Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Test Food
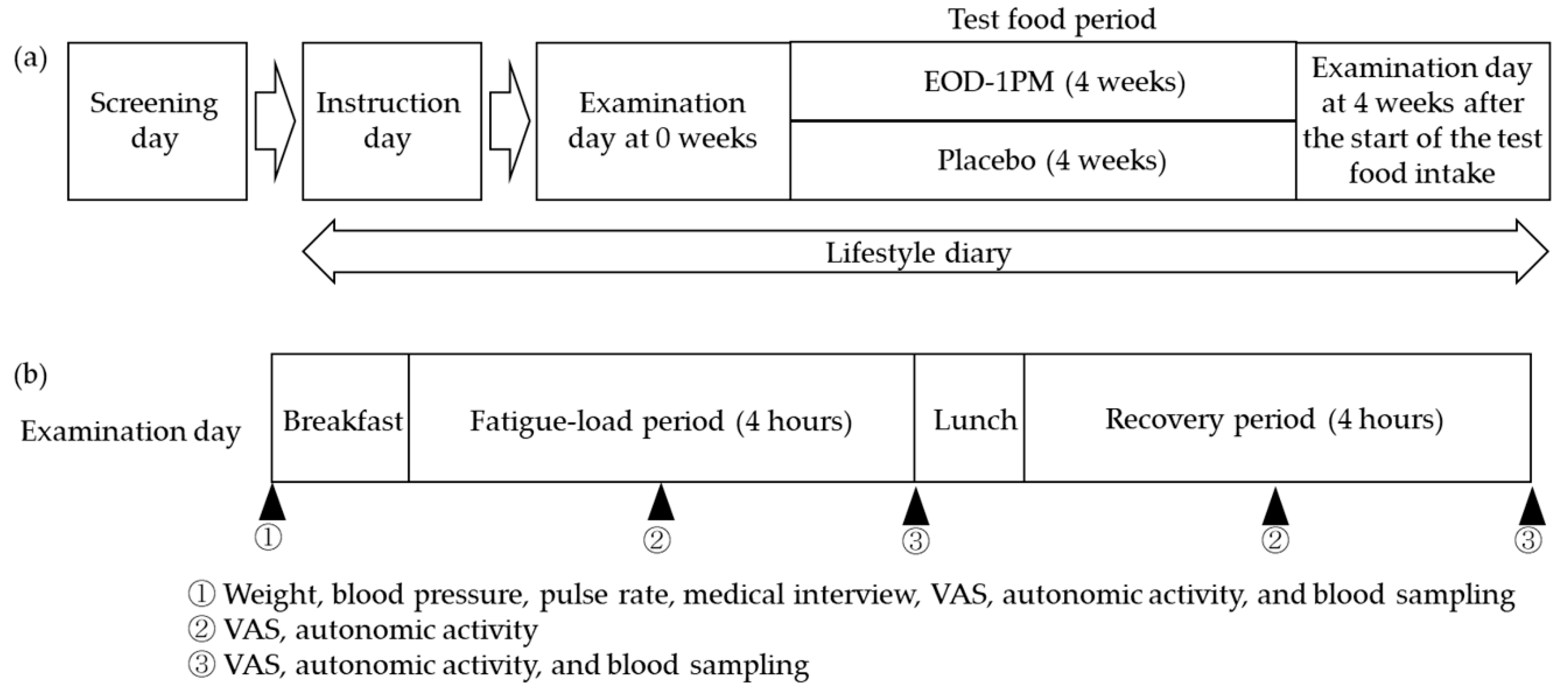
2.3. Study Design and Intake Method
2.4. Method of Fatigue Loading
2.5. Evaluation Method
2.6. Statistical Analysis
3. Results
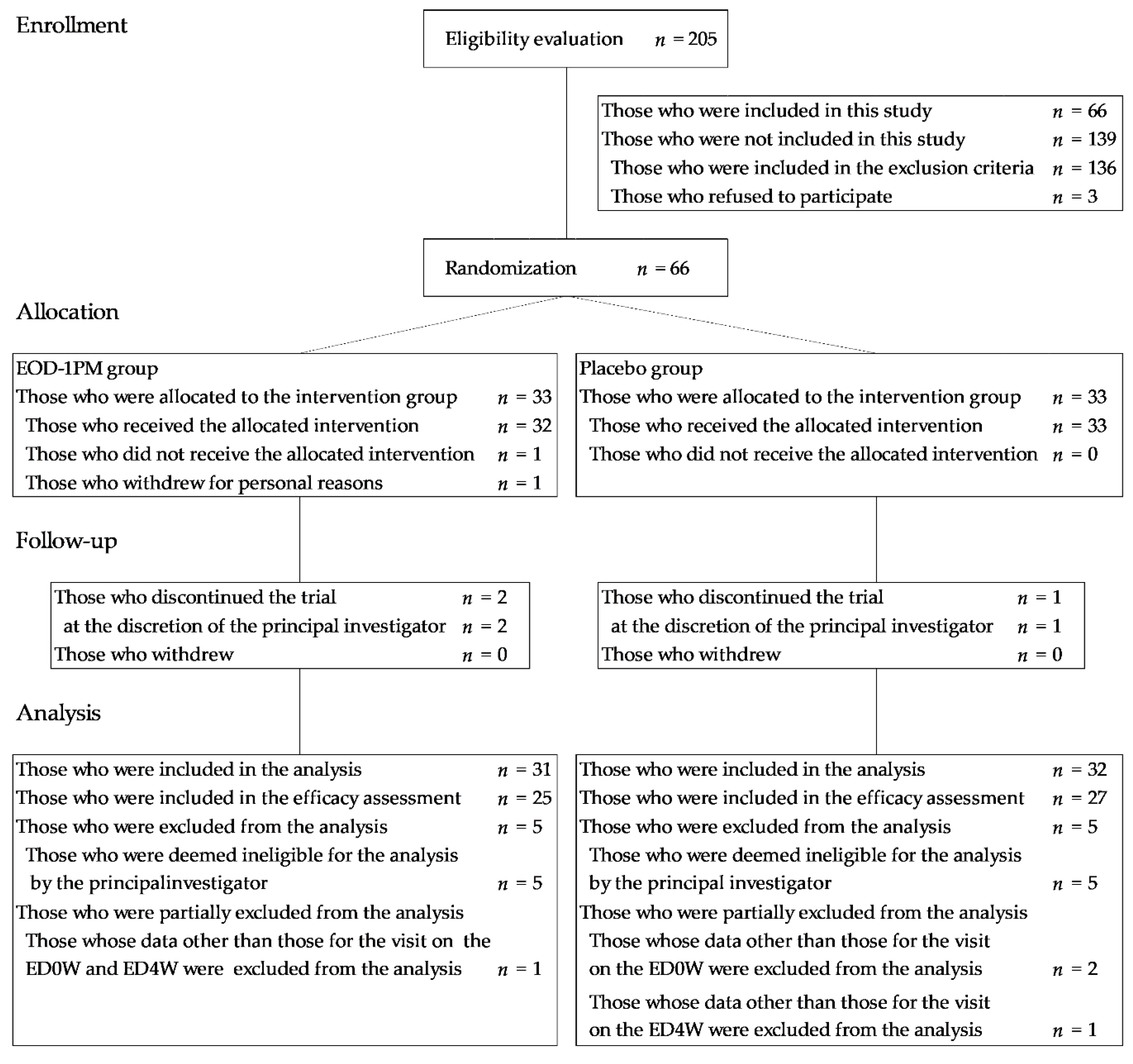
3.1. Subjects
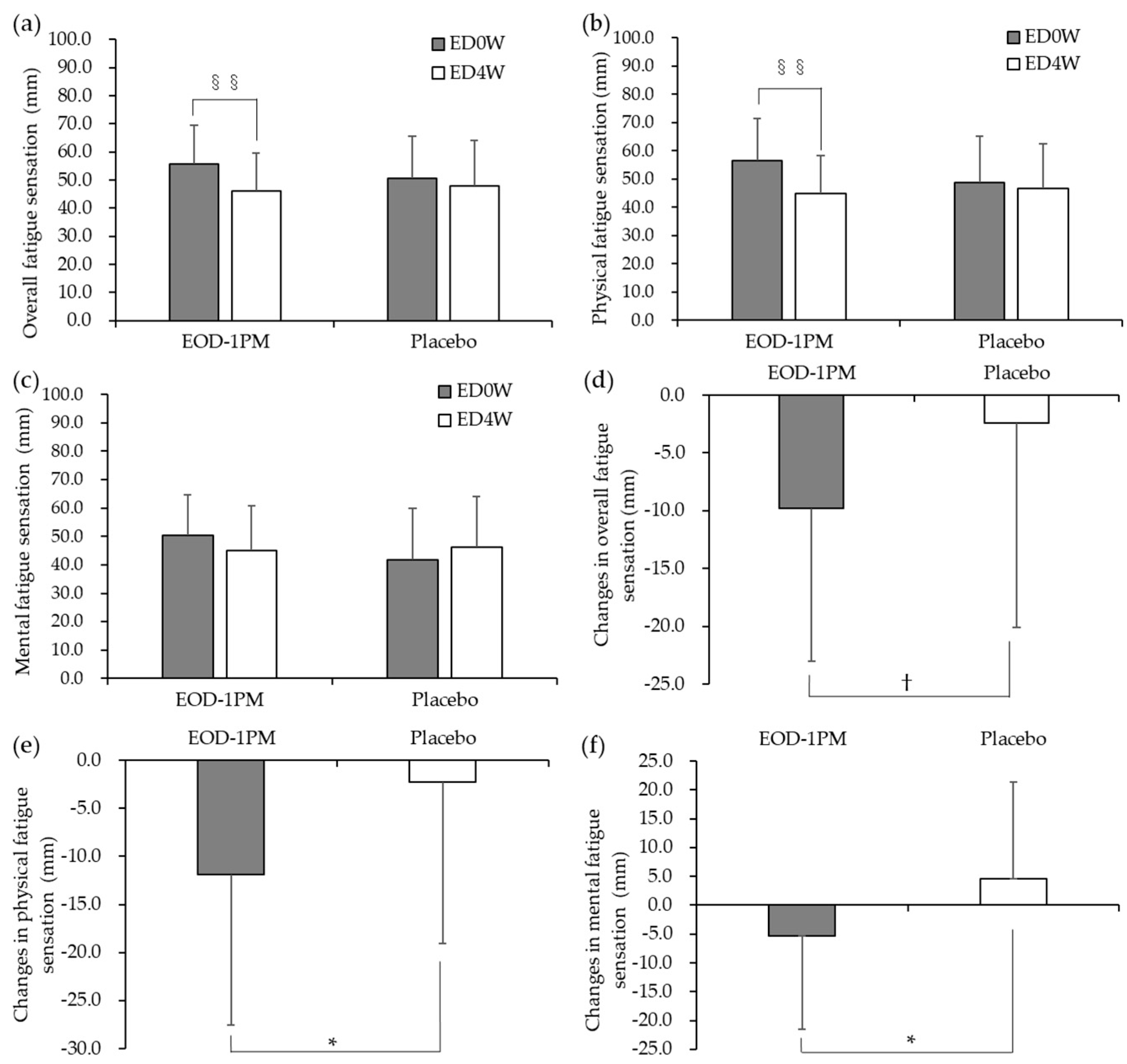
3.2. Subjective Evaluation by the VAS
3.3. Blood Cells and Blood Biochemistry Data
3.4. Oxidative Stress and Antioxidant Markers
3.5. Evaluation of Fatigue Load and Work Efficiency
3.6. Evaluation of Autonomic Nerve Function
3.7. Safety Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Kitani, T. Term Committee of Japanese Society of Fatigue Science. Nihon Hirougakkaishi 2011, 6, 1. [Google Scholar]
- Ishii, A.; Tanaka, M.; Watanabe, Y. Neural mechanisms of mental fatigue. Rev. Neurosci. 2014, 25, 469–479. [Google Scholar] [CrossRef]
- Tanaka, M.; Mizuno, K.; Tajima, S.; Sasabe, T.; Watanabe, Y. Central nervous system fatigue alters autonomic nerve activity. Life Sci. 2009, 84, 235–239. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef]
- Nozaki, S.; Tanaka, M.; Mizuno, K.; Ataka, S.; Mizuma, H.; Tahara, T.; Sugino, T.; Shirai, T.; Eguchi, A.; Okuyama, K.; et al. Mental and physical fatigue-related biochemical alterations. Nutrition 2009, 25, 51–57. [Google Scholar] [CrossRef]
- Watanabe, Y. What is fatigue?-Statistics of fatigue in Japan and what are the problems to be solved by fatigue science? J. Clin. Exp. Med. 2009, 228, 593–597. [Google Scholar]
- Watanabe, Y. Preface and Mini-review: Fatigue Science for Human Health. In Fatigue Science for Human Health; Springer: New York, NY, USA, 2008; pp. 5–11. [Google Scholar]
- Watanabe, Y.; Kuratsune, H.; Kajimoto, O. Biochemical Indices of Fatigue for Anti-fatigue Strategies and Products. In The Handbook of Operator Fatigue; CRC Press: Boca Raton, FL, USA, 2018; pp. 209–224. [Google Scholar]
- Silverman, M.N.; Heim, C.M.; Nater, U.M.; Marques, A.H.; Sternberg, E.M. Neuroendocrine and immune contributors to fatigue. PM R 2010, 2, 338–346. [Google Scholar] [CrossRef]
