Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intervention Diets
2.2. Nutritional Assessment
2.3. Dietary Evaluation at Baseline
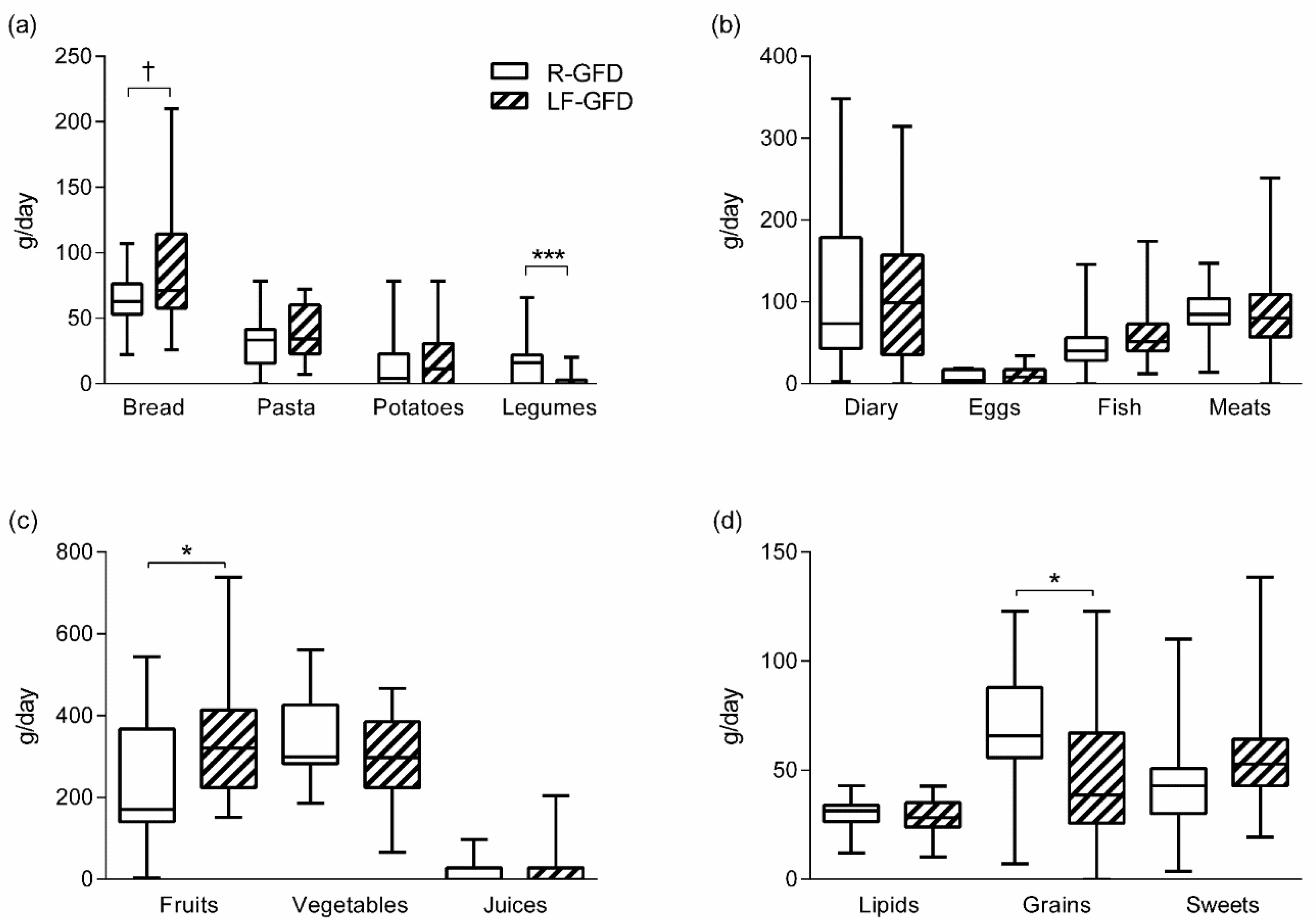
2.4. Dietary Evaluation at the End of Intervention
2.5. Statistical Analysis
3. Results
Nutritional Adequacy of Consumed Diets Compared to Macro- and Micronutrients Recommendations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bascuñán, K.A.; Roncoroni, L.; Branchi, F.; Doneda, L.; Scricciolo, A.; Ferretti, F.; Araya, M.; Elli, L. The 5 Ws of a gluten challenge for gluten-related disorders. Nutr. Rev. 2018, 76, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac disease. Lancet 2017, 391, 70–81. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M. Celiac disease and the gluten-free diet: Consequences and recommendations for improvement. Dig. Dis. 2015, 33, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Newman, J.M. Celiac diet: Its impact on quality of life. J. Am. Diet. Assoc. 2003, 103, 1533–1535. [Google Scholar] [CrossRef]
- Högberg, L.; Grodzinsky, E.; Stenhammar, L. Better dietary compliance in patients with coeliac disease diagnosed in early childhood. Scand. J. Gastroenterol. 2003, 38, 751–754. [Google Scholar]
- Ciacci, C.; Cirillo, M.; Cavallaro, R.; Mazzacca, G. Long-term follow-up of celiac adults on gluten-free diet: Prevalence and correlates of intestinal damage. Digestion 2002, 66, 178–185. [Google Scholar] [CrossRef]
- O’Leary, C.; Wieneke, P.; Buckley, S.; O’Regan, P.; Cronin, C.C.; Quigley, E.M.M.; Shanahan, F. Celiac disease and irritable bowel-type symptoms. Am. J. Gastroenterol. 2002, 97, 1463–1467. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Personal view: Food for thought—Western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment. Pharmacol. Ther. 2005, 21, 1399–1409. [Google Scholar] [CrossRef]
- Roncoroni, L.; Bascuñán, K.A.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Branchi, F.; Ferretti, F.; Dell’osso, B.; Montanari, V.; Bardella, M.T.; et al. A low FODMAP gluten-free diet improves functional gastrointestinal disorders and overall mental health of celiac disease patients: A randomized controlled trial. Nutrients 2018, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Jansen, C.; Martin, L.; Williams, M.; Seamark, L.; Staudacher, H.M.; Irving, P.M.; Whelan, K.; Lomer, M.C. Long-term impact of the low-FODMAP diet on gastrointestinal symptoms, dietary intake, patient acceptability, and healthcare utilization in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13154. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Viti, M.G.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The gluten-free diet: A nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharm. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Tomba, C.; Elli, L.; Sieri, S.; Grioni, S.; Bardella, M.T.; Agostoni, C.; Doneda, L.; et al. Evaluation of a modified italian European prospective investigation into cancer and nutrition food frequency questionnaire for individuals with celiac disease. J. Acad. Nutr. Diet. 2016, 116, 1810–1816. [Google Scholar] [CrossRef]
