Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults
Abstract
1. Introduction
2. Materials and Methods
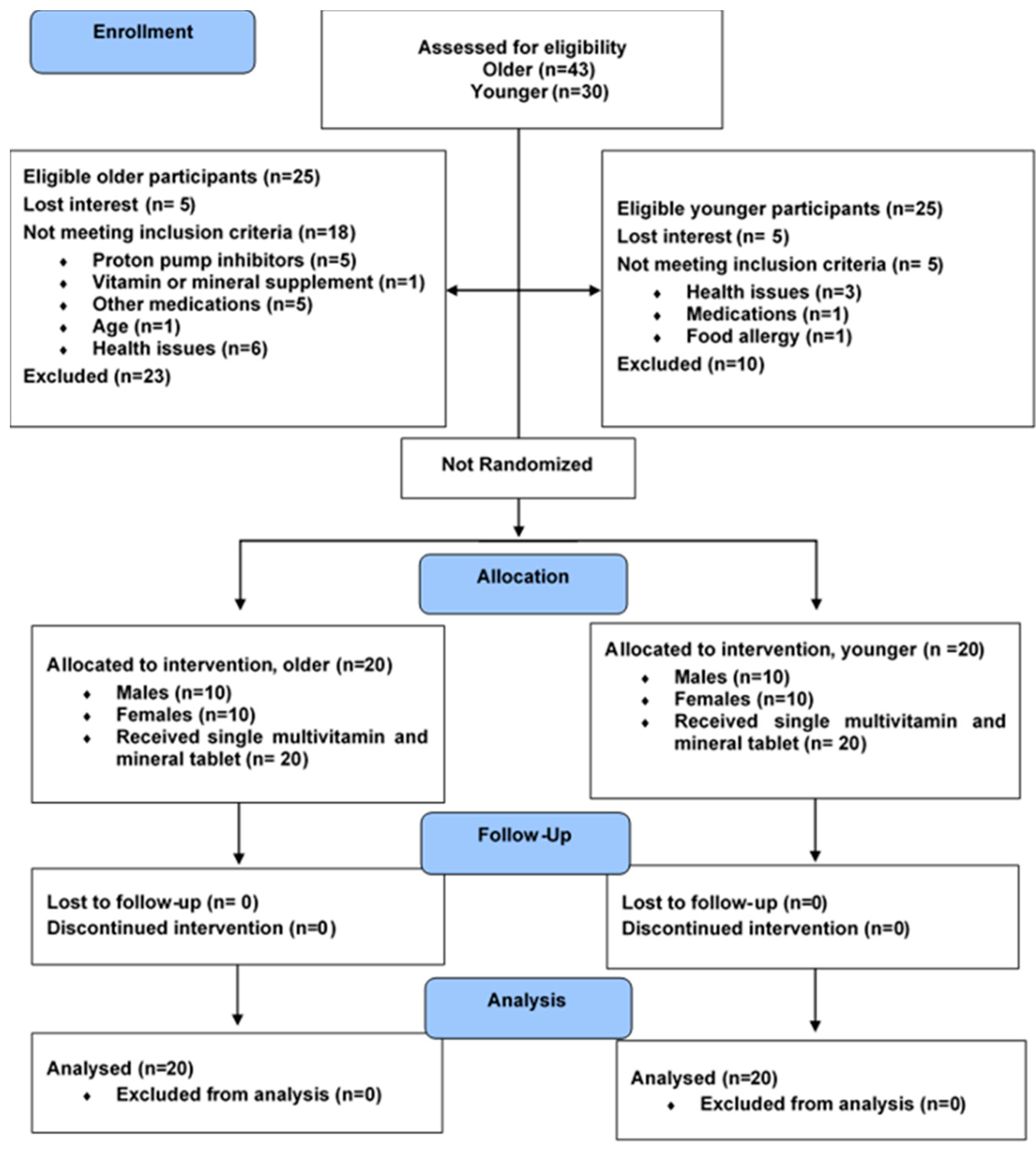
2.1. Study Design
2.2. Participants
2.3. Ethics Approval
2.4. Intervention: Standardized Breakfast Meal
2.5. Outcomes
2.5.1. Anthropometric Measurements
| Vitamins | Supplement | Test Meal | Total Intake |
|---|---|---|---|
| Vitamin B12 (µg) | 22.00 | 0.00 | 22.00 |
| Total Folate (µg) | 0.00 | 79.01 | 79.01 |
| Folic Acid (µg) | 400.00 | 0.00 1 | 400.00 |
| Vitamin B6 (mg) | 6.00 | 0.38 | 6.38 |
| Thiamine (mg) | 2.18 | 0.43 | 2.61 |
| Pantothenic acid (mg) | 10.80 | 0.00 1 | 10.80 |
| Riboflavin (mg) | 3.20 | 0.16 | 3.36 |
| Niacin (mg) | 15.00 | 0.78 | 15.78 |
| Biotin (µg) | 45.00 | 0.00 1 | 45.00 |
2.5.2. Dietary and Meal Intake Analysis
2.5.3. Biochemical Measures
2.5.4. Analysis of B-Vitamins
2.6. Sample Size, Data Interpretation, and Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Estimated B-Vitamin Intake from 3-Day Food Record
3.3. Urine Output and Creatinine Concentration
3.4. Fasting Circulating Vitamin Status and Urinary Concentrations
3.5. Acute Postprandial Response of B-Vitamins and Vitamers Following MVM Supplement Ingestion
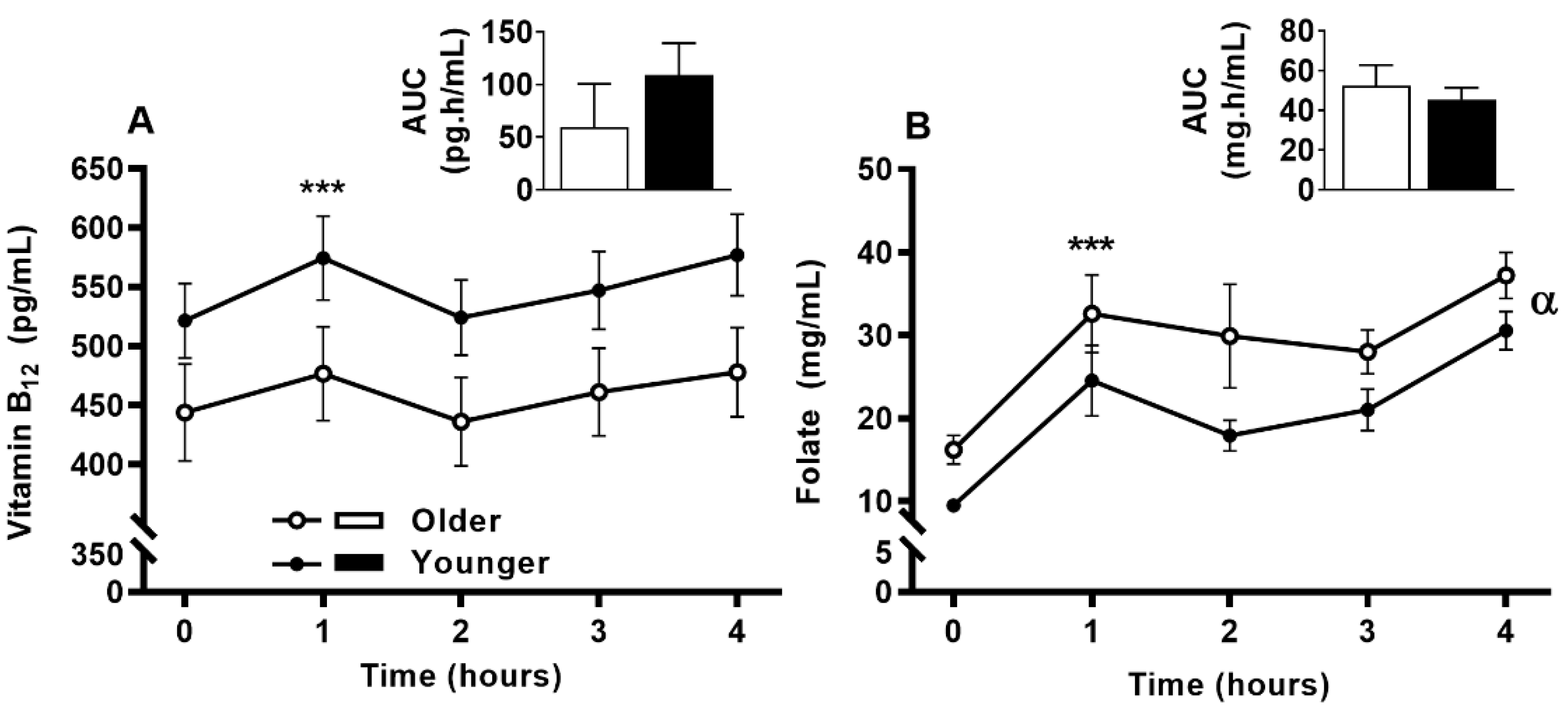
3.6. Impact of Age on Acute Circulating Vitamin B12 and Folate Response to MVM Supplement Ingestion
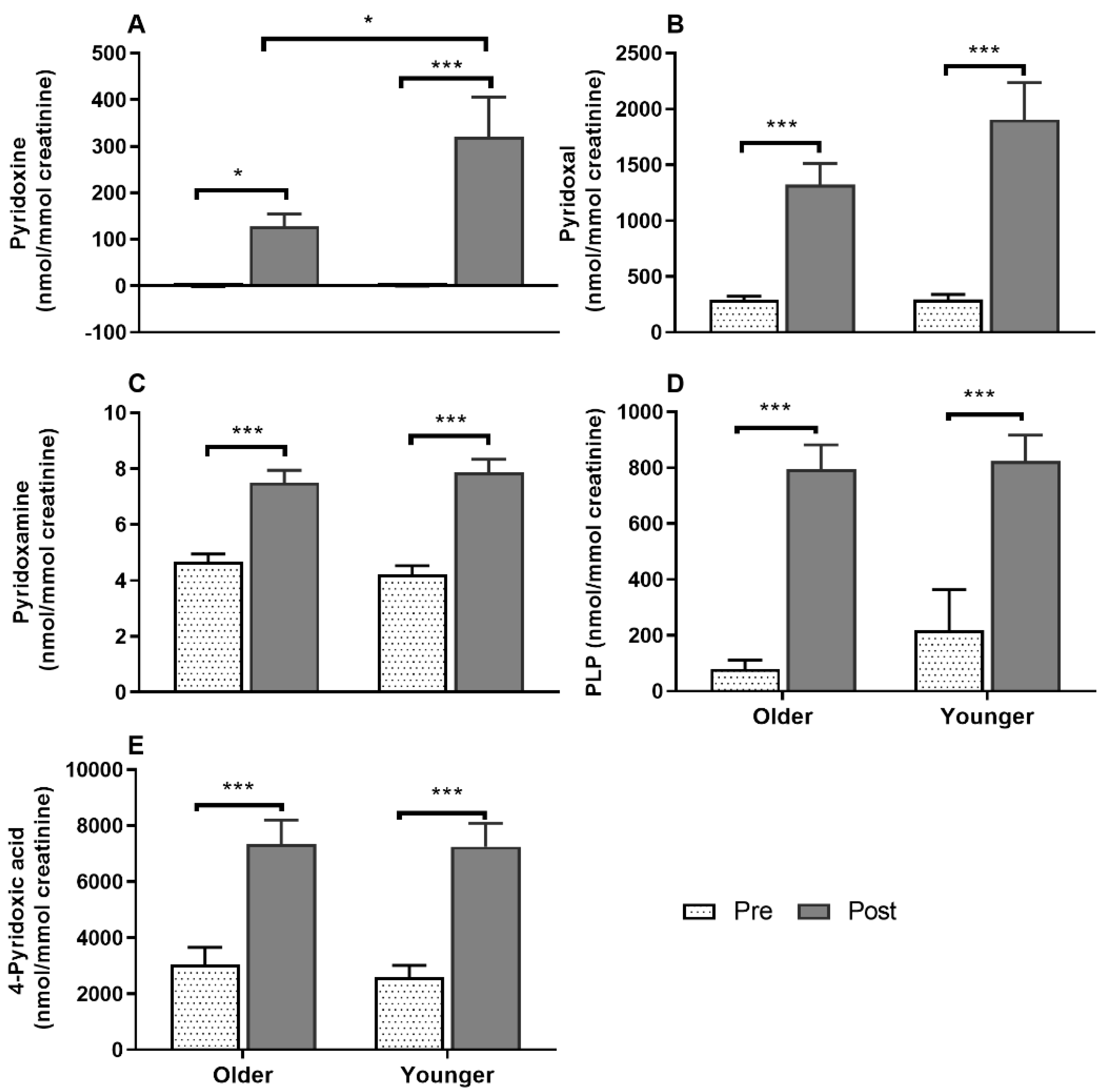
3.7. Impact of Age on Acute Plasma and Urinary B6-Vitamer Responses to MVM Supplement Ingestion
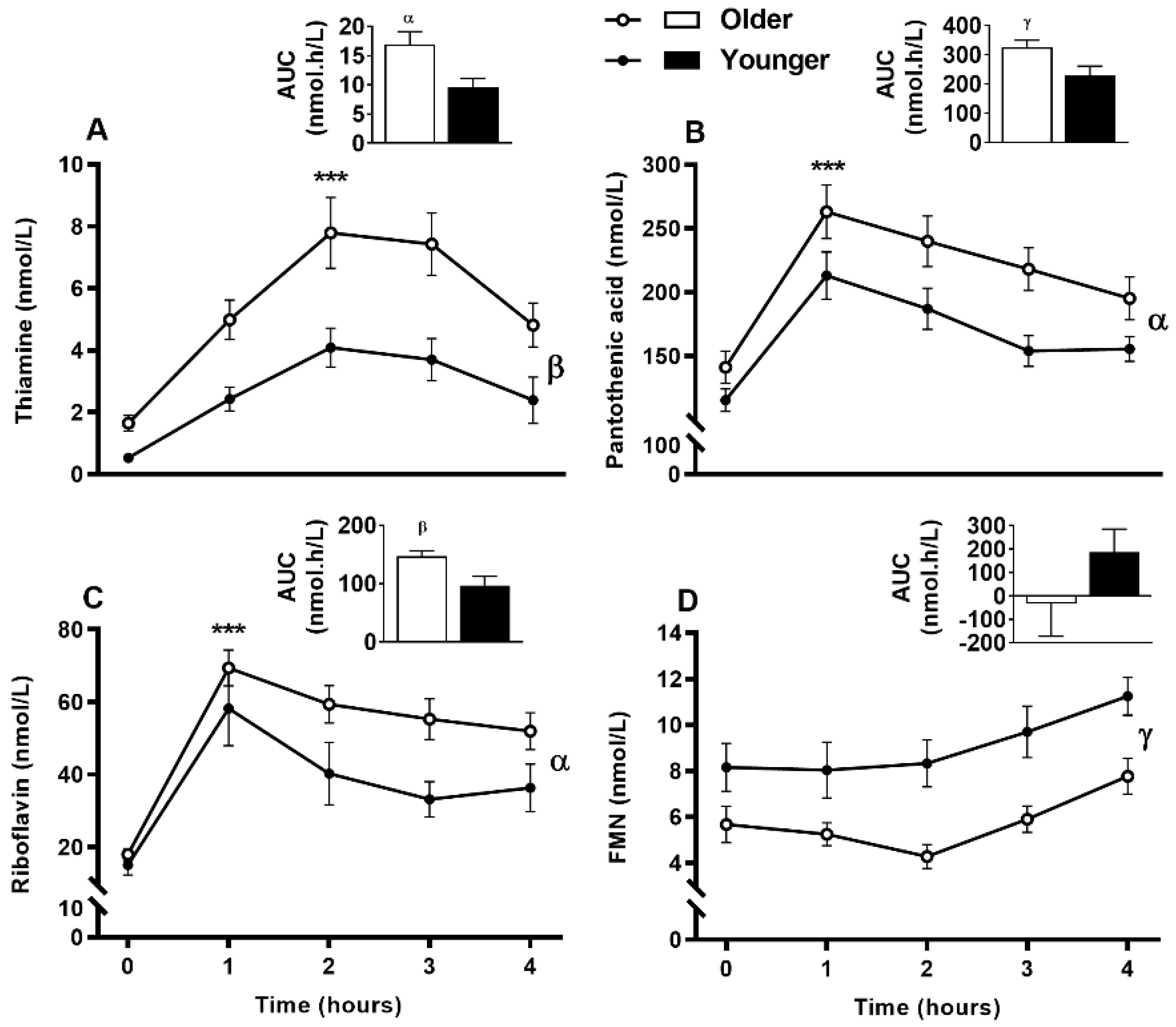
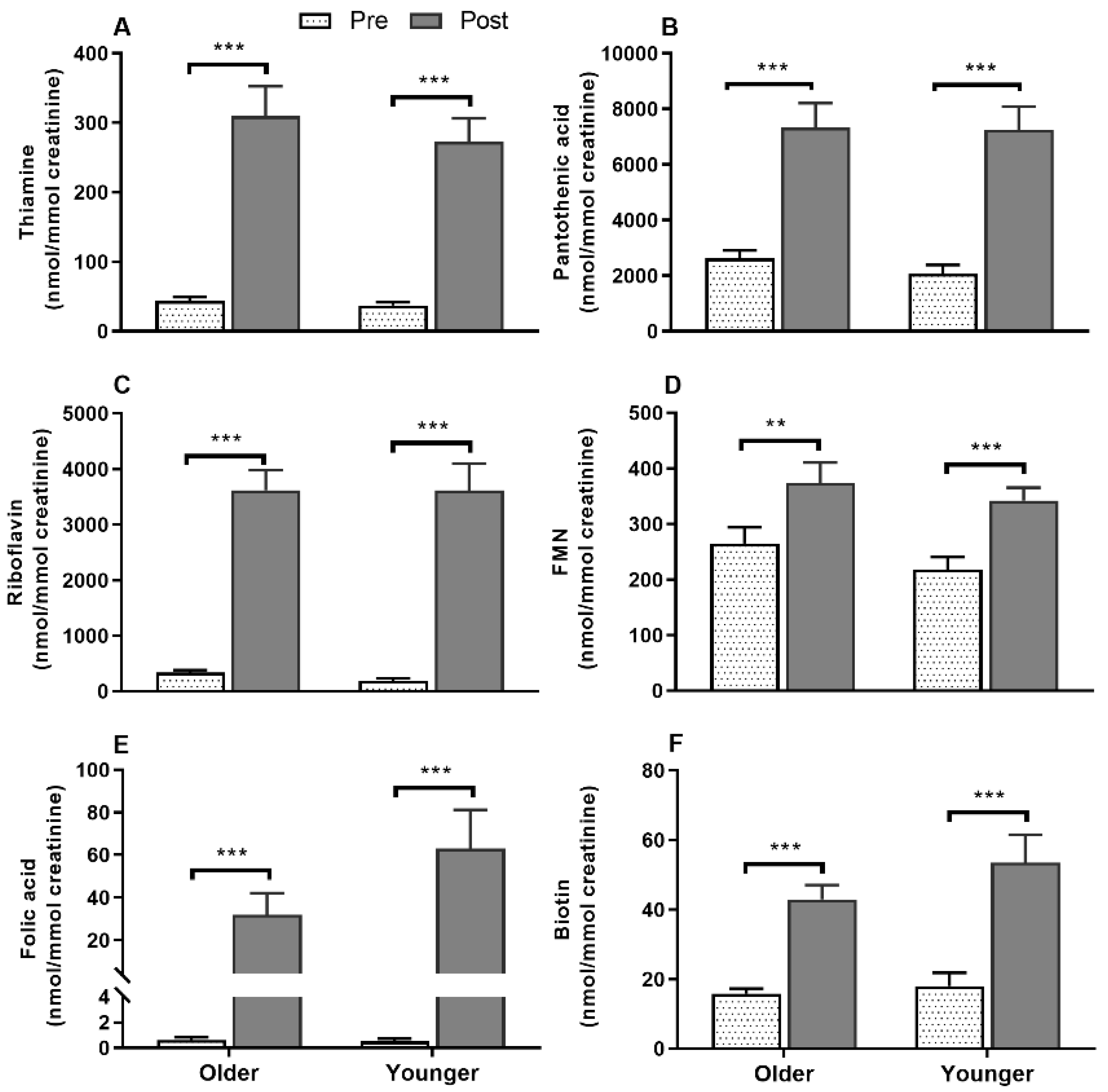