- Mizuno, K.; Watanabe, Y. Utility of an Advanced Trail Making Test as a Neuropsychological Tool for an Objective Evaluation of Work Efficiency during Mental Fatigue. In Fatigue Science for Human Health; Springer: New York, NY, USA, 2008; pp. 47–54. [Google Scholar]
- Fukuda, S.; Nojima, J.; Motoki, Y.; Yamaguti, K.; Nakatomi, Y.; Okawa, N.; Fujiwara, K.; Watanabe, Y.; Kuratsune, H. A potential biomarker for fatigue: Oxidative stress and anti-oxidative activity. Biol. Psychol. 2016, 118, 88–93. [Google Scholar] [CrossRef]
- Kume, S.; Yamato, M.; Tamura, Y.; Jin, G.; Nakano, M.; Miyashige, Y.; Eguchi, A.; Ogata, Y.; Goda, N.; Iwai, K.; et al. Potential biomarkers of fatigue identified by plasma metabolome analysis in rats. PLoS ONE 2015, 10, e0120106. [Google Scholar] [CrossRef]
- Yamano, E.; Sugimoto, M.; Hirayama, A.; Kume, S.; Yamato, M.; Jin, G.; Tajima, S.; Goda, N.; Iwai, K.; Fukuda, S.; et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci. Rep. 2016, 6, 34990. [Google Scholar] [CrossRef] [PubMed]
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sivonova, M.; Zitnanova, I.; Hlincikova, L.; Skodacek, I.; Trebaticka, J.; Durackova, Z. Oxidative stress in university students during examinations. Stress 2004, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y. Molecular and neural mechanisms of fatigue-previous hypotheses and present concepts. J. Clin. Exp. Med. 2009, 228, 598–604. [Google Scholar]
- Takahashi, M.; Kawashima, J.; Nishida, N.; Onaka, N. Beta-Glucan Derived from Euglena (Paramylon). In Trends in Basic Research and Applied Sciences of Beta-Glucan; CMC Publishing Co., Ltd.: Tokyo, Japan, 2018; pp. 174–182. [Google Scholar]
