Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. High Performance Liquid Chromatography (HPLC) Determination of the Principal Circulating Thiols in Plasma and Erythrocytes
2.3. HPLC Determination of Vitamin C (ascorbic acid), Vitamin A (retinol), Vitamin E (α-tocopherol)
2.4. HPLC Determination of Urinary Neopterin
2.5. Statistics
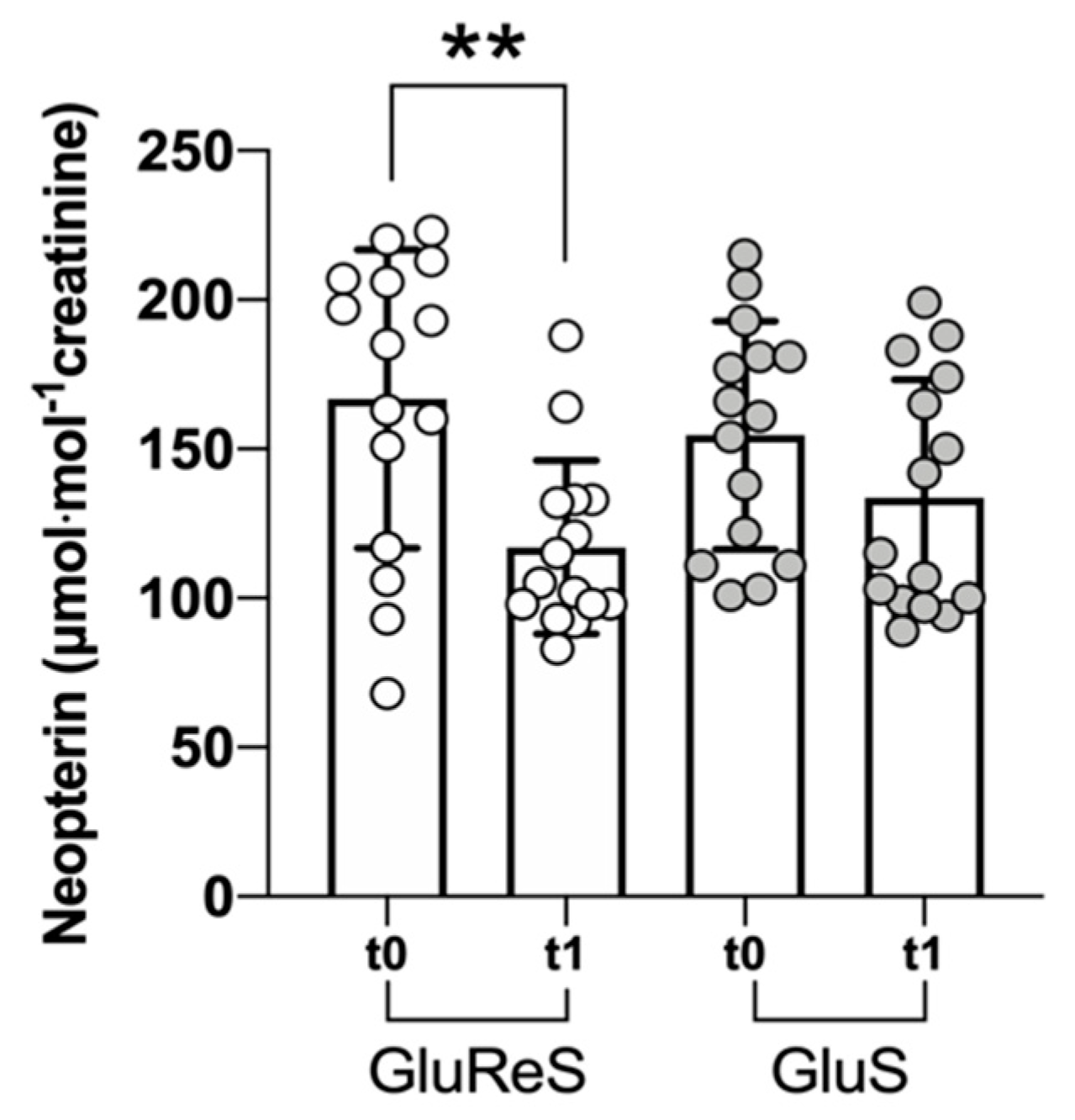
3. Results
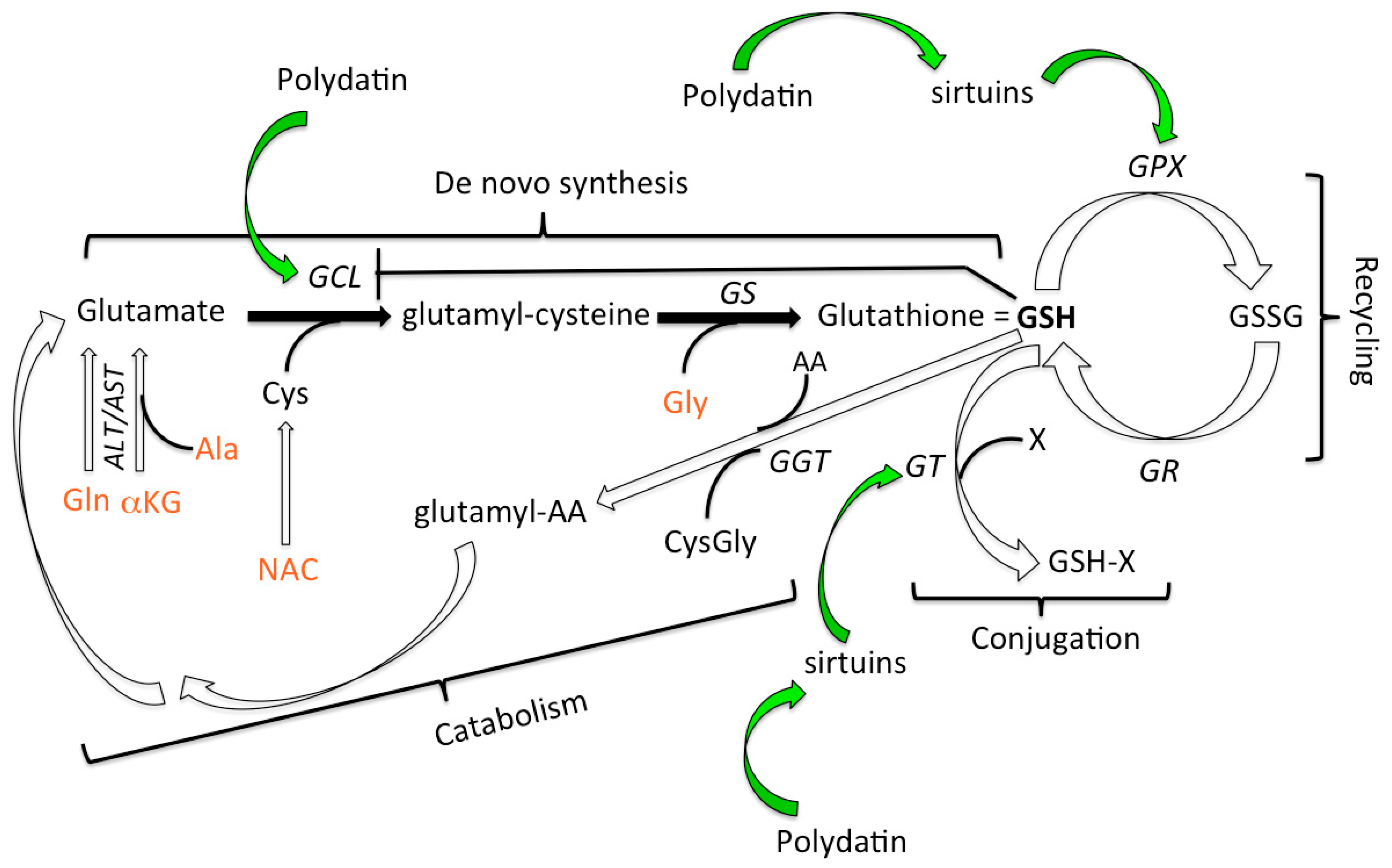
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pomatto, L.C.; Davies, K.J. Adaptive homeostasis and the free radical theory of ageing. Free. Radic. Biol. Med. 2018, 124, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nat. Cell Biol. 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nat. Cell Biol. 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Hornbrook, K. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1997, 1362, 116–127. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 294, 19683–19708. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Aw, T.Y.; Jones, D.P. Glutathione-dependent projection against oxidative injury. Pharmacol. Ther. 1990, 47, 61–71. [Google Scholar] [CrossRef]
- DePonte, M. The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Huang, M.-E. The Unfinished Puzzle of Glutathione Physiological Functions, an Old Molecule That Still Retains Many Enigmas. Antioxid. Redox Signal. 2017, 27, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.E.; Sunal, E.; Kanbak, G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Shenvi, S.; Hagen, T.M.; Liu, R.-M. Glutathione Metabolism during Aging and in Alzheimer Disease. Ann. N. Y. Acad. Sci. 2004, 1019, 346–349. [Google Scholar] [CrossRef]
- Rebrin, I.; Sohal, R.S. Pro-oxidant shift in glutathione redox state during aging. Adv. Drug Deliv. Rev. 2008, 60, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2017, 72, 105–111. [Google Scholar] [CrossRef]
- Gould, R.L.; Pazdro, R. Impact of Supplementary Amino Acids, Micronutrients, and Overall Diet on Glutathione Homeostasis. Nutrition 2019, 11, 1056. [Google Scholar] [CrossRef]
