Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Uptake of Fatty Acids from Algae Oils
2.3. FAME Analysis
2.4. Cell Viability Assay
2.5. Uptake of Phytosterol from Plant Oils
2.6. Phytosterol Analytics
2.7. Statistics
3. Results
3.1. Analysis of Fatty Acid Composition of Algae Oil
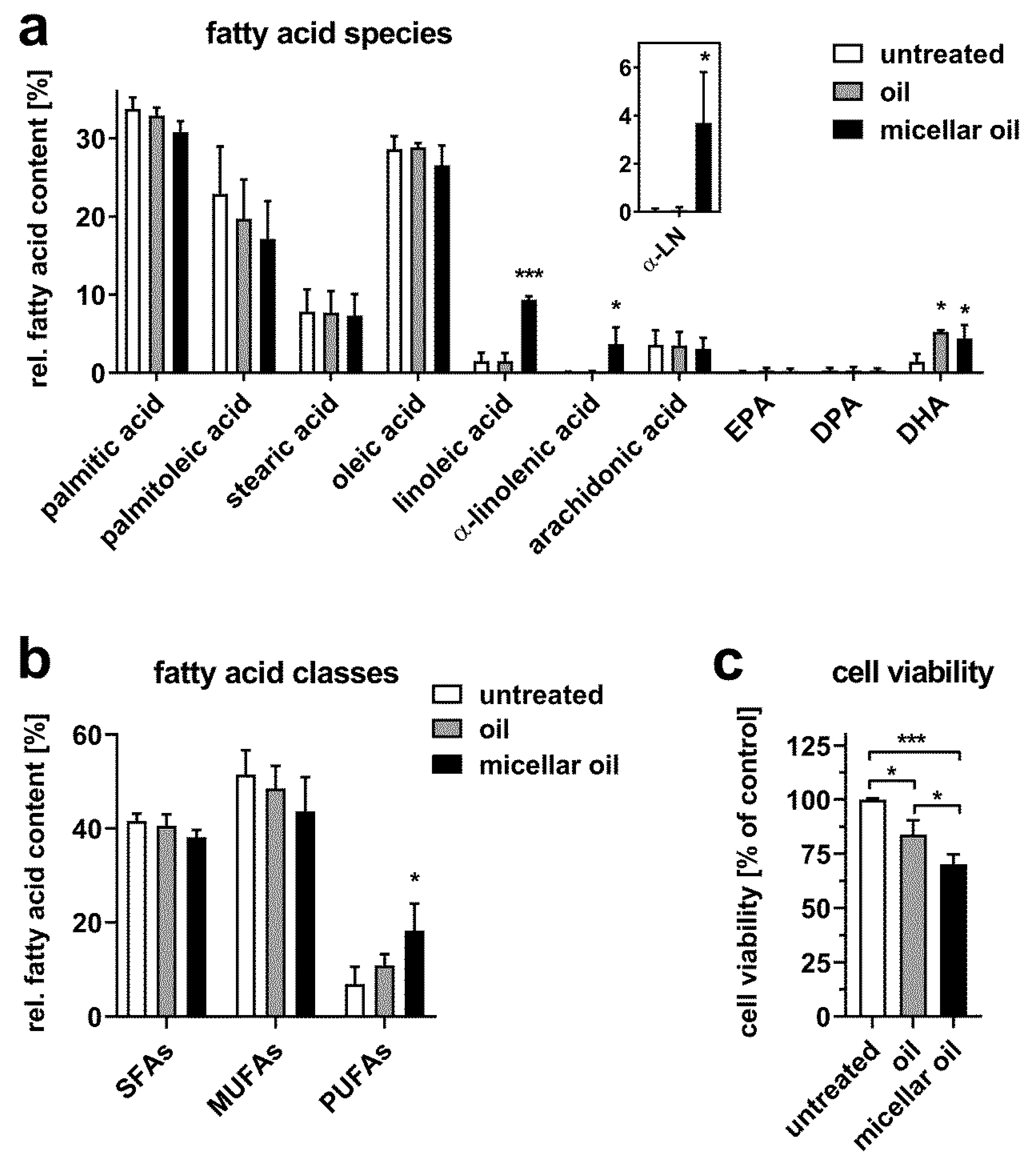
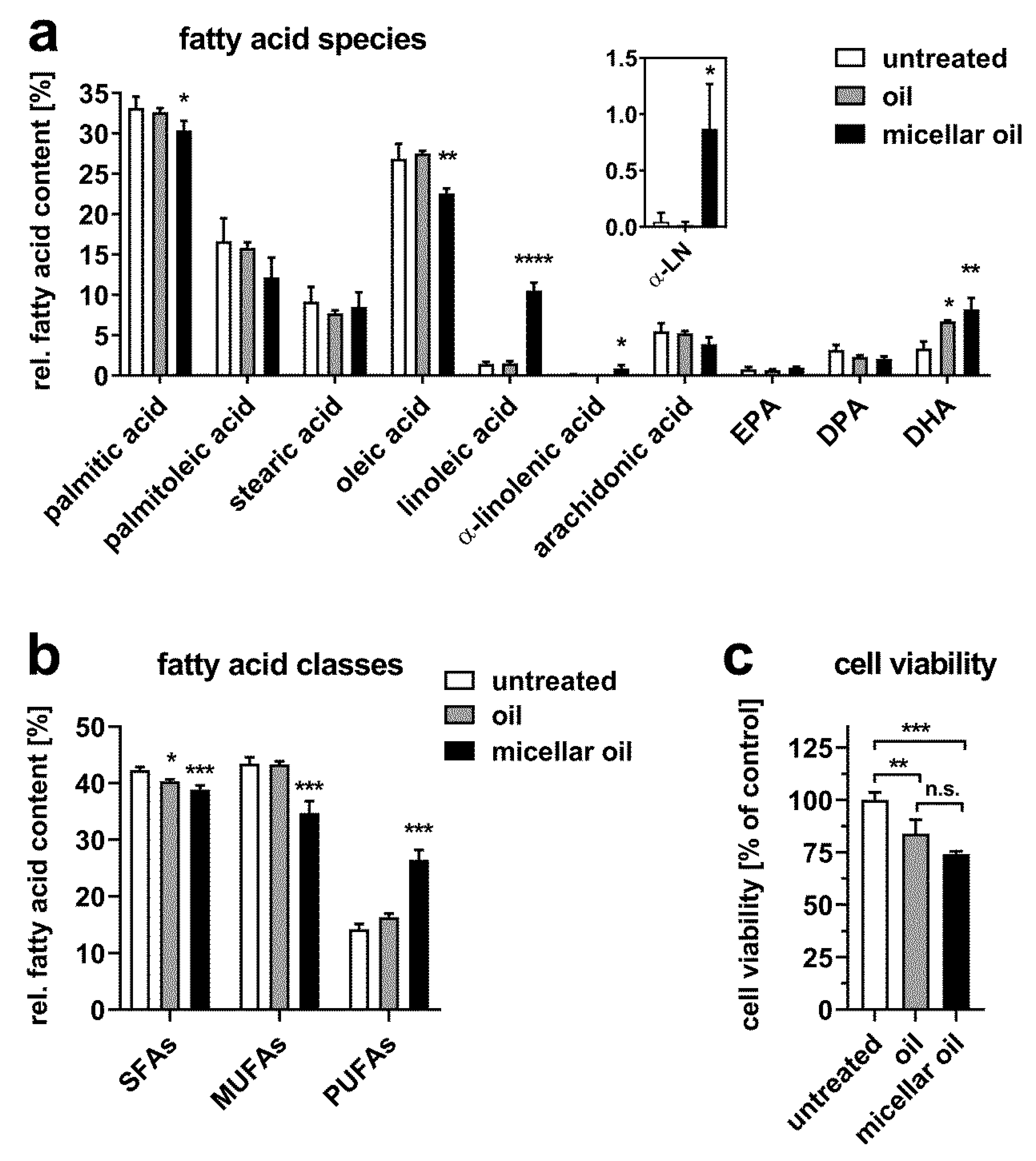
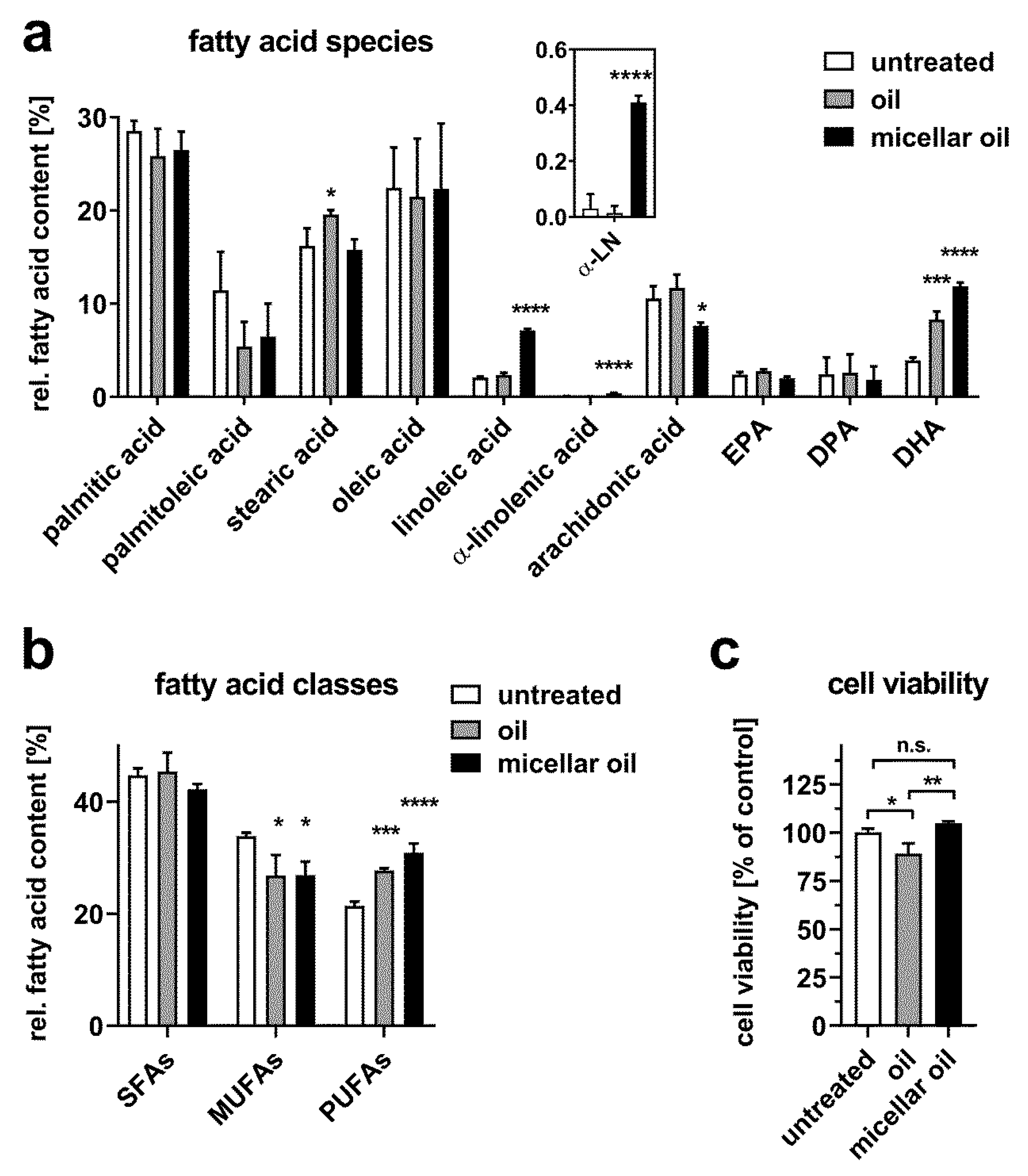
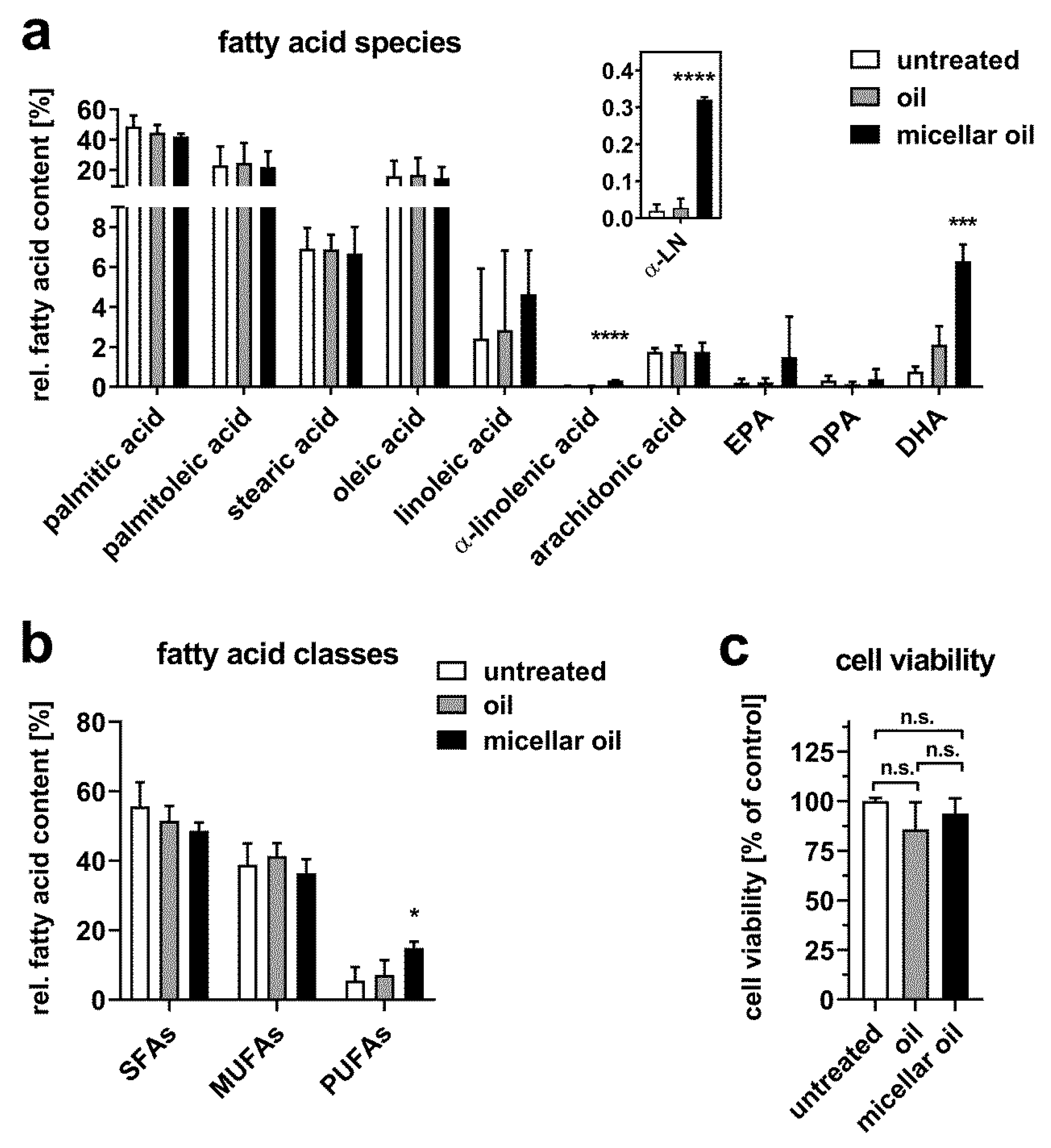
3.2. Uptake of Algae Oil into Cell Models of Enterocytes
3.3. Uptake of Algae Oil into Adipocyte Cell Models
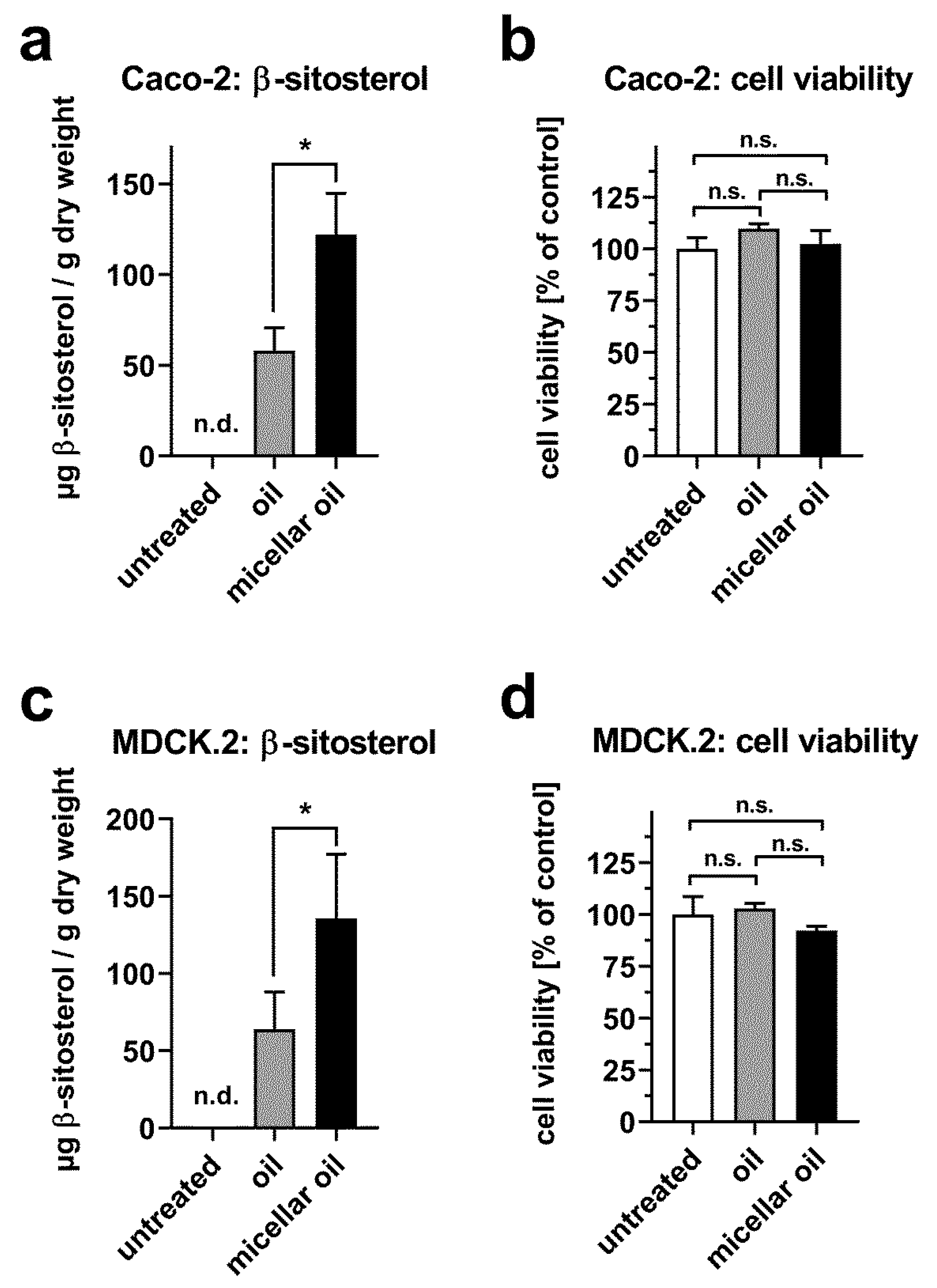
3.4. Uptake of Phytosterols from Phytogenic Oil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Zwol, W.V.; Rimbert, A.; Kuivenhoven, J.A. The Future of Lipid-lowering Therapy. J. Clin. Med. 2019, 8, 1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Sun, J.; Ran, L.; Li, Y.; Wang, N.; Yu, T.; Gao, W.; Jia, W.; Jiang, R.; et al. Microalgal Oil from Schizochytrium sp. Prevents HFD-Induced Abdominal Fat Accumulation in Mice. J. Am. Coll. Nutr. 2017, 36, 347–356. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Volker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Hadley, K.B.; Bauer, J.; Milgram, N.W. The oil-rich alga Schizochytrium sp. as a dietary source of docosahexaenoic acid improves shape discrimination learning associated with visual processing in a canine model of senescence. Prostaglandins Leukot. Essent. Fat. Acids 2017, 118, 10–18. [Google Scholar] [CrossRef][Green