3.8. Impact of Age on Acute Postprandial Plasma and Urinary Thiamine, Pantothenic Acid, Riboflavin, FMN, and Urinary Biotin Responses to Single MVM Supplement Ingestion
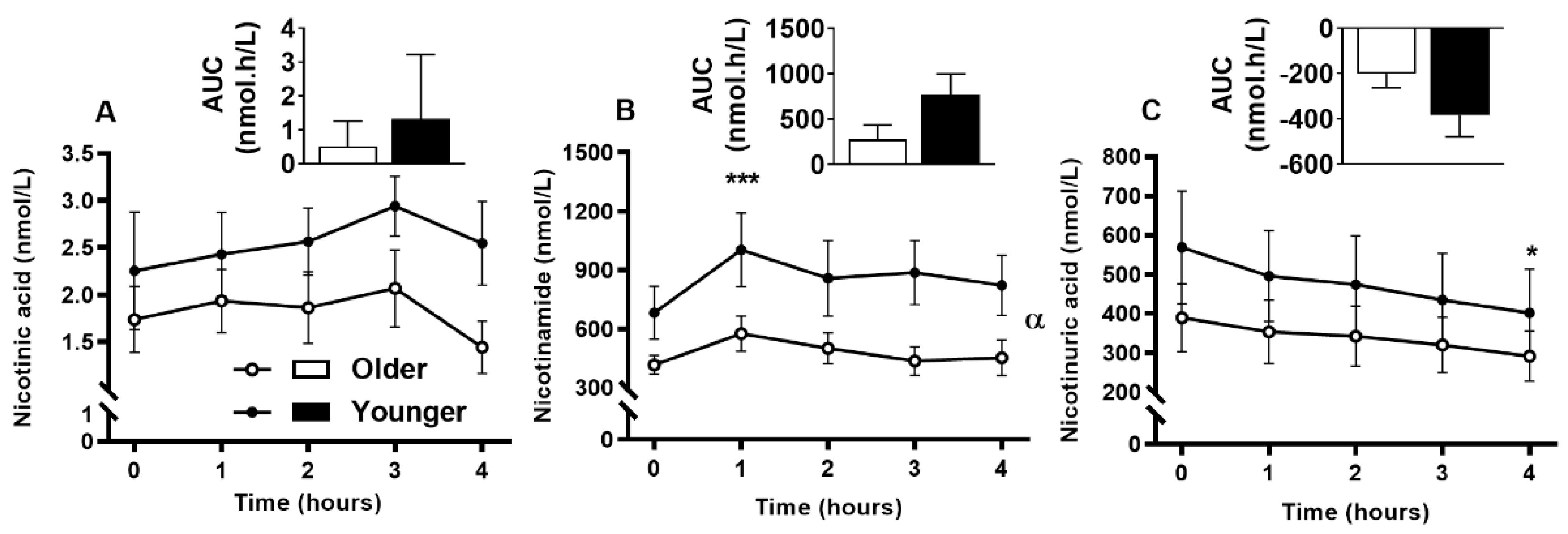
3.9. Impact of Age on Acute Plasma and Urinary B3-Vitamer Responses to Single MVM Supplement Ingestion
3.10. Sex-Specific Effects on Acute Circulating and Urinary B-Vitamin and Vitamer Responses to Supplement Ingestion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janssen, J.J.E.; Grefte, S.; Keijer, J.; De Boer, V.C.J. Mito-nuclear communication by mitochondrial metabolites and its regulation by B-vitamins. Front. Physiol. 2019, 10, 1–23. [Google Scholar] [CrossRef]
- Porter, K.; Hoey, L.; Hughes, C.; Ward, M.; McNulty, H. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Maras, J.E.; Bakun, P.J.; Tucker, K.L. Dietary intake of vitamin B6, plasma pyridoxal 5′-phosphate and homocysteine in Puerto Rican adults. J. Am. Diet. Assoc. 2010, 110, 1660–1668. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and its role in cell metabolism and physiology. Cells 2018, 84, 28. [Google Scholar] [CrossRef]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.Y.; Wang, Y.Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B6, and vitamin B12 can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenetics 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Abbenhardt, C.; Miller, J.W.; Song, X.; Brown, E.C.; David Cheng, T.