Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo Experiments
2.1.1. Animals and Experimental Design
2.1.2. Housing and Diets
2.1.3. Sample Collection and Measurements
Clinical Scores
Blood Sampling and Hematological Analysis
BALF Sampling and Phenotyping
Measurements of Cytokines/Chemokines and M. haemolytica-LPS lgG
Lung Scores
2.2. In Vitro Experiments
2.2.1. M. haemolytica Growth, Quantification and Preparation
2.2.2. Cell Cultures and Treatments
2.2.3. Transepithelial Electrical Resistance (TEER) Measurement and Paracellular Tracer Flux Assay
2.2.4. ELISA Measurement and Lactate dehydrogenase (LDH) Assay
2.2.5. Western Blotting
2.2.6. Immunofluorescence
2.3. Statistical Analysis
3. Results
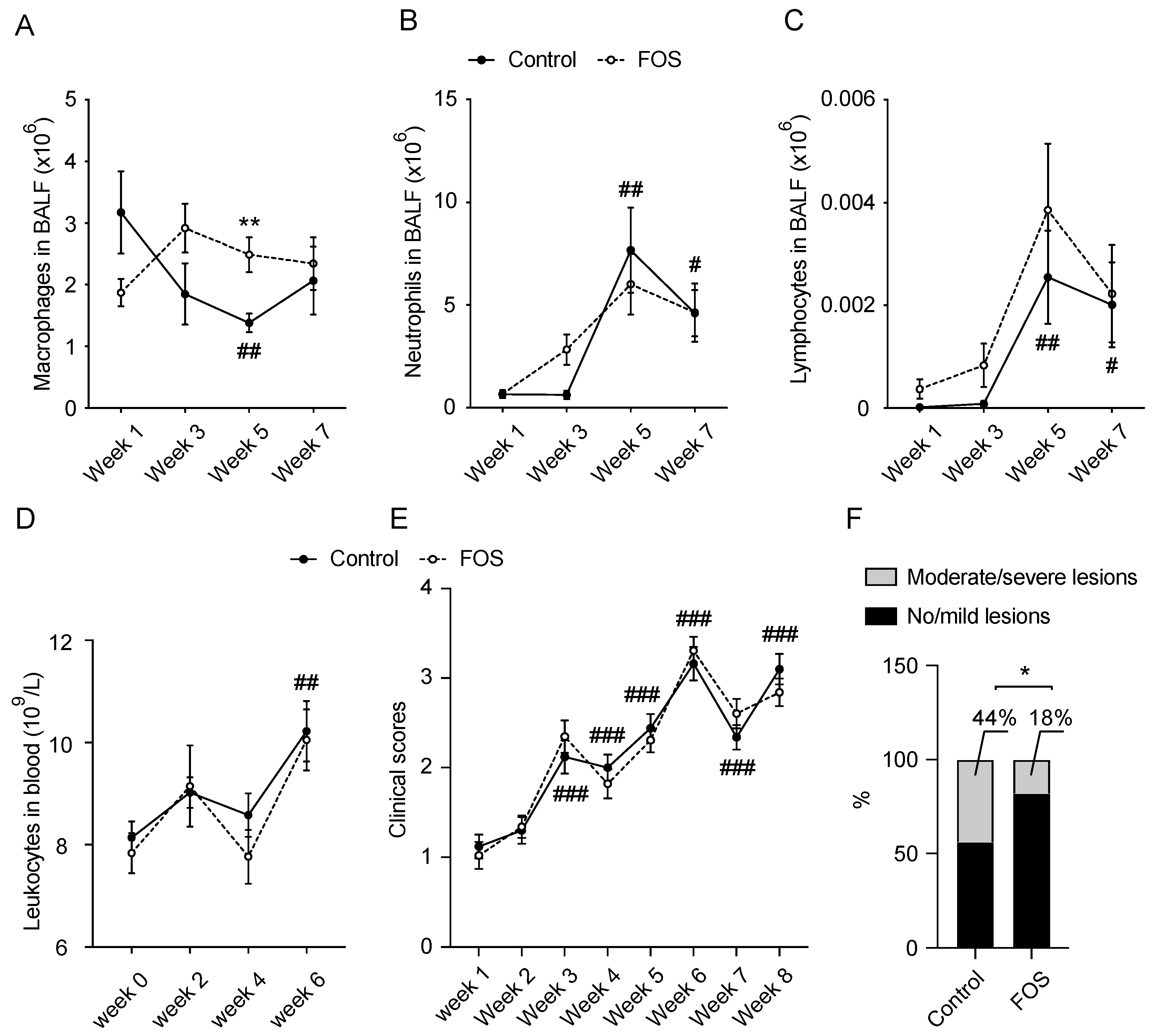
3.1. Effect of FOS on BALF and Blood Cell Compositions and Clinical and Lung Scores in Calves
3.2. Effect of FOS on Cytokine/Chemokine Concentrations in BALF and Blood of Calves
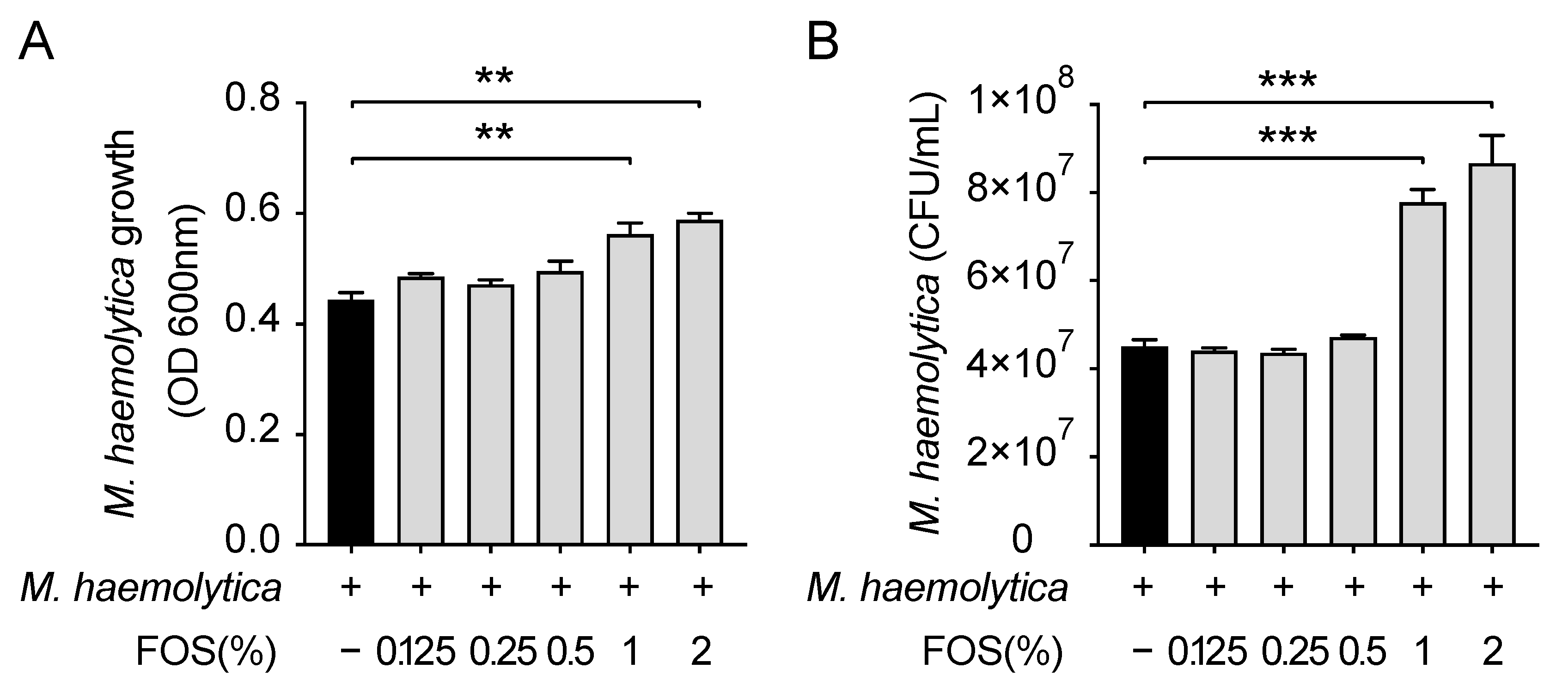
3.3. Effect of FOS on M. haemolytica Growth In Vitro
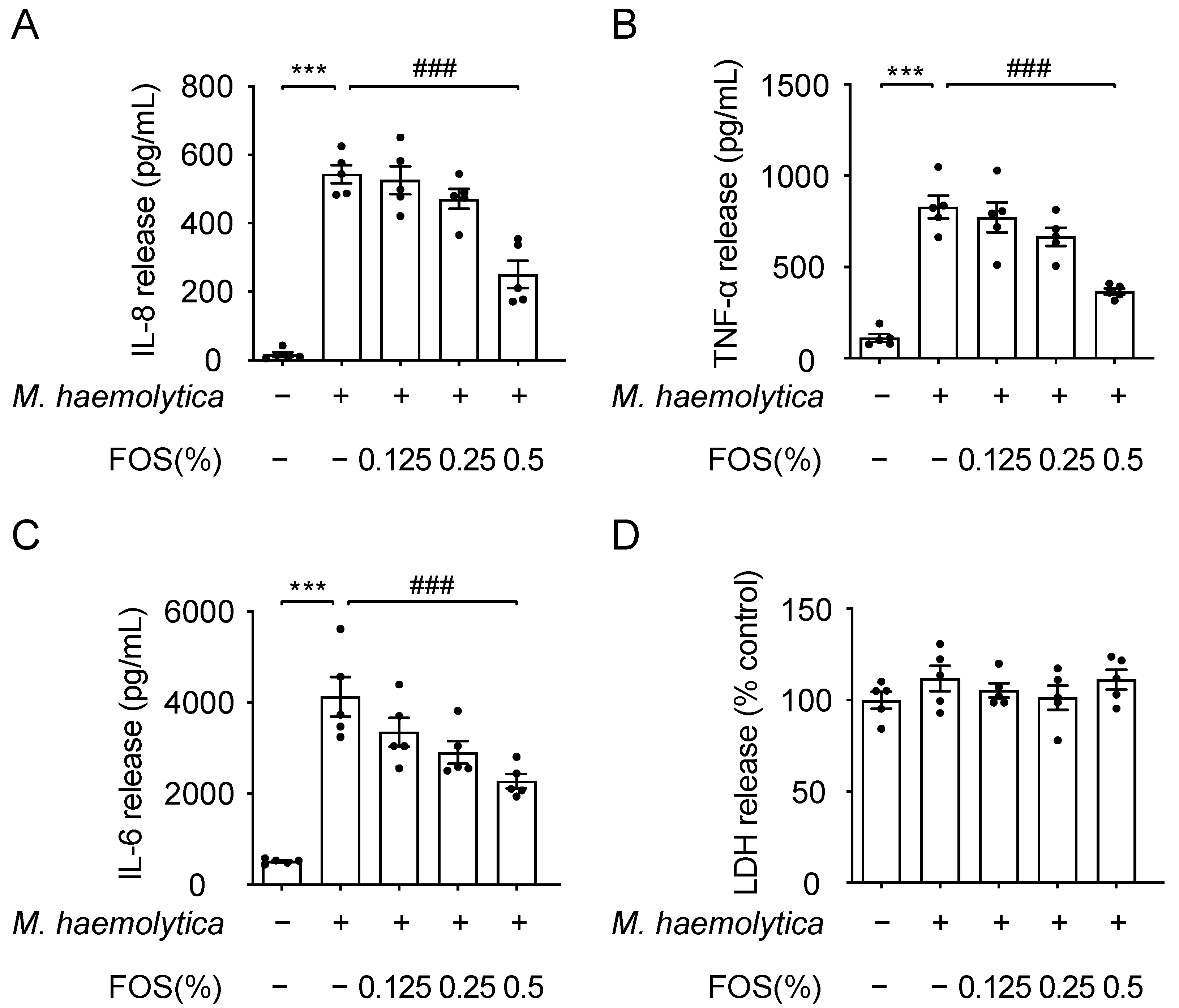
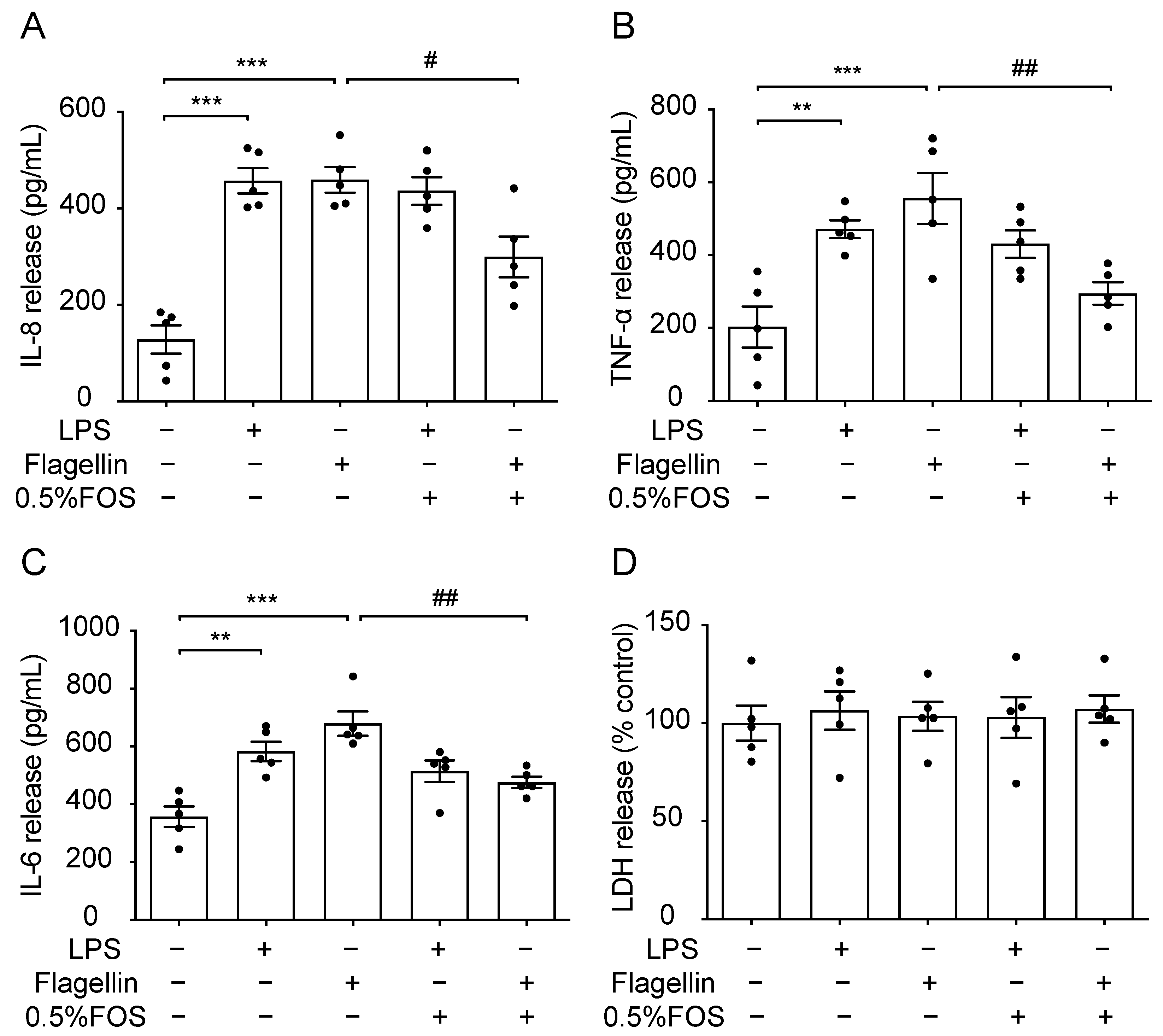
3.4. Effect of FOS on M. haemolytica-Induced Inflammation in PBECs
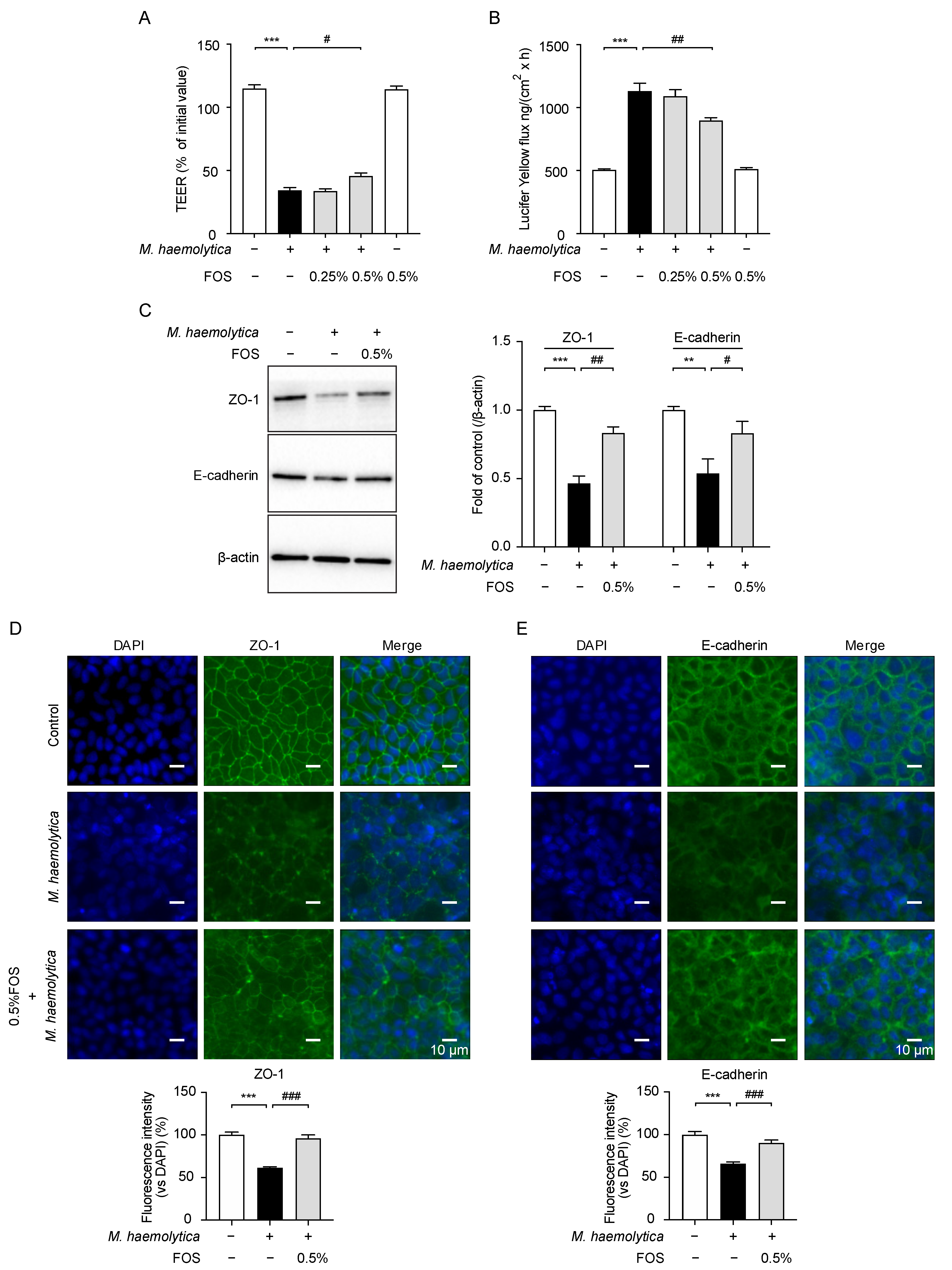
3.5. Effect of FOS on M. haemolytica-Induced Barrier Dysfunction in PBECs
3.6. Effect of FOS on M. haemolytica-Induced Phosphorylation of Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor (NF)-κB p65 in PBECs