Version]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegard, L.; Jessup, W.; Jones, P.J.; Lutjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz, E.; Badimon, L.; Padro, T. Phytosterols and inflammation. Curr. Med. Chem. 2018, 26, 6724–6734. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I.; Rockwood, J.; Zak, D. Effect of orange juice and beverage with phytosterols on cytokines and PAI-1 activity. Clin. Nutr. 2011, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Cubedo, J.; Padro, T.; Sanchez-Hernandez, J.; Antonijoan, R.M.; Perez, A.; Badimon, L. Phytosterols and Omega 3 Supplementation Exert Novel Regulatory Effects on Metabolic and Inflammatory Pathways: A Proteomic Study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Aldini, R.; Micucci, M.; Cevenini, M.; Fato, R.; Bergamini, C.; Nanni, C.; Cont, M.; Camborata, C.; Spinozzi, S.; Montagnani, M.; et al. Antiinflammatory effect of phytosterols in experimental murine colitis model: Prevention, induction, remission study. PLoS ONE 2014, 9, e108112. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, E.; Rincon, F.; Perez-Galvez, A. Developing an emulsifier system to improve the bioaccessibility of carotenoids. J. Agric. Food Chem. 2008, 56, 10384–10390. [Google Scholar] [CrossRef]
- Higgins, S.; Carroll, Y.L.; O’Brien, N.M.; Morrissey, P.A. Use of microencapsulated fish oil as a means of increasing n-3 polyunsaturated fatty acid intake. J. Hum. Nutr. Diet. 1999, 12, 265–271. [Google Scholar] [CrossRef]
