Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets
Abstract
1. Introduction
2. N-Acylethanolamines and Diet
3. ALIAmides, PEA and Food
4. Diet Modify PEA Levels
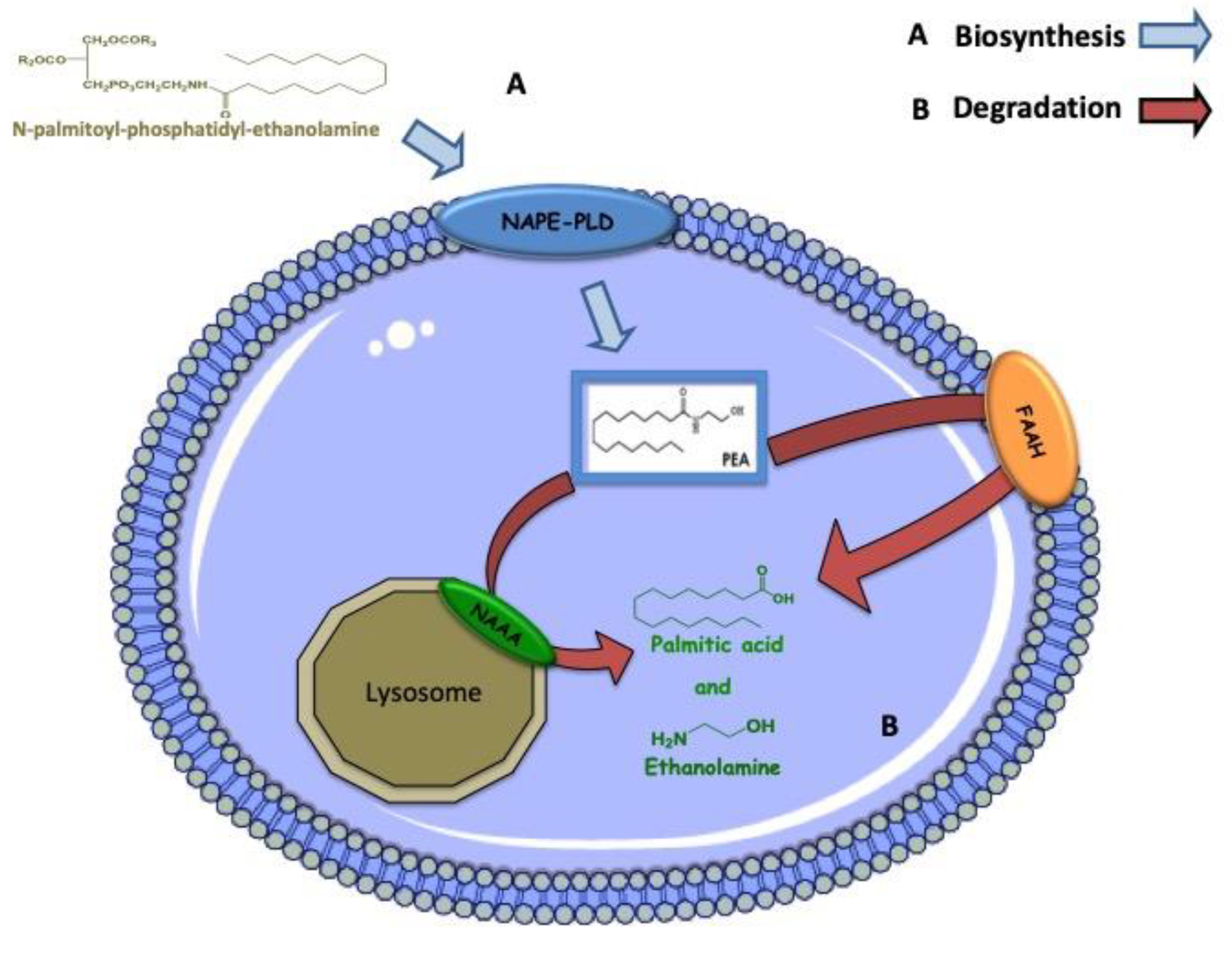
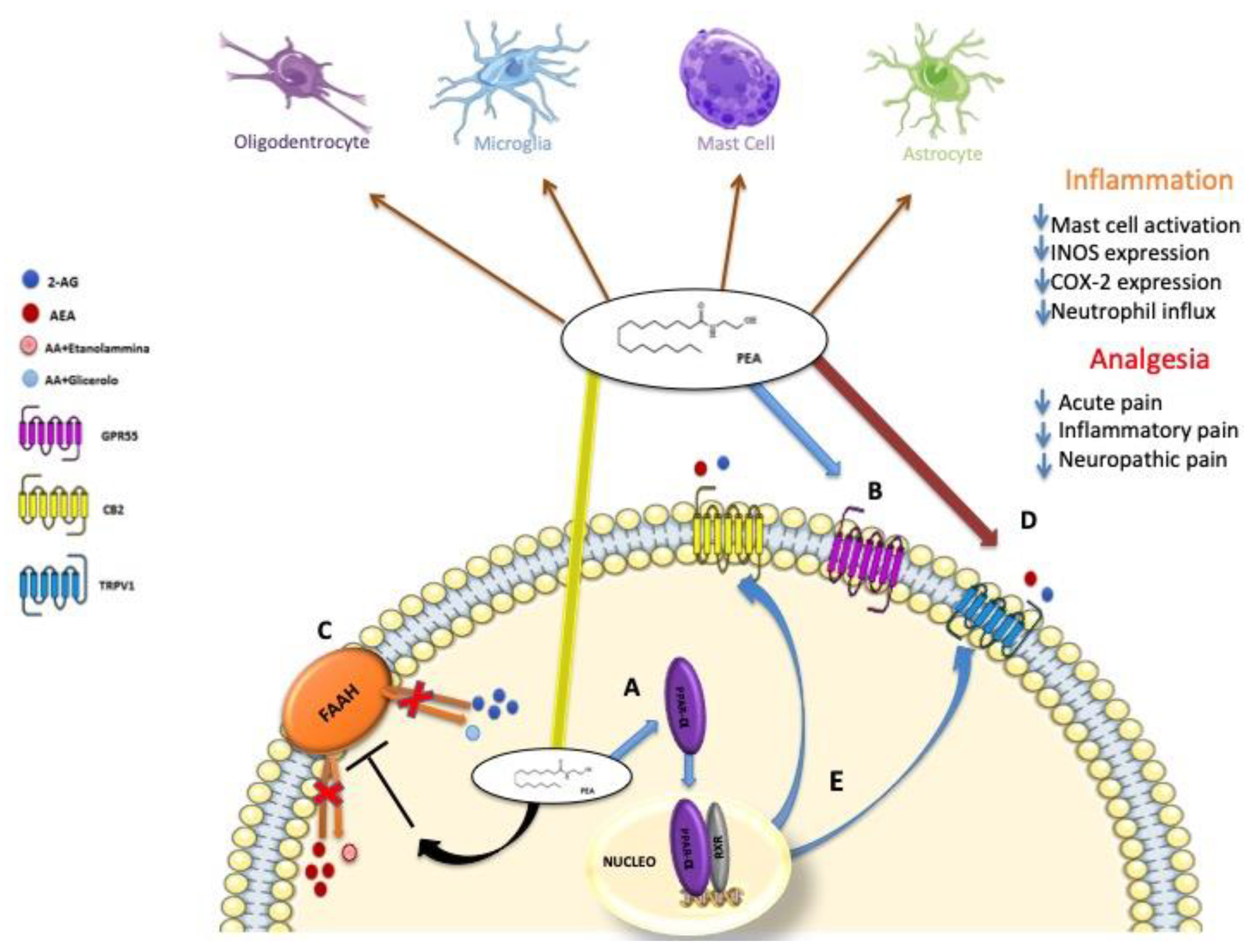
5. Biosynthesis, Degradation and Mechanism of Action of PEA
6. PEA, Synergy with Natural Molecules with Antioxidant Properties
6.1. PEA and Luteolin
6.2. PEA and Polydatin
6.3. PEA and Quercetin
6.4. PEA and Silymarin
6.5. PEA and Baicalein
7. Coffee Identification of a New Form of PEA
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acids |
| EPA | Eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| NAEs | N-Acylethanolamines |
| EE | ethyl ester |
| 2-AG | 2-archidonoylglycerol |
| AA | arachidonic acid |
| AEA | anandamide |
| CB1 and CB2 | cannabinoid receptor type 1 and 2 |
| CLA | conjugated linoleic acid |
| DAG | diacylglycerol |
| FO | fish oil |
| KO | krill oil |
| LA | linoleic acid |
| NAPE | N-acyl-phosphatidylethanolamine |
| NAPE-PLD | N-acyl-phosphatidyl-ethanolamine D |
| OA | oleic acid |
| OEA | N-oleoylethanolamine |
| PEA | N-palmitoylethanolamine |
| GPR55 | G protein coupled receptor 55 |
| PPAR-a/g | peroxisome proliferatoractivated receptor-a/g |
| TRPV1 | transient receptor potential of vanilloid type-1 |
| NAAA | N-acylethanolamine-hydrolysing acid |
| FAAH | fatty acid amide hydrolase |
References
- Audial, S.; Bonnotte, B. Inflammation. Rev. Prat. 2015, 65, 403–408. [Google Scholar] [PubMed]
- Graziottin, A.; Skaper, S.D.; Fusco, M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol. Endocrinol. 2014, 30, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Bucci, L.; Capri, M.; Salvioli, S.; Scurti, M.; Pini, E.; Monti, D.; Franceschi, C. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation 2008, 15, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary phytochemicals: Natural swords combating inflammation and oxidation-mediated degenerative diseases. Oxid. Med. Cell Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Raso, G.M.; Russo, R.; Calignano, A.; Meli, R. Palmitoylethanolamide in cns health and disease. Pharmacol. Res. 2014, 86, 32–41. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Crozier, G.; Bisogno, T.; Cavaliere, P.; Innis, S.; Di Marzo, V. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding n-acylethanolamines in piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Doshi, M.; Hamazaki, T. N-3 polyunsaturated fatty acid (pufa) deficiency elevates and n-3 pufa enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 51–59. [Google Scholar] [CrossRef]