3.7. The Anti-Inflammatory Effect of FOS Might Be Related to the Interference with TLR5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mizgerd, J.P. Acute Lower Respiratory Tract Infection. N. Engl. J. Med. 2008, 358, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caswell, J.L. Failure of Respiratory Defenses in the Pathogenesis of Bacterial Pneumonia of Cattle. Vet. Pathol. 2013, 51, 393–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duff, G.C.; Galyean, M.L. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 2007, 85, 823–840. [Google Scholar] [CrossRef]
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial-host interactions in bovine pneumonia. Vet. Pathol. 2010, 48, 338–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The Airway Epithelium: Soldier in the Fight against Respiratory Viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Varasteh, S.; van Putten, J.; Folkerts, G.; Braber, S. Mannheimia haemolytica and lipopolysaccharide induce airway epithelial inflammatory responses in an extensively developed ex vivo calf model. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Janbazacyabar, H.; Van Bergenhenegouwen, J.; Verheijden, K.A.; Leusink-Muis, T.; Van Helvoort, A.; Garssen, J.; Folkerts, G.; Braber, S. Non-digestible oligosaccharides partially prevent the development of LPS-induced lung emphysema in mice. Pharma Nutr. 2019, 10, 100163. [Google Scholar] [CrossRef]
- Bernard, H.; Desseyn, J.-L.; Bartke, N.; Kleinjans, L.; Stahl, B.; Belzer, C.; Knol, J.; Gottrand, F.; Husson, M.-O. Dietary pectin–derived acidic oligosaccharides improve the pulmonary bacterial clearance of pseudomonas aeruginosalung infection in mice by modulating intestinal microbiota and immunity. J. Infect. Dis. 2015, 211, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Arslanoglu, S.; Moro, G.E.; Boehm, G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J. Nutr. 2007, 137, 2420–2424. [Google Scholar] [CrossRef] [Green Version]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Vazquez, E.; Santos-Fandila, A.; Buck, R.; Rueda, R.; Ramirez, M. Major human milk oligosaccharides are absorbed into the systemic circulation after oral administration in rats. Br. J. Nutr. 2017, 117, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Stroble, C.; Underwood, M.A.; Lebrilla, C.B. Detection of milk oligosaccharides in plasma of infants. Anal. Bioanal. Chem. 2014, 406, 5775–5784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Difilippo, E.; Bettonvil, M.; Willems, R.; Braber, S.; Fink-Gremmels, J.; Jeurink, P.V.; Schoterman, M.H.C..; Gruppen, H.; Schols, H.A. Oligosaccharides in Urine, Blood, and Feces of Piglets Fed Milk Replacer Containing Galacto-oligosaccharides. J. Agric. Food Chem. 2015, 63, 10862–10872. [Google Scholar] [CrossRef] [PubMed]
