Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Extracts
2.3. Probiotic Bacteria Strain and Culture Conditions
2.4. Pathogenic Bacteria Strain and Culture Conditions
2.5. Caco-2 Cell Culture
2.6. Cell Viability Bioassay
2.7. Antimicrobial Activity
2.8. Infection of Caco-2 Cells with EHEC
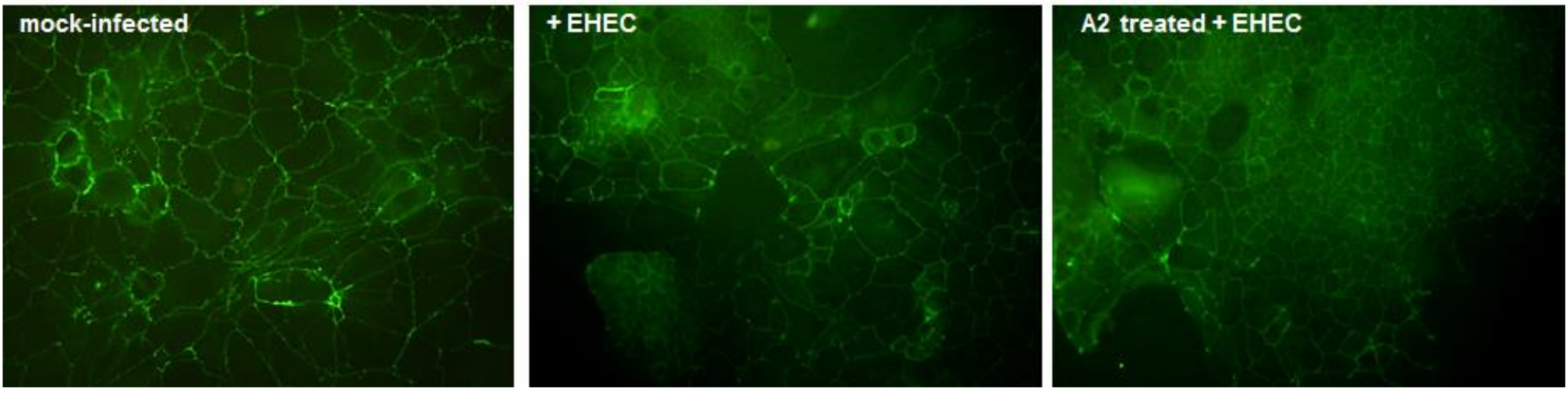
2.9. Immunofluorescence for the Tight Junction-Associated Protein Zona Occludens-1 (ZO-1)
2.10. RNA Extraction and Quantitative Real Time PCR
2.11. ELISA
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Extracts
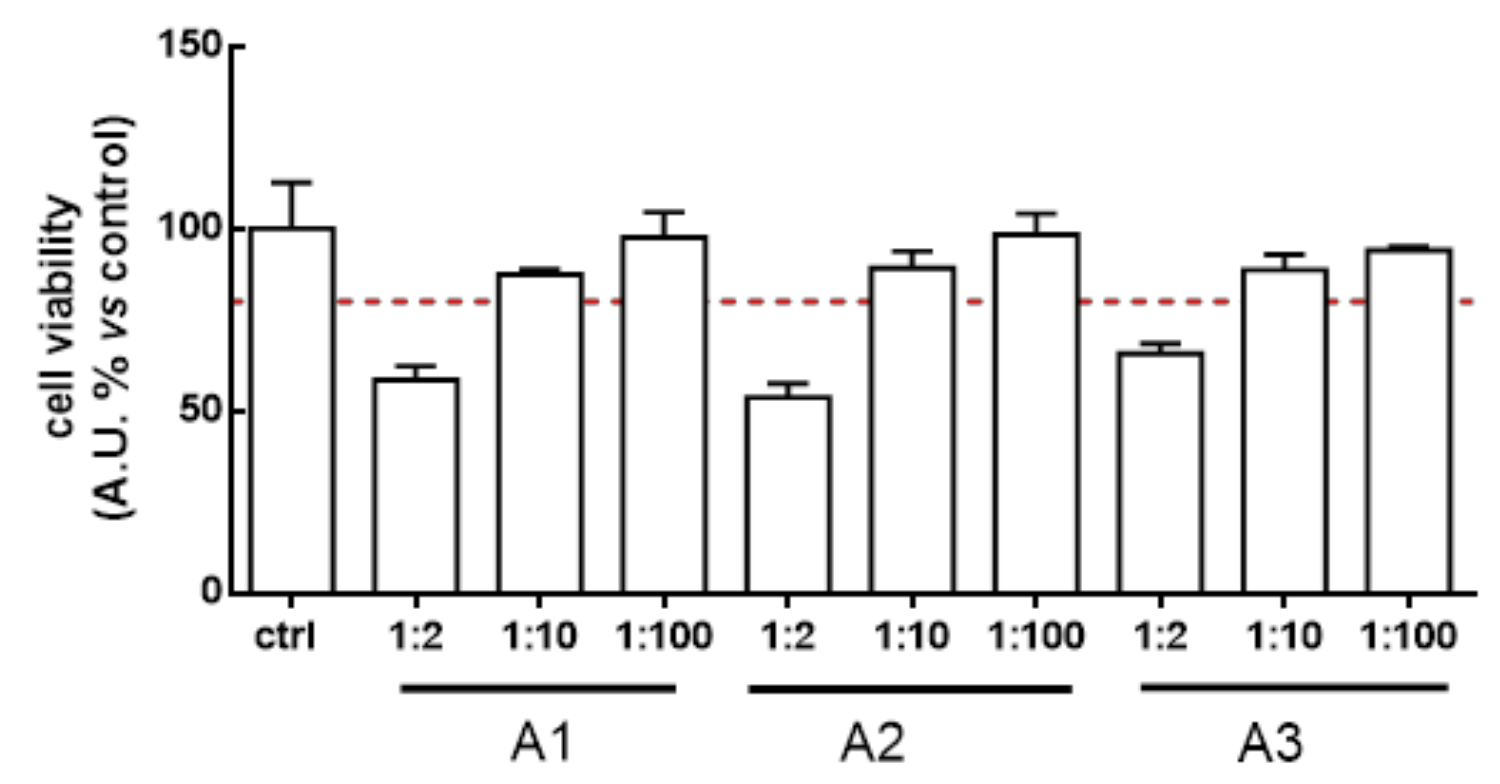
3.2. Lactobacillus Acidophilus Broth’s Safety in Human Intestinal Cells
3.3. Lactobacillus Acidophilus Broth Effects on Human Pathogenic Bacteria
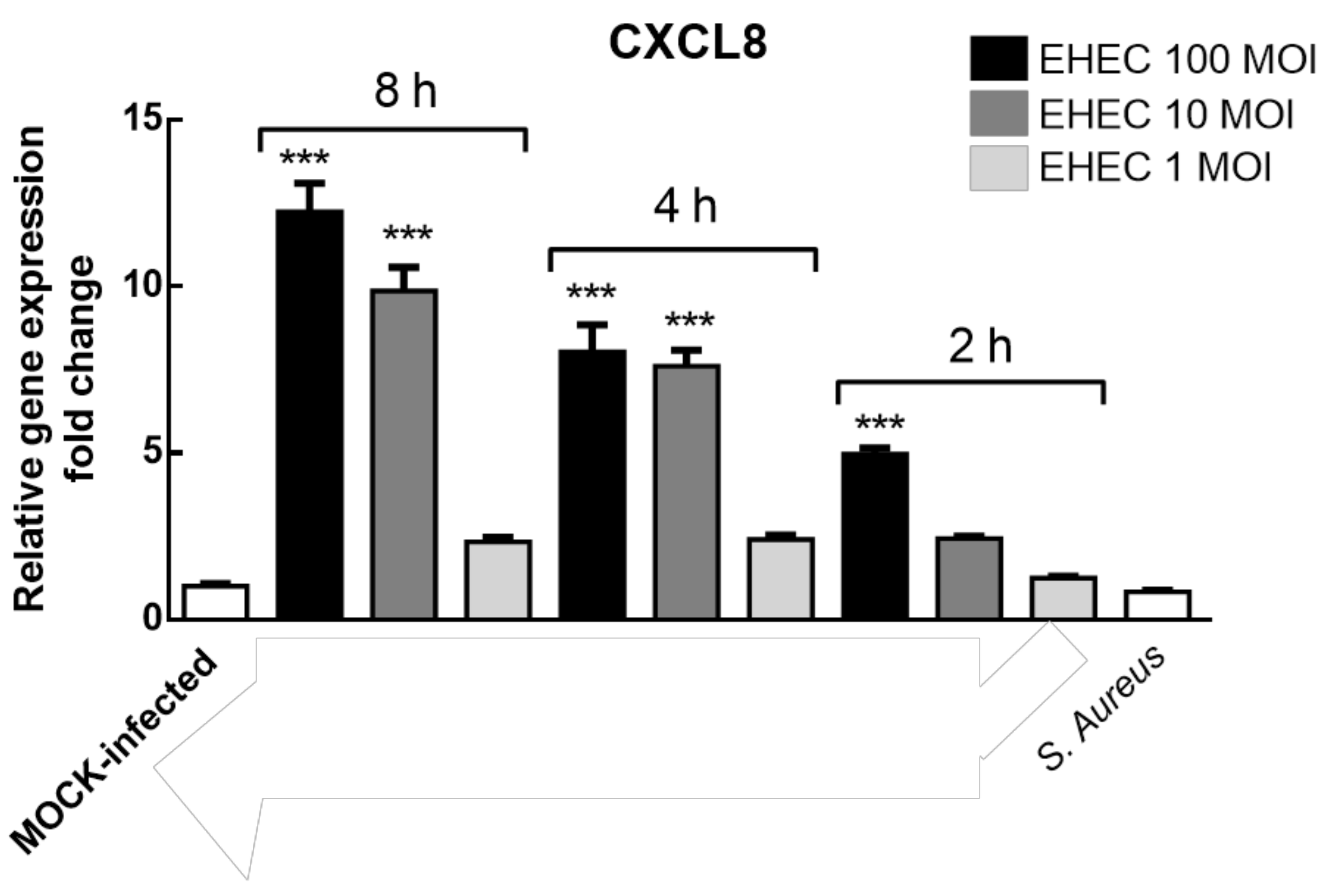
3.4. EHEC Infection of Caco-2 Cells Modelling
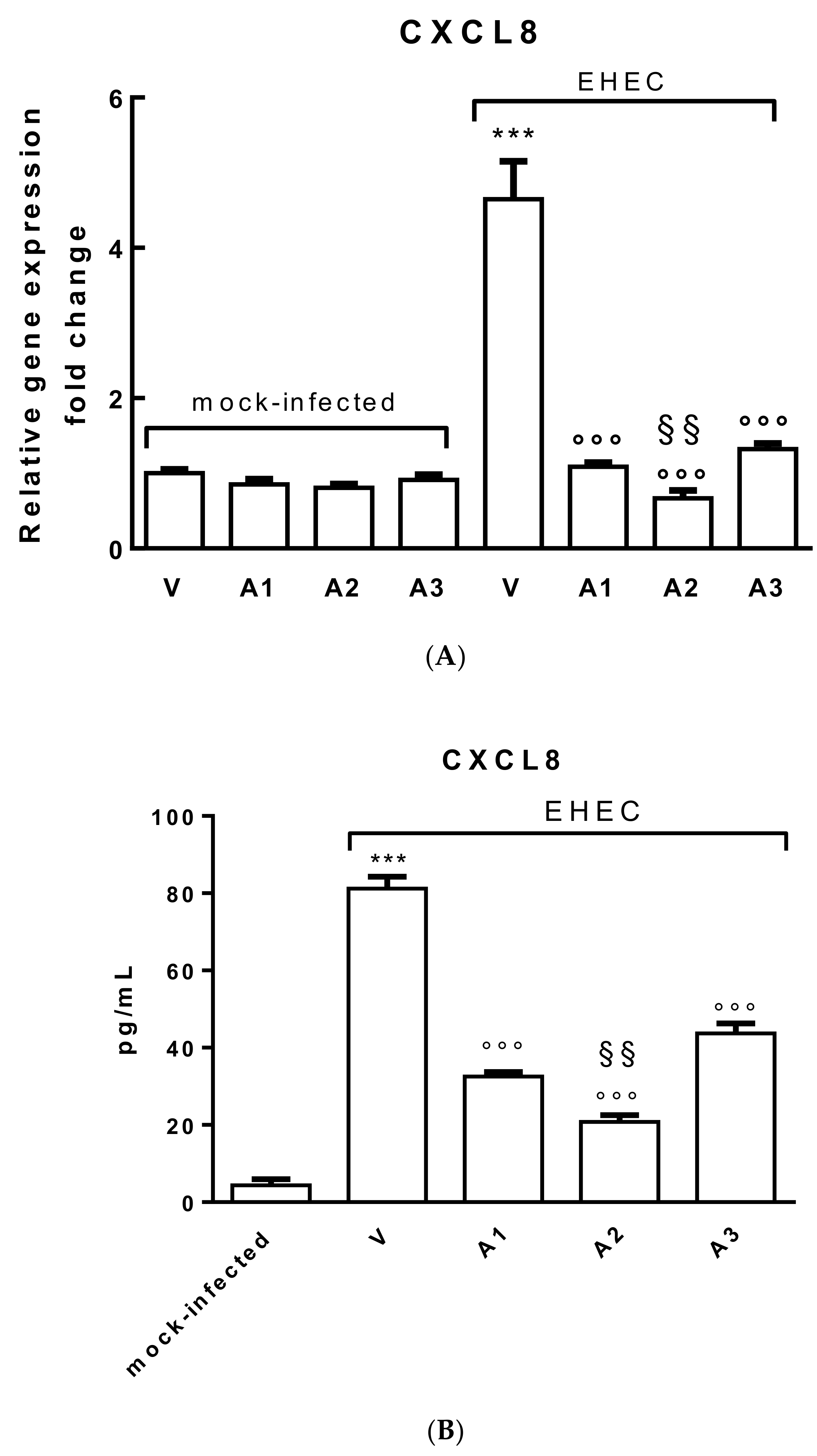
3.5. Effects of Probiotic Metabolites on CXCL8 Production by Polarized Caco-2 Cells Infected with EHEC
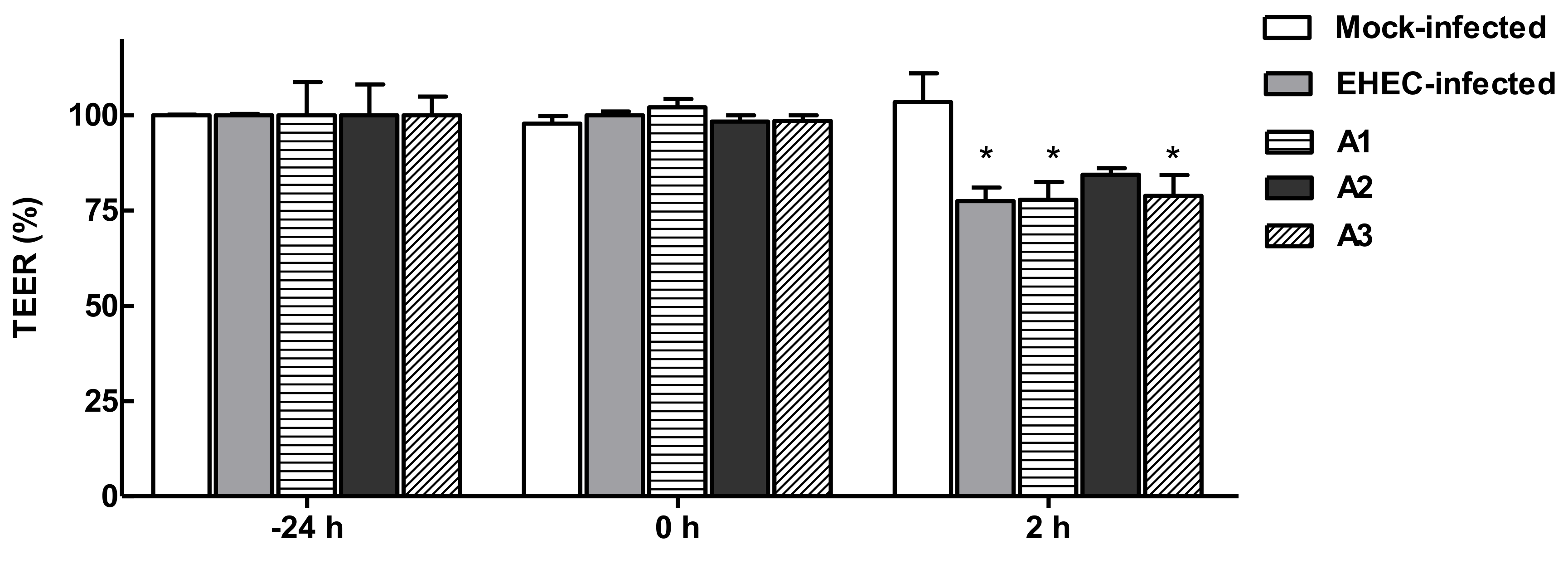