- Matias, I.; Carta, G.; Murru, E.; Petrosino, S.; Banni, S.; Di Marzo, V. Effect of polyunsaturated fatty acids on endocannabinoid and n-acyl-ethanolamine levels in mouse adipocytes. Biochim. Biophys. Acta 2008, 1781, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Alvheim, A.R.; Malde, M.K.; Osei-Hyiaman, D.; Lin, Y.H.; Pawlosky, R.J.; Madsen, L.; Kristiansen, K.; Froyland, L.; Hibbeln, J.R. Dietary linoleic acid elevates endogenous 2-ag and anandamide and induces obesity. Obesity 2012, 20, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Hutchins-Wiese, H.L.; Li, Y.; Hannon, K.; Watkins, B.A. Hind limb suspension and long-chain omega-3 pufa increase mrna endocannabinoid system levels in skeletal muscle. J. Nutr. Biochem. 2012, 23, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.; Cascio, M.G.; Wahle, K.W.; Smoum, R.; Mechoulam, R.; Ross, R.A.; Pertwee, R.G.; Heys, S.D. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 2010, 31, 1584–1591. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharmacol. 2007, 152, 583–593. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of epa and dha into plasma phospholipids in response to different omega-3 fatty acid formulations-a comparative bioavailability study of fish oil vs. Krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Tsuyama, S.; Oikawa, D.; Tsuji, Y.; Akimoto, Y.; Jikuya, H.; Furuse, M. Dietary conjugated linoleic acid modifies the brain endocannabinoid system in mice. Nutr. Neurosci. 2009, 12, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Smoum, R.; Mechoulam, R.; Maccarrone, M. Bioactive lipids aliamides differentially modulate inflammatory responses of distinct subsets of primary human t lymphocytes. FASEB J. 2018, 32, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Coburn, A.F.; Trulson, M.F.; Moore, L.V. further study of the effect of the administration of egg yolk on susceptibility of children to rheumatic infection. Minerva Med. 1954, 45, 1534–1536. [Google Scholar] [PubMed]
- Long, D.A.; Martin, A.J. Factor in arachis oil depressing sensitivity to tuberculin in b.C.G.-infected guineapigs. Lancet 1956, 270, 464–466. [Google Scholar] [CrossRef]
- Venables, B.J.; Waggoner, C.A.; Chapman, K.D. N-acylethanolamines in seeds of selected legumes. Phytochemistry 2005, 66, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.D.; Venables, B.; Markovic, R.; Blair, R.W., Jr.; Bettinger, C. N-acylethanolamines in seeds. Quantification of molecular species and their degradation upon imbibition. Plant Physiol. 1999, 120, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Rawyler, A.J.; Braendle, R.A. N-acylphosphatidylethanolamine accumulation in potato cells upon energy shortage caused by anoxia or respiratory inhibitors. Plant Physiol. 2001, 127, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; Konje, J.C. Simultaneous measurement of three n-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2089–2097. [Google Scholar] [CrossRef]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Vet. J. 2007, 173, 21–30. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and n-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Matias, I.; Capasso, R.; Petrosino, S.; Borrelli, F.; Orlando, P.; Romano, B.; Capasso, F.; Di Marzo, V.; Izzo, A.A. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice. J. Mol. Med. 2008, 86, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.L.; De Micheli, E.; Bogdanov, M.B.; Wurtman, R.J. Effects of ethanolamine (etn) administration on etn and choline (ch) levels in plasma, brain extracellular fluid (ecf) and brain tissue, and on brain phospholipid levels in rats: An in vivo study. Neurosci. Res. Commun. 1996, 18, 87–96. [Google Scholar] [CrossRef]