-Y.; Wener, M.H.; Zheng, Y.; Toriola, A.T.; Neuhouser, M.L.; Beresford, S.A.; et al. Nutritional epidemiology biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J. Nutr. 2014, 144, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Trevisi, L.; Urch, B.; Lin, X.; Speck, M.; Coull, B.A.; Liss, G.; Thompson, A.; Wu, S.; Wilson, A.; et al. B-vitamin supplementation mitigates effects of fine particles on cardiac autonomic dysfunction and inflammation: A pilot human intervention trial. Sci. Rep. 2017, 7, 45322. [Google Scholar] [CrossRef]
- Blumberg, J.; Bailey, R.; Sesso, H.; Ulrich, C. The Evolving Role of Multivitamin/Multimineral Supplement Use among Adults in the Age of Personalized Nutrition. Nutrients 2018, 10, 248. [Google Scholar] [CrossRef]
- Power, S.; Jeffery, I.; Ross, R.; Stanton, C.; O’Toole, P.; O’Connor, E.; Fitzgerald, G. Food and nutrient intake of Irish community-dwelling elderly subjects: Who is at nutritional risk? J. Nutr. Health Aging 2014, 18, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.G.A.; Luiking, Y.C.; De Groot, L.C.P.G.M. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Ferroli, C.E.; Trumbo, P.R. Bioavailability of vitamin B-6 in young and older men. Am. J. Clin. Nutr. 1994, 60, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Shibata, K.; Sugita, C.; Sano, M.; Fukuwatari, T. Urinary excretion of B-group vitamins reflects the nutritional status of B-group vitamins in rats. J. Nutr. Sci. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Said, H.M.M. Intestinal absorption of water-soluble vitamins in health and disease. Biochem. J. 2011, 437, 357–372. [Google Scholar] [CrossRef]
- Morley, J.E. Decreased Food Intake With Aging. J. Gerontol. Ser. A 2001, 56, 81–88. [Google Scholar] [CrossRef]