- Haug, I.J.; Sagmo, L.B.; Zeiss, D.; Olsen, I.C.; Draget, K.I.; Seternes, T. Bioavailability of EPA and DHA delivered by gelled emulsions and soft gel capsules. Eur. J. Lipid Sci. Technol. 2010, 113, 137–145. [Google Scholar] [CrossRef]
- Couedelo, L.; Amara, S.; Lecomte, M.; Meugnier, E.; Monteil, J.; Fonseca, L.; Pineau, G.; Cansell, M.; Carriere, F.; Michalski, M.C.; et al. Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis. Food Funct. 2015, 6, 1726–1735. [Google Scholar] [CrossRef]
- Van Wijk, N.; Balvers, M.; Cansev, M.; Maher, T.J.; Sijben, J.W.; Broersen, L.M. Dietary Crude Lecithin Increases Systemic Availability of Dietary Docosahexaenoic Acid with Combined Intake in Rats. Lipids 2016, 51, 833–846. [Google Scholar] [CrossRef]
- Haselgrubler, R.; Lanzerstorfer, P.; Rohrl, C.; Stubl, F.; Schurr, J.; Schwarzinger, B.; Schwarzinger, C.; Brameshuber, M.; Wieser, S.; Winkler, S.M.; et al. Hypolipidemic effects of herbal extracts by reduction of adipocyte differentiation, intracellular neutral lipid content, lipolysis, fatty acid exchange and lipid droplet motility. Sci. Rep. 2019, 9, 10492. [Google Scholar] [CrossRef]
- Konig, A.; Schwarzinger, B.; Stadlbauer, V.; Lanzerstorfer, P.; Iken, M.; Schwarzinger, C.; Kolb, P.; Schwarzinger, S.; Morwald, K.; Brunner, S.; et al. Guava (Psidium guajava) Fruit Extract Prepared by Supercritical CO2 Extraction Inhibits Intestinal Glucose Resorption in a Double-Blind, Randomized Clinical Study. Nutrients 2019, 11, 1512. [Google Scholar] [CrossRef]