- Shimada, Y.; Morita, T.; Sugiyama, K. Dietary eritadenine and ethanolamine depress fatty acid desaturase activities by increasing liver microsomal phosphatidylethanolamine in rats. J. Nutr. 2003, 133, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide in homeostatic and traumatic central nervous system injuries. CNS Neurol. Disord. Drug Targets 2013, 12, 55–61. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide is a new possible pharmacological treatment for the inflammation associated with trauma. Mini Rev. Med. Chem. 2013, 13, 237–255. [Google Scholar]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase d generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef]
- Ueda, N.; Yamanaka, K.; Yamamoto, S. Purification and characterization of an acid amidase selective for n-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J. Biol. Chem. 2001, 276, 35552–35557. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions 1993, 39, C145–147. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Skaper, S.D.; Dal Toso, R.; Petrelli, L.; Leon, A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996, 19, 514–520. [Google Scholar] [CrossRef]
- Facci, L.; Dal Toso, R.; Romanello, S.; Buriani, A.; Skaper, S.D.; Leon, A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 1995, 92, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Kondo, S.; Kishimoto, S.; Miyashita, T.; Nakane, S.; Kodaka, T.; Suhara, Y.; Takayama, H.; Waku, K. Evidence that 2-arachidonoylglycerol but not n-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid cb2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in hl-60 cells. J. Biol. Chem. 2000, 275, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Conti, S.; Giagnoni, G.; Colleoni, M. Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: Inhibition of nitric oxide and cyclo-oxygenase systems. Br. J. Pharmacol. 2002, 137, 413–420. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Larsson, N.; Sjogren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The orphan receptor gpr55 is a novel cannabinoid receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Davis, J.B.; Di Marzo, V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid vr1 receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Orlando, P.; Bisogno, T.; Zagoory, O.; Bifulco, M.; Vogel, Z.; De Petrocellis, L. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001, 358, 249–255. [Google Scholar] [CrossRef]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. ‘Entourage’ effects of n-palmitoylethanolamide and n-oleoylethanolamide on vasorelaxation to anandamide occur through trpv1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at trpv1 cation channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Ermund, A.; Movahed, P.; Andersson, D.A.; Simonsen, C.; Jonsson, B.A.; Blomgren, A.; Birnir, B.; Bevan, S.; Eschalier, A.; et al. Monoacylglycerols activate trpv1-a link between phospholipase c and trpv1. PLoS ONE 2013, 8, e81618. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Boccella, S.; Giordano, M.E.; Romano, R.; Bellini, G.; Manzo, I.; Furiano, A.; Rizzo, A.; Imperatore, R.; et al. Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: Involvement of the cb2 receptor. Sci. Rep. 2017, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Soldovieri, M.V.; Russo, C.; Taglialatela, M. Activation and desensitization of trpv1 channels in sensory neurons by the pparalpha agonist palmitoylethanolamide. Br. J. Pharmacol. 2013, 168, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Soldovieri, M.V.; De Maria, M.; Russo, C.; Taglialatela, M. Functional and biochemical interaction between pparalpha receptors and trpv1 channels: Potential role in pparalpha agonists-mediated analgesia. Pharmacol. Res. 2014, 87, 113–122. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Barbierato, M.; Zusso, M.; Bruschetta, G.; Impellizzeri, D.; Cuzzocrea, S.; Giusti, P. N-palmitoylethanolamine and neuroinflammation: A novel therapeutic strategy of resolution. Mol. Neurobiol. 2015, 52, 1034–1042. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflamm. 2014, 11, 136. [Google Scholar] [CrossRef]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Jin, Z.Y.; Wang, X.J.; Xu, X.M.; Deng, L.; Zhao, J.W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci. Lett. 2008, 448, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Romanova, D.; Vachalkova, A.; Cipak, L.; Ovesna, Z.; Rauko, P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma 2001, 48, 104–107. [Google Scholar] [PubMed]
- Ghanta, S.; Banerjee, A.; Poddar, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and antioxidant potential of stevia rebaudiana (bertoni) bertoni, a natural sweetener. J. Agric. Food Chem. 2007, 55, 10962–10967. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-e2 formation in raw 264.7 cells. J. Nutr. 2006, 136, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar] [PubMed]
- Riachi, L.G.; De Maria, C.A. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking nf-kappab and ap-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Crupi, R.; Paterniti, I.; Ahmad, A.; Campolo, M.; Esposito, E.; Cuzzocrea, S. Effects of palmitoylethanolamide and luteolin in an animal model of anxiety/depression. CNS Neurol. Disord. Drug Targets 2013, 12, 989–1001. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. A new co-ultramicronized composite including palmitoylethanolamide and luteolin to prevent neuroinflammation in spinal cord injury. J. Neuroinflamm. 2013, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paterniti, I.; Bruschetta, G.; Cordaro, M.; Impellizzeri, D.; Crupi, R.; Cuzzocrea, S.; Esposito, E. The association of palmitoylethanolamide with luteolin decreases autophagy in spinal cord injury. Mol. Neurobiol. 