- Whillier, S.; Garcia, B.; Chapman, B.E.; Kuchel, P.W.; Raftos, J.E. Glutamine and α-ketoglutarate as glutamate sources for glutathione synthesis in human erythrocytes. FEBS J. 2011, 278, 3152–3163. [Google Scholar] [CrossRef]
- Ellinger, J.J.; Lewis, I.A.; Markley, J.L. Role of aminotransferases in glutamate metabolism of human erythrocytes. J. Biomol. NMR 2011, 49, 221–229. [Google Scholar] [CrossRef]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A. N-Acetylcysteine—A safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione Synthesis Is Diminished in Patients with Uncontrolled Diabetes and Restored by Dietary Supplementation with Cysteine and Glycine. Diabetes Care 2010, 34, 162–167. [Google Scholar] [CrossRef]
- Du, Q.-H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Beretta, A.; Accinni, R.; Dellanoce, C.; Tonini, A.; Cardot, J.-M.; Bussière, A. Efficacy of a Standardized Extract ofPrunus mumein Liver Protection and Redox Homeostasis: A Randomized, Double-Blind, Placebo-Controlled Study. Phytotherapy Res. 2016, 30, 949–955. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nat. Cell Biol. 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Henry-Vitrac, C.; Desmoulière, A.; Girard, D.; Mérillon, J.-M.; Krisa, S. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur. J. Nutr. 2006, 45, 376–382. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef]
- Su, D.; Cheng, Y.; Liu, M.; Liu, D.; Cui, H.; Zhang, B.-L.; Zhou, S.; Yang, T.; Mei, Q. Comparision of Piceid and Resveratrol in Antioxidation and Antiproliferation Activities In Vitro. PLoS ONE 2013, 8, e54505. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Labanda, J.F.; Mallavia, R.; Pérez-Fons, L.; Lizama, V.; Saura, A.D.; Micol, V. Determination of Piceid and Resveratrol in Spanish Wines Deriving fromMonastrell(Vitis vinifera L.) Grape Variety. J. Agric. Food Chem. 2004, 52, 5396–5403. [Google Scholar] [CrossRef]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, Z.; Xu, S.; Zhang, Q.; Wang, X.; Gao, Y.; Zhao, K.