- Conzade, R.; Koenig, W.; Heier, M.; Schneider, A.; Grill, E.; Peters, A.; Thorand, B. Prevalence and predictors of subclinical micronutrient deficiency in German older adults: Results from the population-based KORA-Age Study. Nutrients 2017, 9, 1276. [Google Scholar] [CrossRef]
- Kant, A.K.; Moser-Veillon, P.B.; Reynolds, R.D. Effect of age on changes in plasma, erythrocyte, and urinary B-6 vitamers after an oral vitamin B-6 load. Am. J. Clin. Nutr. 1988, 48, 1284–1290. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 31 December 2015).
- Ministry of Health. Food and Nutrition Guidelines for Healthy Older People: A Background Paper; Ministry of Health: Wellington, New Zealand, 2013; ISBN 9780478393996.
- Sebastian, R.S.; Cleveland, L.E.; Goldman, J.D.; Moshfegh, A.J. Older adults who use vitamin/mineral supplements differ from nonusers in nutrient intake adequacy and dietary attitudes. J. Am. Diet. Assoc. 2007, 107, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.W.; Lawrence, J.C.; Knowlden, A.P. The use of fortified foods to treat malnutrition among older adults: A systematic review, Quality in Ageing and Older Adults. Ageing Older Adults 2017, 18, 104–119. [Google Scholar] [CrossRef]
- Eussen, S.J.P.M.; de Groot, L.C.P.G.M.; Clarke, R.; Schneede, J.; Ueland, P.M.; Hoefnagels, W.H.L.; van Staveren, W.A. Oral Cyanocobalamin Supplementation in Older People With Vitamin B12 Deficiency. Arch. Intern. Med. 2005, 165, 1167. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Williams, S.M.; Green, T.J.; McMahon, J.A.; Mann, J.I.; Knight, R.G.; Skeaff, C.M. Differences in Erythrocyte Folate Concentrations in Older Adults Reached Steady-State within One Year in a Two-Year, Controlled, 1 mg/d Folate Supplementation Trial. J. Nutr. 2012, 142, 1633–1637. [Google Scholar] [CrossRef]