3.6. Functional Effects of LAB Broths on Barrier Integrity in Polarized Caco-2 Cells Infected with EHEC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desroches, S.; Lapointe, A.; Dugrenier, M.; Provencher, V.; Lamarche, B.; Desroches, S. A systematic review of the effect of yogurt consumption on chronic diseases risk markers in adults. Eur. J. Nutr. 2016, 56, 1375–1392. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of fermented foods in food guides around the world. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W. Health benefits of Kimchi (Korean Fermented Vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.-H.; Kim, B.-S.; Kim, M.J.; Kim, E.-J.; Kim, H. Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef]
- Ro, S.L.; Burn, M.W.; Sandine, W.E. Vitamin B12 and ascorbic acid in Kimchi inoculated with Propionibacterium freudenreichji ss. shermanii. J. Food Sci. 1979, 44, 873–877. [Google Scholar] [CrossRef]
- Du, R.; Song, G.; Zhao, D.; Sun, J.; Ping, W.; Ge, J. Lactobacillus casei starter culture improves vitamin content, increases acidity and decreases nitrite concentration during sauerkraut fermentation. Int. J. Food Sci. Technol. 2018, 53, 1925–1931. [Google Scholar] [CrossRef]
- Quirante-Moya, S.; García-Ibañez, P.; Quirante-Moya, F.; Villaño, D.; Moreno, D.A. The role of Brassica bioactives on human health: Are we studying it the right way? Molecules 2020, 25, 1591. [Google Scholar] [CrossRef]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.A.P.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1271527. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef]
- Ahrné, N.; Jeppsson, A.; Wold, A.E.; Molin, G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 1998, 85, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Shu, Q.; Lin, H.; Rutherfurd, K.J.; Cross, M.L. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med. Microbiol. Immunol. 2001, 190, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Donato, K.A.; Gareau, M.G.; Wang, Y.J.J.; Sherman, P.M. Lactobacillus rhamnosus GG attenuates interferon-γ and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiology 2010, 156, 3288–3297. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2013, 111, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. J. Dairy Sci. 2016, 99, 2654–2665. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; Rivas, B.D.L.; De Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.