- Levy, E.; Mehran, M.; Seidman, E. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J. 1995, 9, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.; Stubl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Hoglinger, O.; et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium Guajava) Extracts. Mol. Nutr. Food Res. 2018, 62, e1701012. [Google Scholar] [CrossRef] [PubMed]
- Luchoomun, J.; Zhou, Z.; Bakillah, A.; Jamil, H.; Hussain, M.M. Assembly and secretion of VLDL in nondifferentiated Caco-2 cells stably transfected with human recombinant ApoB48 cDNA. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, J.H.; Seidman, E.G.; Menard, D.; Levy, E. Endogenous lipase activity in Caco-2 cells. Biochim. Biophys. Acta 1998, 1393, 119–127. [Google Scholar] [CrossRef]
- Olivecrona, G. Role of lipoprotein lipase in lipid metabolism. Curr. Opin. Lipidol. 2016, 27, 233–241. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr. Metab. 2007, 4, 22. [Google Scholar] [CrossRef]
- Wolins, N.E.; Quaynor, B.K.; Skinner, J.R.; Tzekov, A.; Park, C.; Choi, K.; Bickel, P.E. OP9 mouse stromal cells rapidly differentiate into adipocytes: Characterization of a useful new model of adipogenesis. J. Lipid Res. 2006, 47, 450–460. [Google Scholar] [CrossRef]
- Liu, C.; Han, T.; Stachura, D.L.; Wang, H.; Vaisman, B.L.; Kim, J.; Klemke, R.L.; Remaley, A.T.; Rana, T.M.; Traver, D.; et al. Lipoprotein lipase regulates hematopoietic stem progenitor cell maintenance through DHA supply. Nat. Commun. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Park, H.G.; Lawrence, P.; Engel, M.G.; Kothapalli, K.; Brenna, J.T. Metabolic fate of docosahexaenoic acid (DHA; 22:6n-3) in human cells: Direct retroconversion of DHA to eicosapentaenoic acid (20:5n-3) dominates over elongation to tetracosahexaenoic acid (24:6n-3). FEBS Lett. 2016, 590, 3188–3194. [Google Scholar] [CrossRef]
- Chen, C.T.; Liu, Z.; Ouellet, M.; Calon, F.; Bazinet, R.P. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: An in situ study. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 157–163. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H. The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Kishi, H.; Komatsu, W.; Nagao, M.; Ohhira, S.; Kobashi, G. Lipid and Bile Acid Dysmetabolism in Crohn’s Disease. J. Immunol. Res. 2018, 2018, 7270486. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Alteheld, B.; Terjung, B.; Junghans, B.; Bitterlich, N.; Stehle, P.; Metzner, C. Change in the fatty acid pattern of erythrocyte membrane phospholipids after oral supplementation of specific fatty acids in patients with gastrointestinal diseases. Eur. J. Clin. Nutr. 2010, 64, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Kalivianakis, M.; Minich, D.M.; Bijleveld, C.M.; van Aalderen, W.M.; Stellaard, F.; Laseur, M.; Vonk, R.J.; Verkade, H.J. Fat malabsorption in cystic fibrosis patients receiving enzyme replacement therapy is due to impaired intestinal uptake of long-chain fatty acids. Am. J. Clin. Nutr. 1999, 69, 127–134. [Google Scholar] [CrossRef]