- Kalman, D.S.; Lou, L.; Schwartz, H.I.; Feldman, S.; Krieger, D.R. A pilot trial comparing the availability of vitamins C, B6, and B12 from a vitamin-fortified water and food source in humans. Int. J. Food Sci. Nutr. 2009, 60, 114–124. [Google Scholar] [CrossRef]
- Navarro, M.; Wood, R.J. Plasma changes in micronutrients following a multivitamin and mineral supplement in healthy adults. J. Am. Coll. Nutr. 2003, 22, 124–132. [Google Scholar] [CrossRef]
- Mayengbam, S.; Virtanen, H.; Hittel, D.S.; Elliott, C.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Metabolic consequences of discretionary fortified beverage consumption containing excessive vitamin B levels in adolescents. PLoS ONE 2019, 14, e0209913. [Google Scholar] [CrossRef]
- Meisser Redeuil, K.; Longet, K.; Bénet, S.; Munari, C.; Campos-Giménez, E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2015, 1422, 89–98. [Google Scholar] [CrossRef]
- Kwok, T.; Lee, J.; Ma, R.C.; Wong, S.Y.; Kung, K.; Lam, A.; Ho, C.S.; Lee, V.; Harrison, J.; Lam, L. A randomized placebo controlled trial of vitamin B12 supplementation to prevent cognitive decline in older diabetic people with borderline low serum vitamin B12. Clin. Nutr. 2017, 36, 1509–1515. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December, 2008; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- FZANZ. AUSNUT 2011-13 Food Nutrient Database; FSANZ, Food Standards Australia New Zealand: Canberra, ACT, Australia, 2014.