- Drossman, D.A.; Dumitrascu, D.L. Rome III: New standard for functional gastrointestinal disorders. J. Gastrointest. Liver Dis. 2006, 15, 237–241. [Google Scholar]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–677. [Google Scholar] [CrossRef]
- Muir, J.G.; Rose, R.; Rosella, O.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Scarlato, A.P.; Galaverna, G.; Brighenti, F.; Pellegrini, N. Dietary exposure to fumonisins and evaluation of nutrient intake in a group of adult celiac patients on a gluten-free diet. Mol. Nutr. Food Res. 2012, 56, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Salvini, S.; Parpinel, M. Food Composition Database for Epidemiological Studies in Italy. 2015. Available online: http://www.bda-ieo.it (accessed on 23 October 2018).
- Mazzeo, T.; Cauzzi, S.; Brighenti, F.; Pellegrini, N. The development of a composition database of gluten-free products. Public Health Nutr. 2015, 18, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine of the National Academies Dietary Reference Intakes (DRIs). Guiding Principles for Nutrition Labelling and Fortification; Committee on Use of Dietary Reference Intakes in Nutrition Labeling Food and Nutrition Board, Ed.; The National Academie Press: Washington, DC, USA, 2003. [Google Scholar]
- Parnell, N.D.; Ciclitira, P.J. Review article: Coeliac disease and its management. Aliment. Pharmacol. Ther. 1999, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-free diet in children: An approach to a nutritionally adequate and balanced diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef]
- Dickey, W.; Kearney, N. Overweight in celiac disease: Prevalence, clinical characteristics, and effect of a gluten-free diet. Am. J. Gastroenterol. 2006, 101, 2356–2359. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac disease and obesity: Need for nutritional follow-up after diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized controlled trial comparing the low FODMAP diet vs. modified nice guidelines in US adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. J. Acad. Nutr. Diet. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.; Barrett, J. Re-challenging FODMAPs: The low FODMAP diet phase two. J. Gastroenterol. Hepatol. 2017, 32, 11–15. [Google Scholar] [CrossRef]
- Eswaran, S.; Farida, J.P.; Green, J.; Miller, J.D.; Chey, W.D. Nutrition in the management of gastrointestinal diseases and disorders: The evidence for the low FODMAP diet. Curr. Opin. Pharmacol. 2017, 37, 151–157. [Google Scholar] [CrossRef]