- Eiwegger, T.; Stahl, B.; Haidl, P.; Schmitt, J.; Boehm, G.; Dehlink, E.; Urbanek, R.; Szépfalusi, Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 2010, 21, 1179–1188. [Google Scholar] [CrossRef]
- He, Y.; Lawlor, N.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor–Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, M.D.S.; Ocón, B.; Calvo, I.R.; Anzola, A.; Guadix, E.M.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2013, 58, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, P.; Batra, S.; Zemans, R.L.; Downey, G.P.; Jeyaseelan, S. Divergent functions of toll-like receptors during bacterial lung infections. Am. J. Respir. Crit. Care Med. 2014, 190, 722–732. [Google Scholar] [CrossRef] [Green Version]
- McGuirk, S.M.; Peek, S.F. Timely diagnosis of dairy calf respiratory disease using a standardized scoring system. Anim. Health Res. Rev. 2014, 15, 145–147. [Google Scholar] [CrossRef]
- Caldow, G. Bronchoalveolar lavage in the investigation of bovine respiratory disease. Practice 2001, 23, 41–43. [Google Scholar] [CrossRef]
- Leruste, H.; Brscic, M.; Heutinck, L.; Visser, E.; Wolthuis-Fillerup, M.; Bokkers, E.; Stockhofe-Zurwieden, N.; Cozzi, G.; Gottardo, F.; Lensink, B.; et al. The relationship between clinical signs of respiratory system disorders and lung lesions at slaughter in veal calves. Prev. Vet. Med. 2012, 105, 93–100. [Google Scholar] [CrossRef]