- Anraku, M.; Iohara, D.; Takada, H.; Awane, T.; Kawashima, J.; Takahashi, M.; Hirayama, F. Morphometric analysis of paramylon particles produced by Euglena gracilis EOD-1 using FIB/SEM tomography. Chem. Pharm. Bull. 2020, 68, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.I.; Nishioka, M.; Onaka, N.; Takahashi, M.; Yamanaka, D.; Adachi, Y.; Ohno, N. Effects of Euglena gracilis EOD-1 ingestion on salivary IgA reactivity and health-related quality of life in humans. Nutrients 2019, 11, 1144. [Google Scholar] [CrossRef]
- Aoe, S.; Yamanaka, C.; Koketsu, K.; Nishioka, M.; Onaka, N.; Nishida, N.; Takahashi, M. Effects of paramylon extracted from Euglena gracilis EOD-1 on parameters related to metabolic syndrome in diet-induced obese mice. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef]
- Sugiyama, A.; Suzuki, K.; Mitra, S.; Arashida, R.; Yoshida, E.; Nakano, R.; Yabuta, Y.; Takeuchi, T. Hepatoprotective effects of paramylon, a beta-1, 3-D-glucan isolated from Euglena gracilis Z, on acute liver injury induced by carbon tetrachloride in rats. J. Vet. Med. Sci. 2009, 71, 885–890. [Google Scholar] [CrossRef]
- Kawano, T.; Naito, J.; Nishioka, M.; Nishida, N.; Takahashi, M.; Ando, T.; Ebihara, S.; Watanabe, Y. Effects of consumption of food containing paramylon derived from Euglena gracilis EOD-1 on attenuating feelings of fatigue in daily life—A randomized, double-blind, placebo-controlled, parallel-group comparison study. Jpn. Pharmacol. Ther. 2019, 47, 1851–1859. [Google Scholar]
- Leung, A.W.; Chan, C.C.; Lee, A.H.; Lam, K.W. Visual analogue scale correlates of musculoskeletal fatigue. Percept. Mot. Skills 2004, 99, 235–246. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Okawa, M.; Uchiyama, M. Development of the Japanese version of the Pittsburgh Sleep Quality Index. Jpn. J. Psychiatr. Treat. 1998, 13, 755–763. [Google Scholar]
- Takada, M.; Ebara, T.; Sakai, Y. The Acceleration Plethysmography System as a new physiological technology for evaluating autonomic modulations. Health Eval. Promot. 2008, 35, 373–377. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Nakamura, A.; Hashiguchi, K.; Yamasaki, K.; Takashige, H.; Araki, E.; Saito, M. A randomized, double-blind, placebo-controlled parallel study of effects of food containing aged garlic extract on fatigue. Jpn. Pharmacol. Ther. 2017, 45, 405–421. [Google Scholar]