-W. In vitro probiotic characterization of Lactobacillus strains from fermented radish and their anti-adherence activity against enteric pathogens. Can. J. Microbiol. 2015, 61, 837–850. [Google Scholar] [CrossRef]
- Seong, G.-U.; Hwang, I.-W.; Chung, S.-K. Antioxidant capacities and polyphenolics of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) leaves. Food Chem. 2016, 199, 612–618. [Google Scholar] [CrossRef]
- Fratianni, F.; Pepe, S.; Cardinale, F.; Granese, T.; Cozzolino, A.; Coppola, R.; Nazzaro, F. Eruca sativa might influence the growth, survival under simulated gastrointestinal conditions and some biological features of Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus rhamnosus strains. Int. J. Mol. Sci. 2014, 15, 17790–17805. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 1428, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Arora, S.; Bhatia, A.; Singh, H.; Singh, B.; Arora, S. Molecular targets in cancer prevention by 4-(methylthio) butyl isothiocyanate-A comprehensive review. Life Sci. 2020, 241, 117061. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Mannelli, L.D.C.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1). Phytotherapy Res. 2019, 33, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Hichri, F.; Hichri, A.O.; Maha, M.; Hossan, A.S.M.; Flamini, G.; Ben Jannet, H. Chemical composition, antibacterial, antioxidant and in Vitro antidiabetic activities of essential oils from Eruca vesicaria. Chem. Biodivers. 2019, 16, e1900183. [Google Scholar] [CrossRef]
- Gupta, P.; Wright, S.E.; Kim, S.-H.; Srivastava, S.K. Phenethyl isothiocyanate: A comprehensive review of anti-cancer mechanisms. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2014, 1846, 405–424. [Google Scholar] [CrossRef]
- Naidu, S.D.; Suzuki, T.; Yamamoto, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Phenethyl Isothiocyanate, a dual activator of transcription factors NRF2 and HSF1. Mol. Nutr. Food Res. 2018, 62, 1700908. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 13677–13712. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Kobiela, W.; Herman-Antosiewicz, A.; Węgrzyn, A.; Szalewska-Pałasz, A.; Węgrzyn, G. Phenethyl isothiocyanate inhibits shiga toxin production in enterohemorrhagic Escherichia coli by stringent response induction. Antimicrob. Agents Chemother. 2014, 58, 2304–2315. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, J.; Hovde, C.J. A brief overview of Escherichia coli O157:H7 and its plasmid O157. J. Microbiol. Biotechnol. 2010, 20, 5–14. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.S. Growth-inhibiting activities of Phenethyl Isothiocyanate and its derivatives against intestinal bacteria. J. Food Sci. 2009, 74, M467–M471. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robineleon, S.; Appay, M.D.; Kedinger, M.; Haffen, K.; Fogh, J.; Zweibaum, A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell. 1983, 4, 323–330. [Google Scholar]
- Wathelet, J.-P.; Iori, R.; Leoni, O.; Quinsac, A.; Palmieri, S. Guidelines for glucosinolate analysis in green tissues used for biofumigation. Agroindustria 2004, 3, 257–266. [Google Scholar]
- ISO 9167:2019 Graines et tourteaux de colza-Dosage des glucosinolates-Méthode par chromatographie liquide à haute performance. Available online: https://www.iso.org/fr/standard/72207.html (accessed on 22 January 2019).