- Cozens, D.; Sutherland, E.; Lauder, M.; Taylor, G.; Berry, C.C.; Davies, R.L. Pathogenic Mannheimia haemolytica Invades Differentiated Bovine Airway Epithelial Cells. Infect. Immun. 2019, 87, e00078-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Kiewiet, M.B.; Groeneveld, A.; Nauta, A.; de Vos, P. Human milk oligosaccharides and its acid hydrolysate LNT2 show immunomodulatory effects via TLRs in a dose and structure-dependent way. J. Funct. Foods 2019, 59, 174–184. [Google Scholar] [CrossRef]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amat, S.; Alexander, T.W.; Holman, D.B.; Schwinghamer, T.; Timsit, E. Intranasal Bacterial Therapeutics Reduce Colonization by the Respiratory Pathogen Mannheimia haemolytica in Dairy Calves. mSystems 2020, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Driessche, L.; Valgaeren, B.; Gille, L.; Boyen, F.; Ducatelle, R.; Haesebrouck, F.; Deprez, P.; Pardon, B. A Deep Nasopharyngeal Swab Versus Nonendoscopic Bronchoalveolar Lavage for Isolation of Bacterial Pathogens from Preweaned Calves With Respiratory Disease. J. Vet. Intern. Med. 2017, 31, 946–953. [Google Scholar] [CrossRef] [Green Version]
- van Leenen, K.; Van Driessche, L.; De Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P.; Pardon, B. Comparison of bronchoalveolar lavage fluid bacteriology and cytology in calves classified based on combined clinical scoring and lung ultrasonography. Prev. Veter- Med. 2020, 176, 104901. [Google Scholar] [CrossRef]