- Garla, P.; Sala, P.; Torrinhas, R.S.M.; Machado, N.M.; Fonseca, D.C.; da Silva, M.M.; Ravacci, G.R.; Belarmino, G.; Ishida, R.K.; Guarda, I.; et al. Reduced intestinal FADS1 gene expression and plasma omega-3 fatty acids following Roux-en-Y gastric bypass. Clin. Nutr. 2019, 38, 1280–1288. [Google Scholar] [CrossRef]
- Carriere, F.; Renou, C.; Ransac, S.; Lopez, V.; De Caro, J.; Ferrato, F.; De Caro, A.; Fleury, A.; Sanwald-Ducray, P.; Lengsfeld, H.; et al. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G16–G28. [Google Scholar] [CrossRef]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr. J. 2007, 6, 4. [Google Scholar] [CrossRef]
- Jones, P.J.H.; Shamloo, M.; MacKay, D.S.; Rideout, T.C.; Myrie, S.B.; Plat, J.; Roullet, J.B.; Baer, D.J.; Calkins, K.L.; Davis, H.R.; et al. Progress and perspectives in plant sterol and plant stanol research. Nutr. Rev. 2018, 76, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Sane, A.T.; Sinnett, D.; Delvin, E.; Bendayan, M.; Marcil, V.; Menard, D.; Beaulieu, J.F.; Levy, E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 2006, 47, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Weinglass, A.B.; Kohler, M.G.; Nketiah, E.O.; Liu, J.; Schmalhofer, W.; Thomas, A.; Williams, B.; Beers, L.; Smith, L.; Hafey, M.; et al. Madin-Darby canine kidney II cells: A pharmacologically validated system for NPC1L1-mediated cholesterol uptake. Mol. Pharmacol. 