-S. Polydatin Protecting Kidneys against Hemorrhagic Shock-Induced Mitochondrial Dysfunction via SIRT1 Activation and p53 Deacetylation. Oxidative Med. Cell. Longev. 2016, 2016, 1737185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, Y.; Dai, X.; Xu, S.; Li, T.; Zhang, Q.; Zhao, K.-S.; Chen, Z. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert Opin. Ther. Targets 2016, 20, 645–652. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Cheng, Z.; Xiong, Z.; Lv, J.; Yang, Z.; Li, T.; Jiang, S.; Gu, J.; Sun, D.; et al. Polydatin ameliorates diabetic cardiomyopathy via Sirt3 activation. Biochem. Biophys. Res. Commun. 2017, 493, 1280–1287. [Google Scholar] [CrossRef]
- Cao, K.; Lei, X.; Liu, H.; Zhao, H.; Guo, J.; Chen, Y.; Xu, Y.; Cheng, Y.; Liu, C.; Cui, J.; et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial-mesenchymal transition. J. Cell. Mol. Med. 2017, 21, 3264–3276. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef]
- Ren, Y.; Du, C.; Shi, Y.; Wei, J.; Wu, H.; Cui, H. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 2017, 39, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.-N.; Zhou, H.-Z.; Tang, H.; Cai, X.-F.; Zhang, W.-L.; Ren, J.-H.; Zhou, L.; Chen, X.; Chen, K.; Li, W.-Y.; et al. Sirtuin 3 enhanced drug sensitivity of human hepatoma cells through glutathione S-transferase pi 1/JNK signaling pathway. Oncotarget 2016, 7, 50117–50130. [Google Scholar] [CrossRef] [PubMed]
- Dellanoce, C.; Cozzi, L.; Zuddas, S.; Pratali, L.; Accinni, R. Determination of different forms of aminothiols in red blood cells without washing erythrocytes. Biomed. Chromatogr. 2013, 28, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Svardal, A.M.; Mansoor, M.A.; Ueland, P.M. Determination of reduced, oxidized, and protein-bound glutathione in human plasma with precolumn derivatization with monobromobimane and liquid chromatography. Anal. Biochem. 1990, 184, 338–346. [Google Scholar] [CrossRef]
- Ford, L.; Farr, J.; Morris, P.; Berg, J. The value of measuring serum cholesterol-adjusted vitamin E in routine practice. Ann. Clin. Biochem. Int. J. Lab. Med. 2006, 43, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, P.; Devika, P.T. Assessment of in vitro antioxidant activity study of polydatin. J. Pharmacogn. Phytochem. 2019, 8, 55–58. [Google Scholar]