- Pardon, B.; De Bleecker, K.; Hostens, M.; Callens, J.; Dewulf, J.; Deprez, P. Longitudinal study on morbidity and mortality in white veal calves in Belgium. BMC Vet. Res. 2012, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Verheijden, K.A.T.; Willemsen, L.E.M.; Braber, S.; Leusink-Muis, T.; Delsing, D.J.M.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model. Respir. Res. 2015, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Lohner, S.; Jakobik, V.; Mihályi, K.; Soldi, S.; Vasileiadis, S.; Theis, S.; Sailer, M.; Sieland, C.; Berényi, K.; Boehm, G.; et al. Inulin-Type Fructan Supplementation of 3- to 6-Year-Old Children Is Associated with Higher FecalBifidobacteriumConcentrations and Fewer Febrile Episodes Requiring Medical Attention. J. Nutr. 2018, 148, 1300–1308. [Google Scholar] [CrossRef]
- Philippeau, C.; Respondek, F.; Julliand, V. In vitro effects of fructo-oligosaccharides on bacterial concentration and fermentation profiles in veal calf ileal contents. Anim. Feed. Sci. Technol. 2010, 162, 83–90. [Google Scholar] [CrossRef]
- Siegel, S.J.; Weiser, J.N. Mechanisms of Bacterial Colonization of the Respiratory Tract. Annu. Rev. Microbiol. 2015, 69, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Coyne, C.B.; VanHook, M.K.; Gambling, T.M.; Carson, J.L.; Boucher, R.C.; Johnson, L.G. Regulation of Airway Tight Junctions by Proinflammatory Cytokines. Mol. Biol. Cell 2002, 13, 3218–3234. [Google Scholar] [CrossRef] [Green Version]