- Kajimoto, O.; Mieda, H.; Hiramitsu, M.; Sakaida, K.; Sugino, T.; Kajimoto, Y. Effect of a drink containing lemon citric acid on people frequently feeling fatigue. Jpn. Pharmacol. Ther. 2007, 35, 809–817. [Google Scholar]
- Kirchner, W.K. Age differences in short-term retention of rapidly changing information. J. Exp. Psychol. 1958, 55, 352–358. [Google Scholar] [CrossRef]
- Hirayama, Y. Development of guideline of clinical evaluation of anti-fatigue. J. Clin. Exp. Med. 2009, 228, 733–736. [Google Scholar]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N,N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2020, 26, 253–267. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, M.A.; Mendosa-Nunez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Pingitore, A.; Traghella, I.; Storti, S.; Parri, S.; Berti, S.; Ndreu, R.; Andrenelli, A.; Palmieri, C.; Iervasi, G.; et al. Emerging Biomarkers of Oxidative Stress in Acute and Stable Coronary Artery Disease: Levels and Determinants. Antioxidants 2019, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Satta, M.; Berna-Erro, A.; Carrasco-Garcia, E.; Alberro, A.; Saenz-Antonanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef] [PubMed]
- Katada, S.; Watanabe, T.; Mizuno, T.; Kobayashi, S.; Takeshita, M.; Osaki, N.; Katsuragi, Y. Effects of chlorogenic acid-enriched and hydroxyhydroquinone-reduced coffee on postprandial fat oxidation and antioxidative capacity in healthy men: A randomized, double-blind, placebo-controlled, crossover trial. Nutrients 2018, 10, 525. [Google Scholar] [CrossRef]
- Omi, N.; Shiba, H.; Nishimura, E.; Tsukamoto, S.; Maruki-Uchida, H.; Oda, M.; Morita, M. Effects of enzymatically modified isoquercitrin in supplementary protein powder on athlete body composition: A randomized, placebo-controlled, double-blind trial. J. Int. Soc. Sports Nutr. 