| R-GFD Group | LF-GFD GRoup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 21) | Adequacy † | End of Intervention (n = 21) | Adequacy † | Baseline (n = 25) | Adequacy † | End of Intervention (n = 25) | Adequacy † | p Value R-GFD ‡ | p Value LF-GFD ‡ | |
| Energy, kcal †† | 2212.4 ± 511.7 | 111.9 ± 30.0 | 1556.2 ± 220.4 | 78.8 ± 14.3 | 1837.0 ± 481.5 | 91.5 ± 27.8 | 1578.0 ± 238.9 | 78.9 ± 17.6 | 0.0001 | 0.048 |
| Protein, % | 13.8 ± 2.6 | 19 (90.4) | 15.6 ± 2.2 | 21 (100.0) | 16.2 ± 2.7 | 25 (100.0) | 16.8 ± 3.3 | 24 (96.0) | 0.147 | 0.999 |
| Carbohydrate, % | 44.4 ± 8.2 | 9 (42.8) | 49.3 ± 4.5 | 17 (80.9) | 41.2 ± 7.1 | 5 (20.0) | 50.4 ± 3.8 | 23 (92.0) | 0.025 | 0.0001 |
| Fat, % | 39.4 ± 6.3 | 7 (33.3) | 35.7 ± 4.1 | 8 (38.0) | 42.4 ± 6.2 | 2 (8.0) | 33.9 ± 3.8 | 18 (72.0) | 0.747 | 0.0001 |
| Dietary fiber, g | 26.0 ± 8.4 | 13 (61.9) | 24.2 ± 10.8 | 7 (33.3) | 21.0 ± 5.7 | 10 (40) | 21.9 ± 8.8 | 8 (32.0) | 0.064 | 0.556 |
| Thiamin, mg | 0.9 ± 0.2 | 8 (38.0) | 0.9 ± 0.2 | 5 (23.8) | 0.9 ± 0.2 | 5 (20.0) | 0.9 ± 0.2 | 6 (24.0) | 0.317 | 0.733 |
| Riboflavin, mg | 1.5 ± 0.4 | 19 (90.4) | 1.5 ± 0.4 | 17 (80.9) | 1.6 ± 0.6 | 21 (84.0) | 1.4 ± 0.3 | 23 (92.0) | 0.663 | 0.667 |
| Niacin, mg | 19.5 ± 3.8 | 20 (95.2) | 19.6 ± 4.7 | 19 (90.4) | 20.0 ± 6.7 | 20 (80.0) | 20.6 ± 6.8 | 20 (80.0) | 0.990 | 0.990 |
| Vitamin B6, mg | 2.0 ± 0.4 | 21 (100.0) | 1.9 ± 0.4 | 21 (100.0) | 2.1 ± 0.7 | 24 (96.0) | 1.9 ± 0.3 | 25 (100.0) | - | 0.990 |
| Vitamin C, mg | 120.5 ± 46.5 | 17 (80.9) | 146.0 ± 82.7 | 19 (90.4) | 138.9 ± 92.2 | 22 (88.0) | 207.4 ± 107.0 | 24 (96.0) | 0.663 | 0.609 |
| Vitamin E, mg | 12.7 ± 4.1 | 9 (42.8) | 14.0 ± 2.7 | 7 (33.3) | 11.8 ± 4.0 | 5 (20.0) | 13.6 ± 2.3 | 7 (28.0) | 0.525 | 0.508 |
| Vitamin D, g | 2.9 ± 1.1 | 0 (0) | 2.3 ± 1.1 | 0 (0) | 3.5 ± 3.0 | 1 (4.0) | 2.7 ± 2.4 | 0 (0) | - | 0.990 |
| Folate, g | 261.2 ± 68.6 | 0 (0) | 331.8 ± 126.7 | 7 (33.3) | 274.1 ± 89.1 | 1 (4.0) | 290.3 ± 96.0 | 4 (16.0) | 0.009 | 0.349 |
| Calcium, mg | 804.6 ± 391.3 | 3 (14.2) | 599.7 ± 198.8 | 0 (0) | 879.8 ± 434.8 | 7 (28.0) | 601.2 ± 170.5 | 2 (8.0) | 0.072 | 0.066 |
| Iron, mg | 11.0 ± 3.0 | 3 (14.2) | 10.8 ± 3.6 | 2 (9.5) | 10.4 ± 3.0 | 5 (20.0) | 11.0 ± 3.2 | 9 (36.0) | 0.990 | 0.208 |
| Phosphorus, mg | 1244.9 ± 374.6 | 20 (95.2) | 1002.4 ± 209.5 | 18 (85.7) | 1239.7 ± 454.9 | 24 (96.0) | 1019.2 ± 205.9 | 24 (96.0) | 0.606 | 0.990 |
| Sodium, mg | 2455.8 ± 846.0 | 2 (9.5) | 4056.5 ± 1146.5 | 2 (9.5) | 1861.8 ± 540.6 | 4 (16.0) | 4243.5 ± 1374.5 | 3 (12.0) | 0.990 | 0.684 |
| Potassium, mg | 3193.8 ± 697.4 | 0 (0) | 2937.1 ± 750.9 | 0 (0) | 3122.7 ± 957.6 | 1 (4.0) | 3186.7 ± 772.7 | 2 (8.0) | - | 0.990 |