- Jevnikar, Z.; Östling, J.; Ax, E.; Calvén, J.; Thörn, K.; Israelsson, E.; Öberg, L.; Singhania, A.; Lau, L.C.; Wilson, S.J.; et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 577–590. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; DiFilippo, E.; Schols, H.A.; Schoterman, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size: Structure-activity relationships of non-digestible oligosaccharides. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Johnson, C.G.; Sciurba, J.; Meng, X.; Stober, V.P.; Liu, C.; Cyphert-Daly, J.M.; Bulek, K.; Qian, W.; Solis, A.; et al. TLR5 participates in the TLR4 receptor complex and promotes MyD88-dependent signaling in environmental lung injury. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]


| Experimental Period | Growing Period | |
|---|---|---|
| Ingredient | g/kg | |
| Lactose or fructo-oligosaccharides 1 Lactose | 2.7 32.3 | |
| Whey powder | 527 | 560 |
| Delactosed whey powder | 52 | 93 |
| Whey protein concentrate | 50 | |
| Soy protein concentrate | 60 | 55 |
| Soluble wheat protein | 50 | 41 |
| Pea fiber | 3 | |
| Extruded wheat flour | 41 | |
| Fat | ||
| Lard | 43 | 48 |
| Tallow | 68 | 77 |
| Coconut oil | 54 | 34 |
| Lecithin | 7.2 | 5 |
| Emulsifier | 7.2 | 5 |