2019, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Kuratsune, D.; Tajima, S.; Koizumi, J.; Yamaguti, K.; Sasabe, T.; Mizuno, K.; Tanaka, M.; Okawa, N.; Mito, H.; Tsubone, H.; et al. Changes in reaction time, coefficient of variance of reaction time, and autonomic nerve function in the mental fatigue state caused by long-term computerized Kraepelin test workload in healthy volunteers. World J. Neurosci. 2012, 2, 113–118. [Google Scholar] [CrossRef]
- Kajimoto, O.; Shiraichi, Y.; Kuriyama, A.; Ohtsuka, M.; Kadowaki, T.; Sugino, T. Effects of newly developed LED lighting on living comfort in an indoor environment (Part 3)—Evaluation of indoor work performance in the room under new LED lighting. Jpn. J. Complement. Altern. Med. 2013, 10, 25–37. [Google Scholar]
- Basner, M.; Rubinstein, J. Fitness for duty: A 3-minute version of the Psychomotor Vigilance Test predicts fatigue-related declines in luggage-screening performance. J. Occup. Environ. Med. 2011, 53, 1146–1154. [Google Scholar] [CrossRef]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef]
- Tanaka, M.; Mizuno, K.; Yamaguti, K.; Kuratsune, H.; Fujii, A.; Baba, H.; Matsuda, K.; Nishimae, A.; Takesaka, T.; Watanabe, Y. Autonomic nervous alterations associated with daily level of fatigue. Behav. Brain Funct. 2011, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Carroll, D.; Evans, P.; Bosch, J.A.; Clow, A.; Hucklebridge, F.; Der, G. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav. Immun. 2006, 20, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.M.; Engels, H.J. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med. Sci. Sports Exerc. 2005, 37, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Penailillo, L.; Maya, L.; Nino, G.; Torres, H.; Zbinden-Foncea, H. Salivary hormones and IgA in relation to physical performance in football. J. Sports Sci. 2015, 33, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Tajima, K.; Okada, N.; Rokushima, K.; Watanabe, Y. Effects of mild-stream bathing on recovery from mental fatigue. Med. Sci. Monit. 2010, 16, CR8–14. [Google Scholar]