2008, 73, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Field, F.J.; Born, E.; Mathur, S.N. LXR/RXR ligand activation enhances basolateral efflux of beta-sitosterol in CaCo-2 cells. J. Lipid Res. 2004, 45, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Hellman, N.E.; Spector, J.; Robinson, J.; Zuo, X.; Saunier, S.; Antignac, C.; Tobias, J.W.; Lipschutz, J.H. Matrix metalloproteinase 13 (MMP13) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), regulated by the MAPK pathway, are both necessary for Madin-Darby canine kidney tubulogenesis. J. Biol. Chem. 2008, 283, 4272–4282. [Google Scholar] [CrossRef]
- Fisher, S.; Wachtel, E.J.; Aserin, A.; Garti, N. Solubilization of simvastatin and phytosterols in a dilutable microemulsion system. Colloids Surf. B 2013, 107, 35–42. [Google Scholar] [CrossRef]
| Fatty acid | Formula | Configuration | Oil | Micellar Oil |
|---|---|---|---|---|
| Relative Content | ||||
| lauric acid | C12:0 | n.a. | 0.08% | 0.05% |
| myristic acid | C14:0 | n.a. | 0.35% | 0.43% |
| myristoleic acid | C14:1 | Z | 0.30% | 0.42% |
| C16:0 | n.a. | 11.12% | 12.08% | |
| palmitoleic acid | C16:1 | Z | 0.17% | 0.25% |
| heptadecanoic acid | C17:0 | n.a. | 0.05% | 0.05% |
| heptadecenoic acid | C17:1 | Z | 0.19% | 0.17% |
| stearic acid | C18:0 | n.a. | 1.10% | 1.23% |
| oleic acid | C18:1 n-9 | Z | 6.71% | 5.85% |
| linoleic acid | C18:2 n-6 | all Z | 1.54% | 1.94% |
| alpha-linolenic acid | C18:3 n-3 | all Z | 0.20% | 0.23% |
| arachidic acid | C20:0 | n.a. | 0.07% | 0.08% |
| eicosenoic acid | C20:1 n-9 | Z | 0.03% | 0.03% |