- Gregory, J.F.; Swendseid, M.E.; Jacob, R.A. Urinary excretion of folate catabolites responds to changes in folate intake more slowly than plasma folate and homocysteine concentrations and lymphocyte DNA methylation in postmenopausal women. J. Nutr. 2000, 130, 2949–2952. [Google Scholar] [CrossRef]
- Sharma, P.; Gillies, N.; Pundir, S.; Pileggi, C.A.; Markworth, J.F.; Thorstensen, E.B.; Cameron-Smith, D.; Milan, A.M. Comparison of the acute postprandial circulating B-vitamin and vitamer responses to single breakfast meals in young and older individuals: Preliminary secondary outcomes of a randomized controlled trial. Nutrients 2019, 11, 2893. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Pannérec, A.; Migliavacca, E.; De Castro, A.; Michaud, J.; Karaz, S.; Goulet, L.; Rezzi, S.; Ng, T.P.; Bosco, N.; Larbi, A.; et al. Vitamin B12 deficiency and impaired expression of amnionless during aging. J. Cachexia. Sarcopenia Muscle 2018, 9, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Margalit, I.; Cohen, E.; Goldberg, E.; Krause, I. Vitamin B12 deficiency and the role of gender: A cross-sectional study of a large cohort. Ann. Nutr. Metab. 2018, 72, 265–271. [Google Scholar] [CrossRef]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef]
- Araújo, D.; Noronha, M.; Cunha, N.; Abrunhosa, S.; Rocha, A.; Amaral, T. Low serum levels of vitamin B12 in older adults with normal nutritional status by mini nutritional assessment. Eur. J. Clin. Nutr. 2016, 70, 859–862. [Google Scholar] [CrossRef]
- Albersen, M.; Bosma, M.; Knoers, N.V.V.A.M.; De Ruiter, B.H.B.; Ne, E.; Diekman, F.; De Ruijter, J.; Visser, W.F.; De Koning, T.J.; Verhoeven-Duif, N.M. The intestine plays a substantial role in human vitamin B6 metabolism: A Caco-2 cell model. PLoS ONE 2013, 8, e54113. [Google Scholar] [CrossRef]
- Chiang, E.-P.; Smith, D.E.; Selhub, J.; Dallal, G.; Wang, Y.-C.; Roubenoff, R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res. Ther. 2005, 7, R1254–R1262. [Google Scholar] [CrossRef]
- Pannemans, D.L.E.; van den Berg, H.; Westerterp, K.R. The influence of protein intake on vitamin B-6 metabolism differs in young and elderly humans. J. Nutr. 1994, 124, 1207–1214. [Google Scholar] [CrossRef]
- Bode, W.; Mocking, J.A.J.J.; Van den Berg, H. Influence of age and sex on vitamin B-6 vitamer distribution and on vitam B-6 metabolizing enzymes in Wistar rats. J. Nutr. 1991, 121, 318–329. [Google Scholar] [CrossRef]
- Midttun, Ø.; Ulvik, A.; Ringdal Pedersen, E.; Ebbing, M.; Bleie, Ø.; Schartum-Hansen, H.; Nilsen, R.M.; Nygård, O.; Ueland, P.M. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J. Nutr. 2011, 141, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Yeh, E.-L.; Chen, C.-C.; Huang, S.-C.; Huang, Y.-C. Vitamin B-6, independent of homocysteine, is a significant factor in relation to inflammatory responses for chronic kidney disease and hemodialysis patients. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Schreiner, M.G.; Mayer, H.K. Rapid determination of the various native forms of vitamin B6 and B2 in cow’s milk using ultra-high performance liquid chromatography. J. Chromatogr. A 2017, 1500, 89–95. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, H.; Bode, W.; Mocking, J.; Löwik, M. Effect of aging on vitamin B6 status and metabolism. Ann. N. Y. Acad. Sci. 1990, 585, 96–105. [Google Scholar] [CrossRef]
- Bode, W.; Van Den Berg, H. Pyridoxal-5’-phosphate and pyridoxal biokinetics in aging Wistar rats. Exp. Gerontol. 1991, 26, 589–599. [Google Scholar] [CrossRef]
- van den Berg, H. Vitamin B 6 status and requirements in older adults. Br. J. Nutr. 1999, 81, 175–176. [Google Scholar] [CrossRef]
- Roubenoff, R. Catabolism of aging: Is it an inflammatory process? Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 295–299. [Google Scholar] [CrossRef]
- Russell, R.M. Factors in aging that effect the bioavailability of nutrients. Bioavailab. Nutr. Other Bioact. Components Diet. Suppl. 2001, 131, 1359S–1361S. [Google Scholar] [CrossRef]
- Manore, M.M. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am. J. Clin. Nutr. 2000, 72, 598S–606S. [Google Scholar] [CrossRef]
- Ukuwatari, T.F.; Ada, H.W.; Hibata, K.S. Age-Related Alterations of B-Group Vitamin Contents in Urine, Blood and Liver from Rats. J. Nutr. Sci. Vitaminol. 2008, 54, 357–362. [Google Scholar] [CrossRef]
- Shibatai, K.; Fukuwatari, T.; Ohta, M.; Okamoto, H.; Watanabe, T.; Fukui, T.; Nishimuta, M.; Totani, M.; Kimura, M.; Ohishi, N.; et al. Values of water-soluble vitamins in blood and urine of Japanese young men and women consuming a semi-purified diet based on the Japanese dietary reference intakes. J. Nutr. Sci. Vitaminol. 2005, 51, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Using Dietary Reference Intakes in Planning Diets for Individuals. In Dietary Reference Intakes: Applications in Dietary Planning; National Academies Press (US): Washington, DC, USA, 2003. [Google Scholar]