- Onaka, N.; Nishioka, M.; Naito, J.; Nishida, N.; Takahashi, M.; Namba, T.; Ebihara, S.; Ishibashi, K.; Ohno, N. Safety evaluation of long-term intake of Euglena gracilis EOD-1—A randomized, double-blind, placebo-controlled, parallel-group comparison study. Jpn. Pharmacol. Ther. 2020, 48, 665–673. [Google Scholar]
| n | Men | Women | Age (Years) | BMI (kg/m2) | |
|---|---|---|---|---|---|
| EOD-1PM | 25 | 13 | 12 | 49.2 ± 6.5 | 23.4 ± 2.4 |
| Placebo | 27 | 12 | 15 | 48.9 ± 10.3 | 22.7 ± 2.6 |
| Values at the Screening Test | Number of ATMT Trials at the Time of Instruction | ||||
|---|---|---|---|---|---|
| VAS (Overall Fatigue Sensation) Score | Pittsburgh Sleep Quality Index Score | Evaluated Autonomic Nerve Function (Total Power) | Evaluated Autonomic Nerve Function (LF/HF) | ||
| EOD-1PM | 59.4 ± 8.0 | 5.2 ± 1.9 | 1350.9 ± 961.9 | 1.202 ± 1.232 | 797 ± 128 |
| Placebo | 59.9 ± 7.2 | 5.1 ± 1.9 | 1447.2 ± 732.9 | 1.200 ± 1.253 | 803 ± 129 |
| ED0W EOD-1PM (n = 25) Placebo (n = 27) | ED4W EOD-1PM (n = 25) Placebo (n = 27) | Changes before and after Intake EOD-1PM (n = 25) Placebo (n = 27) | |||||
|---|---|---|---|---|---|---|---|
| d-ROMs (U.CARR) | EOD-1PM | 307 ± 58 | 309 ± 54 | 3 ± 21 | |||
| Placebo | 326 ± 41 | 329 ± 39 | 2 ± 31 | ||||
| BAP (mmol/L) | EOD-1PM | 2085 ± 131 | 2238 ± 130 | * | §§ | 153 ± 171 | * |
| Placebo | 2097 ± 190 | 2147 ± 168 | 50 ± 187 | ||||
| BAP/d-ROMs | EOD-1PM | 7.0 ± 1.4 | 7.4 ± 1.4 | ** | § | 0.4 ± 0.8 | |
| Placebo | 6.5 ± 0.9 | 6.6 ± 0.8 | 0.1 ± 0.9 |
| ED0W Total for All Sets EOD-1PM (n = 24) Placebo (n = 25) | ED4W Total for All Sets EOD-1PM (n = 24) Placebo (n = 26) | Changes before and after Intake Total for All Sets EOD-1PM (n = 24) Placebo (n = 24) | |||||
|---|---|---|---|---|---|---|---|
| Task A | |||||||
| Mean reaction time (sec) | EOD-1PM | 1.673 ± 0.532 | 1.836 ± 0.684 | 0.164 ± 0.241 | |||
| Placebo | 1.639 ± 0.337 | 1.993 ± 0.752 | 0.164 ± 0.208 | ||||
