T.; Lin, C.-H. Impact of Carbohydrate on Glucose Variability in Patients with Type 1 Diabetes Assessed Through Professional Continuous Glucose Monitoring: A Retrospective Study. Diabetes Ther. 2019, 10, 2289–2304. [Google Scholar] [CrossRef] [Green Version]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am. J. Clin. Nutr. 2005, 82, 69–75. [Google Scholar] [CrossRef]
- Buscemi, S.; Cosentino, L.; Rosafio, G.; Morgana, M.; Mattina, A.; Sprini, D.; Verga, S.; Rini, G.B. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin. Nutr. 2013, 32, 346–352. [Google Scholar] [CrossRef]
- Henry, C.J.; Kaur, B.; Quek, R.Y.C.; Camps, S.G. A Low Glycaemic Index Diet Incorporating Isomaltulose Is Associated with Lower Glycaemic Response and Variability, and Promotes Fat Oxidation in Asians. Nutrients 2017, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.P.; Iliescu, R.G.; Thomas, C.E.; Aronne, L.J. Food Order Has a Significant Impact on Postprandial Glucose and Insulin Levels. Diabetes Care 2015, 38, e98–e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Skipping breakfast is associated with glycemic variability in patients with type 2 diabetes. Nutrition 2020, 71, 110639. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Christensen, M.L.; Poulsen, C.W.; Rud, C.; Christensen, A.S.; Andersen, J.R.; Kampmann, U.; Ovesen, P.G. Effect of High Versus Low Carbohydrate Intake in the Morning on Glycemic Variability and Glycemic Control Measured by Continuous Blood Glucose Monitoring in Women with Gestational Diabetes Mellitus—A Randomized Crossover Study. Nutrients 2020, 12, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Abbreviation | Full Name | Description |
|---|---|---|
| Short-Term GV | Measures GV fluctuations in minute to hour increments | Typically measured using a CGM and reported with a variety of statistical measures |
| Long-Term GV | Measures GV on the scale of weeks to months | Typically reported as standard deviation of inter-visit HbA1C |
| HbA1C | GlycatedHemoglobin Assay | Reported as percentage of glycated hemoglobin |
| CGM | Continuous Glucose Monitor | A wearable medical device that regularly records blood sugar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watt, C.; Sanchez-Rangel, E.; Hwang, J.J. Glycemic Variability and CNS Inflammation: Reviewing the Connection. Nutrients 2020, 12, 3906. https://doi.org/10.3390/nu12123906
Watt C, Sanchez-Rangel E, Hwang JJ. Glycemic Variability and CNS Inflammation: Reviewing the Connection. Nutrients. 2020; 12(12):3906. https://doi.org/10.3390/nu12123906
Chicago/Turabian StyleWatt, Charles, Elizabeth Sanchez-Rangel, and Janice Jin Hwang. 2020. "Glycemic Variability and CNS Inflammation: Reviewing the Connection" Nutrients 12, no. 12: 3906. https://doi.org/10.3390/nu12123906