- Ukuwatari, T.F.; Oshida, E.Y.; Akahashi, K.T.; Hibata, K.S. Effect of Fasting on the Urinary Excretion of Water-Soluble Vitamins in Humans and Rats. J. Nutr. Sci. Vitaminol. 2010, 56, 19–26. [Google Scholar] [CrossRef][Green Version]
- Fernyhough, L.K.; Horwath, C.C.; Campbell, A.J.; Robertson, M.C.; Busby, W.J. Changes in dietary intake during a 6-year follow-up of an older population. Eur. J. Clin. Nutr. 1999, 53, 216–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gillies, N.; Cameron-Smith, D.; Pundir, S.; Wall, C.; Milan, A. Exploring trajectories in dietary adequacy of the B vitamins folate, riboflavin, vitamins B 6 and B 12, with advancing older age: A systematic review. Br. J. Nutr. 2020, 1–32, (online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Wham, C.; Teh, R.; Moyes, S.A.; Rolleston, A.; Muru-Lanning, M.; Hayman, K.; Kerse, N.; Adamson, A. Micronutrient intake in advanced age: Te Puāwaitanga o Ngā Tapuwae Kia ora Tonu, Life and Living in Advanced Age: A Cohort Study in New Zealand (LiLACS NZ). Br. J. Nutr. 2016, 116, 1754–1769. [Google Scholar] [CrossRef] [PubMed]
- Ongena, K.P.S. B-Vitamin Status and Homocysteine in Older Adults in Residential Aged Care Facilities in New Zealand: A Cross-Sectional Study. Ph.D. Dissertation, University of Otago, Otago, New Zealand, 6 April 2020. [Google Scholar]
- O’Keeffe, S.T. Thiamine deficiency in elderly people. Age Ageing 2000, 29, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Hanger, H.C.; George, P.M.; Sainsbury, R. Is thiamine deficiency in elderly people related to age or co-morbidity? Age Ageing 2000, 29, 111–116. [Google Scholar] [CrossRef]
- Leblanc, V.; Bégin, C.; Corneau, L.; Dodin, S.; Lemieux, S. Gender differences in dietary intakes: WHAT is the contribution of motivational variables? J. Hum. Nutr. Diet. 2015, 28, 37–46. [Google Scholar] [CrossRef]
- Manippa, V.; Padulo, C.; Van Der Laan, L.N.; Brancucci, A.; Herrmann, M.J. Gender differences in food choice: Effects of superior temporal sulcus stimulation. Front. Hum. Neurosci. 2017, 11, 597. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Gender differences in metabolism; nutrition and supplements. J. Sci. Med. Sport 2000, 3, 287–298. [Google Scholar] [CrossRef]
- Veitch, R.L.; Lumeng, L.; Li, T.K. Vitamin B6 metabolism in chronic alcohol abuse. The effect of ethanol oxidation on hepatic pyridoxal 5’ phosphate metabolism. J. Clin. Investig. 1975, 55, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Azar, D.; Yun, Y.M.; Ghausi, O.; Ix, J.; Fitzgerald, R.L. Serum methylmalonic acid and holotranscobalamin-II as markers for vitamin B12 deficiency in end-stage renal disease patients. Ann. Clin. Lab. Sci. 2013, 43, 243–249. [Google Scholar] [PubMed]
- Herrmann, W.; Obeid, R.; Schorr, H.; Geisel, J. The Usefulness of Holotranscobalamin in Predicting Vitamin B12 Status in Different Clinical Settings. Curr. Drug Metab. 2005, 6, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Matsumoto, T.; Shino, Y.; Hashizume, N. Assay values for thiamine or thiamine phosphate esters in whole blood do not depend on the anticoagulant used. J. Clin. Lab. Anal. 2005, 19, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Portari, G.V.; Vannucchi, H.; Jordao, A.A. Liver, plasma and erythrocyte levels of thiamine and its phosphate esters in rats with acute ethanol intoxication: A comparison of thiamine and benfotiamine administration. Eur. J. Pharm. Sci. 2013, 48, 799–802. [Google Scholar] [CrossRef]
- Said, H.M.; Mohammed, Z.M.; Hamid, S.M.; Mohammed, Z.M.; Said, H.M.; Mohammed, Z.M. Intestinal absorption of water-soluble vitamins: An update. Curr. Opin. Gastroenterol. 2006, 22, 140–146. [Google Scholar] [CrossRef]
- Said, H.M.; Rose, R.; Seetharam, B. Intestinal absorption of water-soluble vitamins: Cellular and molecular aspects. In Gastrointestinal Transport: Molecular Physiology; Current Topics in Membranes; Barnett, K.E., Donwitz, M., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 50, pp. 35–75. ISBN 0121533506. [Google Scholar]
- Laird, E.; Casey, M.C.; Ward, M.; Hoey, L.; Hughes, C.F.; McCarroll, K.; Cunningham, C.; Strain, J.J.; McNulty, H.; Molloy, A.M. Dairy intakes in older Irish adults and effects on vitamin micronutrient status: Data from the TUDA study. J. Nutr. Health Aging 2017, 21, 954–961. [Google Scholar] [CrossRef]
- Andrews, K.W.; Roseland, J.M.; Gusev, P.A.; Palachuvattil, J.; Dang, P.T.; Savarala, S.; Han, F.; Pehrsson, P.R.; Douglass, L.W.; Dwyer, J.T.; et al. Analytical ingredient content and variability of adult multivitamin/mineral products: National estimates for the Dietary Supplement Ingredient Database. Am. J. Clin. Nutr. 2017, 105, 526–539. [Google Scholar] [CrossRef]
- Lin, S.K.; Lambert, J.R.; Wahlqvist, M.L. Nutrition and gastrointestinal disorders. Asia Pac. J. Clin. Nutr. 1992, 1, 37–42. [Google Scholar]