- Webster, B.R.; Lu, Z.; Sack, M.N.; Scott, I. The role of sirtuins in modulating redox stressors. Free. Radic. Biol. Med. 2012, 52, 281–290. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.-Y.; Kong, A.-N.T. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tang, Q.; Jin, J.; Zheng, G.; Xu, J.; Huang, W.; Li, X.; Shang, P.; Liu, H.-X. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018, 9, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss. Arch. Ophthalmol. 2001, 119, 1417–1436. [CrossRef] [PubMed]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2010, 105, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.; Jorgenson, D.; Landry, E.J.; Whiting, S.J. Vitamin and mineral supplement use in medically complex, community-living, older adults. Appl. Physiol. Nutr. Metab. 2019, 44, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Guallar, E.; Stranges, S.; Mulrow, C.; Appel, L.J.; Miller, E.R. Enough Is Enough: Stop Wasting Money on Vitamin and Mineral Supplements. Ann. Intern. Med. 2013, 159, 850–851. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Lou, T.; Jiang, W.; Xu, D.; Chen, T.; Fu, Y. Inhibitory Effects of Polydatin on Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflammation 2015, 38, 1213–1220. [Google Scholar] [CrossRef]
- Jiang, Q.; Yi, M.; Guo, Q.; Wang, C.; Wang, H.; Meng, S.; Liu, C.; Fu, Y.; Ji, H.; Chen, T. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway. Int. Immunopharmacol. 2015, 29, 370–376. [Google Scholar] [CrossRef]
- Jiang, K.-F.; Zhao, G.; Deng, G.-Z.; Wu, H.-C.; Yin, N.-N.; Chen, X.-Y.; Qiu, C.-W.; Peng, X.-L. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol. Sin. 2016, 38, 211–222. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, K.; Wu, H.; Qiu, C.; Deng, G.; Peng, X. Polydatin reducesStaphylococcus aureuslipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR2-NFκB signalling. J. Cell. Mol. Med. 2017, 21, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L.; et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017, 7, 11895. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lan, Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food Funct. 2017, 8, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.; Campbell, M.; Eccles, M.; Steen, N. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam. Pr. 2000, 17, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Borges-Santos, M.D.; Moreto, F.; Pereira, P.C.M.; Ming-Yu, Y.; Burini, R.C. Plasma glutathione of HIV+ patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 2012, 28, 753–756. [Google Scholar] [CrossRef]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef]
- Mandal, P.K.; Shukla, D.; Tripathi, M.; Ersland, L. Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward. J. Alzheimer’s Dis. 2019, 68, 531–535. [Google Scholar] [CrossRef]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [CrossRef]
- Lian, G.; Gnanaprakasam, J.R.; Wang, T.; Wu, R.; Chen, X.; Liu, L.; Shen, Y.; Yang, M.; Yang, J.J.; Chen, Y.; et al. Glutathione de novo synthesis but not recycling process coordinates with glutamine catabolism to control redox homeostasis and directs murine T cell differentiation. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]


| GluReS | GluS | |
|---|---|---|
| Weight (kg) | 68.65 (14.61) | 75.36 (13.24) |
| Height (m) | 1.69 (0.11) | 1.70 (0.09) |
| BMI (kg/m2) | 23.80 (2.45) | 25.87 (3.62) |
| Waistline (cm) | 90.54 (8.05) | 98.83 (11.95) |
| Heart rate | 68.46 (9.39) | 65.08 (8.55) |
| Sistolic blood pressure | 122.92 (10.77) | 125.58 (12.72) |
| Diastolic blood pressure | 86.77 (4.73) | 83.08 (8.71) |
| GluReS | GluS | |
|---|---|---|
| mg | mg | |
| glutamine, α-ketoglutarate | 217 | 217 |
| N-acetylcysteine | 210 | 210 |
| glycine | 105 | 105 |
| alanine | 126 | 126 |
| sodium selenite | 7 | 7 |
| polydatin | 35 | --- |
| total | 700 | 665 |
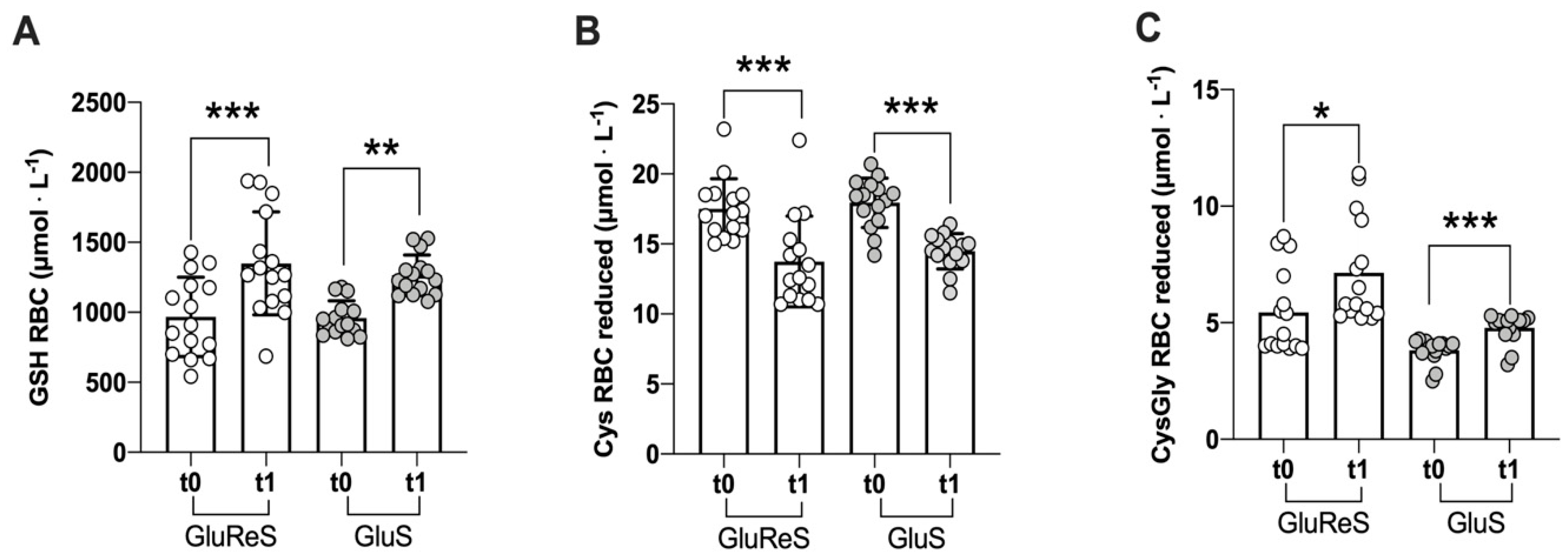
| Erythrocytes | GluReS | GluS | ||||
|---|---|---|---|---|---|---|
| t0 | t1 | %∆ | t0 | t1 | %∆ | |