| Standard deviation of reaction time (sec) | EOD-1PM | 1.213 ± 0.457 | 1.445 ± 0.693 | * | 0.233 ± 0.382 | ||
| Placebo | 1.345 ± 0.492 | 2.053 ± 1.096 | 0.476 ± 0.535 | ||||
| CV of reaction time | EOD-1PM | 72.8 ± 19.8 | 78.6 ± 24.5 | * | 5.8 ± 11.3 | * | |
| Placebo | 80.8 ± 19.3 | 100.2 ± 33.3 | 17.9 ± 20.9 | ||||
| Number of errors | EOD-1PM | 25.0 ± 27.1 | 29.1 ± 32.7 | 4.0 ± 10.7 | |||
| Placebo | 35.0 ± 37.4 | 38.2 ± 38.5 | 4.5 ± 23.6 | ||||
| Task B | |||||||
| Mean reaction time (sec) | EOD-1PM | 2.230 ± 0.616 | 2.392 ± 0.714 | 0.162 ± 0.242 | |||
| Placebo | 2.220 ± 0.465 | 2.693 ± 0.984 | 0.274 ± 0.446 | ||||
| Standard deviation of reaction time (sec) | EOD-1PM | 1.893 ± 0.554 | 2.183 ± 0.842 | * | 0.289 ± 0.530 | ||
| Placebo | 2.196 ± 0.751 | 3.034 ± 1.600 | 0.638 ± 1.261 | ||||
| CV of reaction time | EOD-1PM | 85.8 ± 16.8 | * | 91.2 ± 21.7 | * | 5.4 ± 12.7 | |
| Placebo | 97.7 ± 20.0 | 109.6 ± 30.4 | 11.9 ± 23.2 | ||||
| Number of errors | EOD-1PM | 23.6 ± 27.2 | 31.0 ± 35.4 | 7.4 ± 13.4 | |||
| Placebo | 38.7 ± 36.0 | 41.4 ± 40.0 | 5.0 ± 17.9 | ||||
| Task C | |||||||
| Mean reaction time (sec) | EOD-1PM | 3.303 ± 0.587 | 3.534 ± 0.735 | 0.231 ± 0.402 | |||
| Placebo | 3.341 ± 0.571 | 3.963 ± 1.047 | 0.390 ± 0.433 | ||||
| Standard deviation of reaction time (sec) | EOD-1PM | 2.099 ± 0.598 | 2.434 ± 0.904 | ** | 0.335 ± 0.673 | * | |
| Placebo | 2.359 ± 0.773 | 3.502 ± 1.651 | 0.832 ± 0.840 | ||||
| CV of reaction time | EOD-1PM | 63.0 ± 11.3 | 67.8 ± 14.8 | ** | 4.8 ± 9.3 | * | |
| Placebo | 69.5 ± 14.7 | 85.3 ± 25.6 | 13.7 ± 15.2 | ||||
| Number of errors | EOD-1PM | 31.0 ± 33.5 | 32.4 ± 38.4 | 1.5 ± 13.0 | * | ||
| Placebo | 35.8 ± 36.5 | 44.8 ± 40.3 | 10.7 ± 17.6 |
| ED0W Total for All Sets EOD-1PM (n = 24) Placebo (n = 25) | ED4W Total for All Sets EOD-1PM (n = 24) Placebo (n = 26) | ||
|---|---|---|---|
| Rate of correct responses | EOD-1PM | 0.863 ± 0.102 | 0.853 ± 0.127 |
| Placebo | 0.850 ± 0.093 | 0.841 ± 0.102 |