- Jankowska, M.; Rutkowski, B.; Dębska-Ślizień, A. Vitamins and microelement bioavailability in different stages of chronic kidney disease. Nutrients 2017, 9, 282. [Google Scholar] [CrossRef]
- Iwakawa, H.; Nakamura, Y.; Fukui, T.; Fukuwatari, T.; Ugi, S.; Maegawa, H.; Doi, Y.; Shibata, K. Concentrations of water-soluble vitamins in blood and urinary excretion in patients with diabetes mellitus. Nutr. Metab. Insights 2016, 9, 85–92. [Google Scholar] [CrossRef] [PubMed]


| Breakfast Items | Older Adults | Younger Adults | ||
|---|---|---|---|---|
| M (n = 10) | F (n = 10) | M (n = 10) | F (n = 10) | |
| Toast (g) | 76 ± 1.3 | 73.6 ± 2.4 | 75.9 ± 1.5 | 72.5 ± 0.7 |
| Butter (g) | 9.3 ± 0.4 | 9.5 ± 0.3 | 9.1 ± 0.6 | 8.4 ± 1.0 |
| Honey (g) * | 18.9 ± 0.6 | 19.2 ± 0.5 | 13.8 ± 2.1 | 17.3 ± 1.5 |
| Applesauce (g)* | 100 ± 0 | 100 ± 0 | 77.3 ± 12.1 | 82 ± 12.1 |
| Orange Juice (mL) | 242.1 ± 7.9 | 250 ± 0 | 250 ± 0 | 235 ± 15 |
| Variable | Older Adults (n = 20) | Younger Adults (n = 20) | Effect | ||||
|---|---|---|---|---|---|---|---|
| M (n = 10) | F (n = 10) | M (n = 10) | F (n = 10) | Age | Sex | Age × Sex | |
| Age (years) | 71.1 ± 0.9 | 69.1 ± 0.7 | 23.3 ± 1.1 | 25.0 ± 0.5 | <0.001 * | 0.857 | 0.031 * |
| Weight (kg) | 80.4 ± 4.1 | 69.4 ± 3.3 | 77.9 ± 3.6 | 62.7 ± 2.4 | 0.185 | <0.001 * | 0.539 |
| Height (cm) | 176.0 ± 2.3 | 165.4 ± 1.6 | 174.4 ± 1.6 | 165.3 ± 2.0 | 0.653 | <0.001 * | 0.696 |
| BMI (kg/m2) | 25.9 ± 1.0 | 25.3 ± 1.0 | 25.6 ± 1.2 | 23.0 ± 0.9 | 0.226 | 0.135 | 0.321 |
| Waist circumference (cm) | 90.5 ± 3.5 | 86.3 ± 3.1 | 83.1 ± 3.0 | 75.3 ± 2.0 | 0.004 * | 0.051 | 0.545 |
| Systolic BP (mmHg) | 148 ± 4 | 128 ± 4 | 121 ± 3 | 110 ± 2 | <0.001 * | <0.001 * | 0.189 |
| Diastolic BP (mmHg) | 79 ± 3 | 73 ± 3 | 67 ± 1 | 71 ± 2 | 0.004 * | 0.723 | 0.059 |
| HOMA-IR | 1.31 ± 0.32 | 1.47 ± 0.22 | 1.97 ± 0.41 | 1.38 ± 0.28 | 0.358 | 0.490 | 0.234 |
| Fasting Serum Measures | |||||||
| Glucose (mmol/L) | 5.27 ± 0.18 | 4.74 ± 0.09 | 4.78 ± 0.15 | 4.49 ± 0.13 | 0.014 * | 0.007 * | 0.405 |
| Insulin (µU/mL) | 8.24 ± 1.65 | 6.51 ± 1.38 | 6.24 ± 1.46 | 7.39 ± 1.05 | 0.690 | 0.838 | 0.310 |
| Cholesterol (mmol/L) | 5.75 ± 0.39 | 6.61 ± 0.42 | 4.70 ± 0.33 | 4.57 ±0.27 | <0.001 * | 0.318 | 0.172 |
| HDL-C (mmol/L) | 1.40 ± 0.18 | 1.85 ± 0.09 | 1.47 ± 0.15 | 1.77 ± 0.14 | 0.960 | 0.006 * | 0.559 |
| LDL-C (mmol/L) | 3.85 ± 0.39 | 4.40 ± 0.39 | 2.84 ± 0.28 | 2.63 ± 0.26 | <0.001 * | 0.624 | 0.263 |
| Triglyceride (mmol/L) | 1.51± 0.19 | 1.31 ± 0.21 | 1.06 ± 0.15 | 0.79 ± 0.07 | 0.005 * | 0.156 | 0.803 |
| B-Vitamins | RDIo | Older Adults | RDIy | Younger Adults | Effect | ||||
|---|---|---|---|---|---|---|---|---|---|
| M, F | M (n = 10) | F (n = 10) | M, F | M (n = 9) 1 | F (n = 9) 1 | Age | Sex | Age × Sex | |
| Vitamin B12 (μg) | 2.4 | 2.95 ± 0.4 | 3.12 ± 0.2 | 2.4 | 6.34 ± 1.1 | 4.31 ± 1.0 | 0.003 * | 0.209 | 0.140 |
| Total Folate (μg) | 290.57 ± 62.5 | 377.45 ± 51.0 | 443.96 ± 105.8 | 168.40 ± 36.8 | 0.685 | 0.174 | 0.012 * | ||
| Folate, total DFE (μg) | 400 | 437.81 ± 91.8 | 448.93 ± 83.3 | 400 | 465.11 ± 116.2 | 178.04 ± 37.8 | 0.172 | 0.123 | 0.097 |
| Vitamin B6 (mg) | 1.7, 1.5 | 2.33 ± 0.3 | 2.69 ± 0.3 | 1.3 | 3.83 ± 0.6 | 1.38 ± 0.2 | 0.782 | 0.005 * | <0.001 * |
| Thiamin (mg) | 1.2, 1.1 | 1.60 ± 0.2 | 1.37 ± 0.1 | 1.2, 1.1 | 1.74 ± 0.4 | 0.77 ± 0.1 | 0.3 | 0.009 * | 0.098 |
| Riboflavin (mg) | 1.3, 1.1 2 | 1.88 ± 0.2 | 1.99 ± 0.2 | 1.3, 1.1 | 2.48 ± 0.4 | 1.20 ± 0.2 | 0.74 | 0.036 * | 0.013 * |
| Niacin (mg) | 19.35 ± 2.4 | 18.30 ± 1.5 | 32.66 ± 4.3 | 12.48 ± 2.2 | 0.18 | <0.001 * | 0.001 * | ||
| Niacin E (mg) | 16, 14 | 36.88 ± 3.1 | 33.59 ± 2.2 | 16, 14 | 63.59 ± 7.3 | 24.84 ± 3.4 | 0.044 * | <0.001 * | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Han, S.M.; Gillies, N.; Thorstensen, E.B.; Goy, M.; Barnett, M.P.G.; Roy, N.C.; Cameron-Smith, D.; Milan, A.M. Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults. Nutrients 2020, 12, 3529. https://doi.org/10.3390/nu12113529
Sharma P, Han SM, Gillies N, Thorstensen EB, Goy M, Barnett MPG, Roy NC, Cameron-Smith D, Milan AM. Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults. Nutrients. 2020; 12(11):3529. https://doi.org/10.3390/nu12113529
Chicago/Turabian StyleSharma, Pankaja, Soo Min Han, Nicola Gillies, Eric B. Thorstensen, Michael Goy, Matthew P. G. Barnett, Nicole C. Roy, David Cameron-Smith, and Amber M. Milan. 2020. "Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults" Nutrients 12, no. 11: 3529. https://doi.org/10.3390/nu12113529
APA StyleSharma, P., Han, S. M., Gillies, N., Thorstensen, E. B., Goy, M., Barnett, M. P. G., Roy, N. C., Cameron-Smith, D., & Milan, A. M. (2020). Circulatory and Urinary B-Vitamin Responses to Multivitamin Supplement Ingestion Differ between Older and Younger Adults. Nutrients, 12(11), 3529. https://doi.org/10.3390/nu12113529