- Lazzeri, L.; Malaguti, L.; Bagatta, M.; D’Avino, L.; Ugolini, L.; De Nicola, G.; Casadei, N.; Cinti, S.; Matteo, R.; Iori, R. Characterization of the main glucosinolate content and fatty acid composition in non-food Brassicaceae seeds. Acta Hortic. 2013, 331–338. [Google Scholar] [CrossRef]
- Matteo, R.; D’Avino, L.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under low-input management systems in northern Italy: Yields, chemical characterization and environmental sustainability. Ital. J. Agron. 2020. [Google Scholar] [CrossRef]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Mannelli, L.D.C.; et al. Eruca sativa meal against diabetic neuropathic pain: An H2S-mediated effect of glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef]
- Barillari, J.; Gueyrard, D.; Rollin, P.; Iori, R. Barbarea verna as a source of 2-phenylethyl glucosinolate, precursor of cancer chemopreventive phenylethyl isothiocyanate. Fitoterapia 2001, 72, 760–764. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Chiozzi, R.Z.; Montone, C.M.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P.; et al. Peptides from cauliflower by-products, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxidative Med. Cell. Longev. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Bonvicini, F.; Manet, I.; Belluti, F.; Gobbi, S.; Rampa, A.; Gentilomi, G.A.; Bisi, A. Targeting the bacterial membrane with a new polycyclic privileged structure: A powerful tool to face Staphylococcus aureus infections. ACS Infect. Dis. 2019, 5, 1524–1534. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement techniques for In Vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Caliceti, C.; Aquila, G.; Pannella, M.; Morelli, M.B.; Fortini, C.; Pinton, P.; Bonora, M.; Hrelia, S.; Pannuti, A.; Miele, L.; et al. 17β-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-Notch1 axis in human endothelial cells. PLoS ONE 2013, 8, e71440. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, A.A.; Urbán, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state. Toxicol. Vitr. 2017, 45, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Dewald, B.; Moser, B. Human chemokines: An update. Annu. Rev. Immunol. 1997, 15, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Henry, K.C.; Pinnell, L.J.; Waskow, A.M.; Irrazabal, T.; Martin, A.; Hausner, M.; Sherman, P.M. Short-chain fructo-oligosaccharide and inulin modulate inflammatory responses and microbial communities in Caco2-bbe cells and in a mouse model of intestinal injury. J. Nutr. 2014, 144, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Gross, V.; Andus, T.; Daig, R.; Aschenbrenner, E.; Schölmerich, J.; Falk, W. Regulation of interleukin-8 production in a human colon epithelial cell line (HT-29). Gastroenterology 1995, 108, 653–661. [Google Scholar] [CrossRef]
- Fitzpatrick, M.M.; Shah, V.; Trompeter, R.S.; Dillon, M.J.; Barratt, T.M. Interleukin-8 and polymorphoneutrophil leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 1992, 42, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Darfeuille-Michaud, A.; Egan, L.J.; Miyamoto, Y.; Kagnoff, M.F. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 2002, 4, 635–648. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Iimura, M.; Kaper, J.B.; Torres, A.G.; Kagnoff, M.F. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell. Microbiol. 2006, 8, 869–879. [Google Scholar] [CrossRef]
- Thorpe, C.M.; Hurley, B.P.; Lincicome, L.L.; Jacewicz, M.S.; Keusch, G.T.; Acheson, D.W.K. Shiga Toxins stimulate secretion of Interleukin-8 from intestinal epithelial cells. Infect. Immun. 1999, 67, 5985–5993. [Google Scholar] [CrossRef]
- He, X.; Mishchuk, D.O.; Shah, J.; Weimer, B.C.; Slupsky, C.M. Cross-talk between E. coli strains and a human colorectal adenocarcinoma-derived cell line. Sci. Rep. 2013, 3, 3416. [Google Scholar] [CrossRef]