| 2 h after Load EOD-1PM (n = 24) Placebo (n = 24) | 4 h after Load EOD-1PM (n = 24) Placebo (n = 24) | 2 h after Recovery EOD-1PM (n = 24) Placebo (n = 24) | 4 h after Recovery EOD-1PM (n = 24) Placebo (n = 24) | |||
|---|---|---|---|---|---|---|
| LF (msec2) | EOD-1PM | 9.8 ± 373.5 | 41.9 ± 571.1 | −34.8 ± 401.4 | −88.1 ± 370.0 | |
| Placebo | −112.7 ± 509.0 | −89.1 ± 889.0 | 9.7 ± 512.9 | −21.5 ± 675.2 | ||
| HF (msec2) | EOD-1PM | 98.0 ± 270.3 | 72.0 ± 426.6 | 183.7 ± 344.7 | −50.0 ± 366.5 | |
| Placebo | 30.7 ± 405.0 | 50.1 ± 535.9 | 38.1 ± 365.4 | −8.9 ± 583.1 | ||
| Total Power (msec2) | EOD-1PM | 469.9 ± 1780.1 | 460.9 ± 1548.5 | 421.2 ± 1281.5 | −163.0 ± 1248.0 | |
| Placebo | −60.0 ± 1435.1 | 154.4 ± 2270.9 | 121.4 ± 1967.4 | 437.0 ± 2888.1 | ||
| LF/HF | EOD-1PM | −0.623 ± 1.903 | −0.719 ± 1.555 | −0.389 ± 1.024 | * | −0.364 ± 1.187 |
| Placebo | −0.209 ± 1.913 | −0.302 ± 2.379 | 0.463 ± 1.568 | 0.025 ± 1.400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, T.; Naito, J.; Nishioka, M.; Nishida, N.; Takahashi, M.; Kashiwagi, S.; Sugino, T.; Watanabe, Y. Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Nutrients 2020, 12, 3098. https://doi.org/10.3390/nu12103098
Kawano T, Naito J, Nishioka M, Nishida N, Takahashi M, Kashiwagi S, Sugino T, Watanabe Y. Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Nutrients. 2020; 12(10):3098. https://doi.org/10.3390/nu12103098
Chicago/Turabian StyleKawano, Takanori, Junko Naito, Machiko Nishioka, Norihisa Nishida, Madoka Takahashi, Shinichi Kashiwagi, Tomohiro Sugino, and Yasuyoshi Watanabe. 2020. "Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial" Nutrients 12, no. 10: 3098. https://doi.org/10.3390/nu12103098
APA StyleKawano, T., Naito, J., Nishioka, M., Nishida, N., Takahashi, M., Kashiwagi, S., Sugino, T., & Watanabe, Y. (2020). Effect of Food Containing Paramylon Derived from Euglena gracilis EOD-1 on Fatigue in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial. Nutrients, 12(10), 3098. https://doi.org/10.3390/nu12103098