| Glutathione | ||||||
| reduced | 967.59 ± 283.23 | 1349.87 ± 367.62 | +40 | 958.42 ± 123.74 | 1265.09 ± 144.95 | +32 |
| oxidized | 1262.95 ± 321.77 | 560.47 ± 251.47 | −56 | 1115.35 ± 346.66 | 231.67 ± 196.89 | −79 |
| Cysteine | ||||||
| reduced | 17.51 ± 2.17 | 13.74 ± 3.24 | −22 | 17.94 ± 1.77 | 14.49 ± 1.26 | −19 |
| oxidized | 28.46 ± 3.04 | 18.72 ± 1.72 | −34 | 27.24 ± 3.30 | 20.77 ± 2.89 | −24 |
| Cysteinylglycine | ||||||
| reduced | 5.43 ± 1.81 | 7.14 ± 2.26 | +32 | 3.81 ± 0.51 | 4.77 ± 0.63 | +25 |
| oxidized | 3.45 ± 0.67 | 1.92 ± 0.37 | −44 | 3.40 ± 0.70 | 1.79 ± 0.28 | −47 |
| Plasma | GluReS | GluS | ||||
| t0 | t1 | %∆ | t0 | t1 | %∆ | |
| Cysteine | ||||||
| reduced | 14.59 ± 1.67 | 20.73 ± 5.05 | +42 | 16.71 ± 1.36 | 19.35 ± 2.80 | +16 |
| oxidized | 300.81 ± 75.58 | 215.48 ± 46.55 | −28 | 292.28 ± 34.31 | 213.65 ± 39.17 | −27 |
| Cysteinylglycine | ||||||
| reduced | 5.63 ± 0.83 | 8.19 ± 2.30 | +45 | 7.62 ± 2.06 | 9.45 ± 2.13 | +24 |
| oxidized | 77.55 ± 25.95 | 53.95 ± 12.50 | −30 | 74.99 ± 10.48 | 47.28 ± 10.72 | −37 |
| vitamin C | p | vitamin A | p | vitamin E | p | |
|---|---|---|---|---|---|---|
| ∆% | ∆% | ∆% | ||||
| GluReS | +37 | <0.01 | +33 | <0.01 | +58 | <0.0001 |
| GluS | +11 | <0.001 | +14 | <0.001 | +39 | <0.0008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients 2020, 12, 3224. https://doi.org/10.3390/nu12113224
Biswas P, Dellanoce C, Vezzoli A, Mrakic-Sposta S, Malnati M, Beretta A, Accinni R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients. 2020; 12(11):3224. https://doi.org/10.3390/nu12113224
Chicago/Turabian StyleBiswas, Priscilla, Cinzia Dellanoce, Alessandra Vezzoli, Simona Mrakic-Sposta, Mauro Malnati, Alberto Beretta, and Roberto Accinni. 2020. "Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors" Nutrients 12, no. 11: 3224. https://doi.org/10.3390/nu12113224
APA StyleBiswas, P., Dellanoce, C., Vezzoli, A., Mrakic-Sposta, S., Malnati, M., Beretta, A., & Accinni, R. (2020). Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients, 12(11), 3224. https://doi.org/10.3390/nu12113224