- Pearson, J.S.; Hartland, E.L. The inflammatory response during enterohemorrhagic Escherichia coli infection. Microbiol. Spectr. 2014, 2, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Walana, W.; Ye, Y.; Li, M.; Wang, J.; Wang, B.; Cheng, J.-W.; Gordon, J.R.; Li, F. IL-8 antagonist, CXCL8 (3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed. Pharmacother. 2018, 103, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, P.; Lang, F.; Wu, Z.; Pan, D.; Zeng, X.; Lian, L. Adhesion-related immunomodulatory activity of the screened Lactobacillus plantarum from Sichuan Pickle. Curr. Microbiol. 2018, 76, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, J.; Wang, S.; Zhao, X.; Lu, G.; Tang, X. Lactobacillus plantarum L9 but not Lactobacillus acidophilus LA reduces tumour necrosis factor induced bacterial translocation in Caco-2 cells. Benef. Microbes 2017, 8, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kim, H. Protective effects of lactic acid bacteria against TLR4 induced inflammatory response in hepatoma HepG2 cells through modulation of toll-like receptor negative regulators of mitogen-activated protein kinase and NF-κB signaling. Front. Immunol. 2018, 9, 1537. [Google Scholar] [CrossRef]
- Barillari, J.; Canistro, D.; Paolini, M.; Ferroni, F.; Pedulli, G.F.; Iori, R.; Valgimigli, L. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J. Agric. Food Chem. 2005, 53, 2475–2482. [Google Scholar] [CrossRef]
- Dignass, A.U. Mechanisms and Modulation of Intestinal Epithelial Repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane normalizes intestinal flora and enhances gut barrier in mice with BBN-induced bladder cancer. Mol. Nutr. Food Res. 2018, 62, 1800427. [Google Scholar] [CrossRef]
- Martelli, A.; Piragine, E.; Citi, V.; Testai, L.; Pagnotta, E.; Ugolini, L.; Lazzeri, L.; Mannelli, L.D.C.; Manzo, O.L.; Bucci, M.; et al. Erucin exhibits vasorelaxing effects and antihypertensive activity by H2S-releasing properties. Br. J. Pharmacol. 2019, 177, 824–835. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Medica 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Blackler, R.W.; De Palma, G.; Manko, A.; Da Silva, G.; Flannigan, K.L.; Bercik, P.; Surette, M.G.; Buret, A.G.; Wallace, J.L. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am. J. Physiol. Liver Physiol. 2015, 308, G994–G1003. [Google Scholar] [CrossRef] [PubMed]
- Blackler, R.; Syer, S.; Bolla, M.; Ongini, E.; Wallace, J.L. Gastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defence. PLoS ONE 2012, 7, e35196. [Google Scholar] [CrossRef] [PubMed]


| Exctract | Final Volume (mL) | Total GSLs (µmoL mL−1) | Recovery (%) |
|---|---|---|---|
| Eruca sativa | 20 | 201 ± 5 | 98 |
| Barbarea verna | 26 | 142 ± 4 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonvicini, F.; Pagnotta, E.; Punzo, A.; Calabria, D.; Simoni, P.; Mirasoli, M.; Passerini, N.; Bertoni, S.; Ugolini, L.; Lazzeri, L.; et al. Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients 2020, 12, 3064. https://doi.org/10.3390/nu12103064
Bonvicini F, Pagnotta E, Punzo A, Calabria D, Simoni P, Mirasoli M, Passerini N, Bertoni S, Ugolini L, Lazzeri L, et al. Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients. 2020; 12(10):3064. https://doi.org/10.3390/nu12103064
Chicago/Turabian StyleBonvicini, Francesca, Eleonora Pagnotta, Angela Punzo, Donato Calabria, Patrizia Simoni, Mara Mirasoli, Nadia Passerini, Serena Bertoni, Luisa Ugolini, Luca Lazzeri, and et al. 2020. "Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells" Nutrients 12, no. 10: 3064. https://doi.org/10.3390/nu12103064
APA StyleBonvicini, F., Pagnotta, E., Punzo, A., Calabria, D., Simoni, P., Mirasoli, M., Passerini, N., Bertoni, S., Ugolini, L., Lazzeri, L., Gentilomi, G. A., Caliceti, C., & Roda, A. (2020). Effect of Lactobacillus acidophilus Fermented Broths Enriched with Eruca sativa Seed Extracts on Intestinal Barrier and Inflammation in a Co-Culture System of an Enterohemorrhagic Escherichia coli and Human Intestinal Cells. Nutrients, 12(10), 3064. https://doi.org/10.3390/nu12103064