| dihomo-gamma-linolenic acid | C20:3 n-6 | all Z | 0.11% | 0.08% |
| arachidonic acid methyl ester | C20:4 n-6 | all Z | 0.09% | 0.10% |
| eicosapentaenoic acid (EPA) | C20:5 n-3 | all Z | 0.42% | 0.51% |
| behenic acid | C22:0 | n.a. | 0.11% | 0.13% |
| decosapentaenoic acid (DPA) | C22:5 n-3 | all Z | 13.95% | 11.97% |
| decosahexaenoic acid (DHA) | C22:6 n-3 | all Z | 63.41% | 64.40% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röhrl, C.; Stübl, F.; Maier, M.; Schwarzinger, B.; Schwarzinger, C.; Pitsch, J.; Lanzerstorfer, P.; Iken, M.; Weghuber, J. Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil. Nutrients 2020, 12, 150. https://doi.org/10.3390/nu12010150
Röhrl C, Stübl F, Maier M, Schwarzinger B, Schwarzinger C, Pitsch J, Lanzerstorfer P, Iken M, Weghuber J. Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil. Nutrients. 2020; 12(1):150. https://doi.org/10.3390/nu12010150
Chicago/Turabian StyleRöhrl, Clemens, Flora Stübl, Martin Maier, Bettina Schwarzinger, Clemens Schwarzinger, Johannes Pitsch, Peter Lanzerstorfer, Marcus Iken, and Julian Weghuber. 2020. "Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil" Nutrients 12, no. 1: 150. https://doi.org/10.3390/nu12010150
APA StyleRöhrl, C., Stübl, F., Maier, M., Schwarzinger, B., Schwarzinger, C., Pitsch, J., Lanzerstorfer, P., Iken, M., & Weghuber, J. (2020). Increased Cellular Uptake of Polyunsaturated Fatty Acids and Phytosterols from Natural Micellar Oil. Nutrients, 12(1), 150. https://doi.org/10.3390/nu12010150