| Premix | 10 2 | 10 3 |
| Calcium formate | 9.7 | 10 |
| Citric acid | 2.0 | 2 |
| Sodium bicarbonate | 4.0 | 4 |
| Mono ammonium phosphate | 3.5 | 2 |
| Lysine | 9.8 | 8 |
| Methionine | 2.4 | 3 |
| Threonine | 1.3 | 2 |
| Aroma 4 | 0.2 | |
| Nutrient | g/kg of Dry Matter unless Noted | |
| Dry matter, g/kg | 975 | 976 |
| Crude ash | 82 | 74 |
| Crude protein, N × 6.25 | 231 | 191 |
| Crude fat | 193 | 184 |
| Lactose 5 | 453 | 455 |
| Iron, ppm in dry matter | 48 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Gilbert, M.S.; Gerrits, W.J.J.; Folkerts, G.; Braber, S. Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells. Nutrients 2021, 13, 3514. https://doi.org/10.3390/nu13103514
Cai Y, Gilbert MS, Gerrits WJJ, Folkerts G, Braber S. Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells. Nutrients. 2021; 13(10):3514. https://doi.org/10.3390/nu13103514
Chicago/Turabian StyleCai, Yang, Myrthe S. Gilbert, Walter J. J. Gerrits, Gert Folkerts, and Saskia Braber. 2021. "Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells" Nutrients 13, no. 10: 3514. https://doi.org/10.3390/nu13103514
APA StyleCai, Y., Gilbert, M. S., Gerrits, W. J. J., Folkerts, G., & Braber, S. (2021). Anti-Inflammatory Properties of Fructo-Oligosaccharides in a Calf Lung Infection Model and in Mannheimia haemolytica-Infected Airway Epithelial Cells. Nutrients, 13(10), 3514. https://doi.org/10.3390/nu13103514