| Zinc, mg | 9.7 ± 2.3 | 17 (80.9) | 8.8 ± 1.7 | 15 (71.4) | 9.5 ± 3.3 | 17 (68.0) | 8.6 ± 1.7 | 14 (56.0) | 0.719 | 0.382 |
| R-GFD (n = 21) | LF-GFD (n = 25) | p Value | |
|---|---|---|---|
| Energy, kcal | 1556.2 ± 220.4 | 1578.0 ± 238.9 | 0.750 |
| Energy adequacy, % | 78.8 ± 14.3 | 79.0 ± 20.2 | 0.959 |
| Total protein, g | 59.6 ± 10.9 | 65.7 ± 17.3 | 0.154 |
| Protein, % of energy | 15.6 ± 2.2 | 16.8 ± 3.3 | 0.133 |
| Animal protein, g | 36.3 ± 9.4 | 44.2 ± 15.4 | 0.037 |
| Vegetal protein, g | 23.1 ± 7.0 | 21.2 ± 5.1 | 0.311 |
| Total carbohydrate, g | 190.9 ± 31.4 | 198.4 ± 32.7 | 0.431 |
| Carbohydrate, % of energy | 49.3 ± 4.5 | 50.4 ± 3.8 | 0.376 |
| Total fat, g | 62.1 ± 11.7 | 59.6 ± 11.0 | 0.463 |
| Fat, % of energy | 35.7 ± 4.1 | 33.9 ± 3.8 | 0.123 |
| Cholesterol, mg | 148.3 ± 35.0 | 178.4 ± 42.6 | 0.011 |
| Dietary fiber, g | 24.2 ± 10.8 | 21.9 ± 8.8 | 0.433 |
| Thiamin, mg | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.820 |
| Riboflavin, mg | 1.5 ± 0.4 | 1.4 ± 0.3 | 0.741 |
| Niacin, mg | 19.6 ± 4.7 | 20.6 ± 6.8 | 0.573 |
| Vitamin B6, mg | 1.9 ± 0.4 | 1.9 ± 0.3 | 0.637 |
| Vitamin C, mg | 146.0 ± 82.7 | 207.4 ± 107.0 | 0.033 |
| Vitamin E, mg | 14.0 ± 2.7 | 13.6 ± 2.3 | 0.642 |
| Vitamin D, g | 2.3 ± 1.1 | 2.7 ± 2.4 | 0.477 |
| Folate, g | 331.8 ± 126.7 | 290.3 ± 96.0 | 0.225 |
| Calcium, mg | 599.7 ± 198.8 | 601.2 ± 170.5 | 0.978 |
| Iron, mg | 10.8 ± 3.6 | 11.0 ± 3.2 | 0.874 |
| Phosphorus, mg | 1002.4 ± 209.5 | 1019.2 ± 205.9 | 0.786 |
| Sodium, mg | 4056.5 ± 1146.5 | 4243.5 ± 1374.5 | 0.617 |
| Potassium, mg | 2937.1 ± 750.9 | 3186.7 ± 772.7 | 0.274 |
| Zinc, mg | 8.8 ± 1.7 | 8.6 ± 1.7 | 0.695 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bascuñán, K.A.; Elli, L.; Pellegrini, N.; Scricciolo, A.; Lombardo, V.; Doneda, L.; Vecchi, M.; Scarpa, C.; Araya, M.; Roncoroni, L. Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet. Nutrients 2019, 11, 2220. https://doi.org/10.3390/nu11092220
Bascuñán KA, Elli L, Pellegrini N, Scricciolo A, Lombardo V, Doneda L, Vecchi M, Scarpa C, Araya M, Roncoroni L. Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet. Nutrients. 2019; 11(9):2220. https://doi.org/10.3390/nu11092220
Chicago/Turabian StyleBascuñán, Karla A., Luca Elli, Nicoletta Pellegrini, Alice Scricciolo, Vincenza Lombardo, Luisa Doneda, Maurizio Vecchi, Cecilia Scarpa, Magdalena Araya, and Leda Roncoroni. 2019. "Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet" Nutrients 11, no. 9: 2220. https://doi.org/10.3390/nu11092220
APA StyleBascuñán, K. A., Elli, L., Pellegrini, N., Scricciolo, A., Lombardo, V., Doneda, L., Vecchi, M., Scarpa, C., Araya, M., & Roncoroni, L. (2019). Impact of FODMAP Content Restrictions on the Quality of Diet for Patients with Celiac Disease on a Gluten-Free Diet. Nutrients, 11(9), 2220. https://doi.org/10.3390/nu11092220