2016, 53, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Paterniti, I.; Bruschetta, G.; Siracusa, R.; De Stefano, D.; Cuzzocrea, S.; Esposito, E. Neuroprotective effects of co-ultrapealut on secondary inflammatory process and autophagy involved in traumatic brain injury. J. Neurotrauma 2016, 33, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. The association of palmitoylethanolamide with luteolin decreases neuroinflammation and stimulates autophagy in parkinson’s disease model. CNS Neurol. Disord. Drug Targets 2015, 14, 1350–1365. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Cordaro, M.; Campolo, M.; Siracusa, R.; Cornelius, C.; Navarra, M.; Cuzzocrea, S.; Esposito, E. Neuroprotection by association of palmitoylethanolamide with luteolin in experimental alzheimer’s disease models: The control of neuroinflammation. CNS Neurol. Disord. Drug Targets 2014, 13, 1530–1541. [Google Scholar] [CrossRef]
- Barbierato, M.; Facci, L.; Marinelli, C.; Zusso, M.; Argentini, C.; Skaper, S.D.; Giusti, P. Co-ultramicronized palmitoylethanolamide/luteolin promotes the maturation of oligodendrocyte precursor cells. Sci. Rep. 2015, 5, 16676. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Esposito, E.; Di Paola, R.; Ahmad, A.; Campolo, M.; Peli, A.; Morittu, V.M.; Britti, D.; Cuzzocrea, S. Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type ii in mice. Arthritis Res. Ther. 2013, 15, R192. [Google Scholar] [CrossRef]
- Caltagirone, C.; Cisari, C.; Schievano, C.; Di Paola, R.; Cordaro, M.; Bruschetta, G.; Esposito, E.; Cuzzocrea, S.; Stroke Study, G. Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: From rodent to man. Transl. Stroke Res. 2016, 7, 54–69. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial effects of co-ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Lunardelli, M.L.; Crupi, R.; Siracusa, R.; Cocuzza, G.; Cordaro, M.; Martini, E.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S. Co-ultrapealut: Role in preclinical and clinical delirium manifestations. CNS Neurol. Disord. Drug Targets 2019. [Google Scholar] [CrossRef]
- Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Paterniti, I.; Siracusa, R.; Cuzzocrea, S.; Esposito, E. Co-ultramicronized palmitoylethanolamide/luteolin promotes neuronal regeneration after spinal cord injury. Front. Pharmacol. 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Barbierato, M.; Borri, M.; Facci, L.; Zusso, M.; Skaper, S.D.; Giusti, P. Expression and differential responsiveness of central nervous system glial cell populations to the acute phase protein serum amyloid a. Sci. Rep. 2017, 7, 12158. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Barbierato, M.; Facci, L.; Borri, M.; Contarini, G.; Zusso, M.; Giusti, P. Co-ultramicronized palmitoylethanolamide/luteolin facilitates the development of differentiating and undifferentiated rat oligodendrocyte progenitor cells. Mol. Neurobiol. 2018, 55, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Franceschini, D.; Facci, L.; Barbierato, M.; Giusti, P.; Zusso, M. A co-ultramicronized palmitoylethanolamide/luteolin composite mitigates clinical score and disease-relevant molecular markers in a mouse model of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2019, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.J.; Yang, C.S. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, L.N.; Xing, W.W.; Huang, B.K.; Jia, M.; Wu, J.Z.; Zhang, H.; Qin, L.P. Lipid-lowering effects of polydatin from polygonum cuspidatum in hyperlipidemic hamsters. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 652–658. [Google Scholar] [CrossRef]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant activity and inhibition of alpha-glucosidase by trans-resveratrol, piceid, and a novel trans-stilbene from the roots of israeli rumex bucephalophorus L. J. Agric. Food Chem. 2006, 54, 1243–1247. [Google Scholar] [CrossRef]
- Li, R.P.; Wang, Z.Z.; Sun, M.X.; Hou, X.L.; Sun, Y.; Deng, Z.F.; Xiao, K. Polydatin protects learning and memory impairments in a rat model of vascular dementia. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19, 677–681. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Li, G.; Wang, X.; Zeng, Z.; Cai, S.; Li, F.; Chen, Z. Polydatin attenuates ipopolysaccharide-induced acute lung injury in rats. Int. J. Clin. Exp. Pathol. 2014, 7, 8401–8410. [Google Scholar]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am. J. Reprod. Immunol. 2008, 60, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y. Medical treatment of endometriosis. Chang. Gung Med. J. 2008, 31, 431–440. [Google Scholar] [PubMed]
- Milewski, L.; Barcz, E.; Dziunycz, P.; Radomski, D.; Kaminski, P.; Roszkowski, P.I.; Korczak-Kowalska, G.; Malejczyk, J. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. J. Reprod. Immunol. 2008, 79, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Anaf, V.; Chapron, C.; El Nakadi, I.; De Moor, V.; Simonart, T.; Noel, J.C. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil. Steril. 2006, 86, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Indraccolo, U.; Barbieri, F. Effect of palmitoylethanolamide-polydatin combination on chronic pelvic pain associated with endometriosis: Preliminary observations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 150, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; Trabucco, E.; De Franciscis, P.; Torella, M.; Colacurci, N. Effectiveness of the association micronized n-palmitoylethanolamine (pea)-transpolydatin in the treatment of chronic pelvic pain related to endometriosis after laparoscopic assessment: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 82–86. [Google Scholar] [CrossRef]
- Giugliano, E.; Cagnazzo, E.; Soave, I.; Lo Monte, G.; Wenger, J.M.; Marci, R. The adjuvant use of n-palmitoylethanolamine and transpolydatin in the treatment of endometriotic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 209–213. [Google Scholar] [CrossRef]
- Lo Monte, G.; Soave, I.; Marci, R. administration of micronized palmitoylethanolamide (pea)-transpolydatin in the treatment of chronic pelvic pain in women affected by endometriosis: Preliminary results. Minerva Ginecol. 2013, 65, 453–463. [Google Scholar]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Indraccolo, U.; Indraccolo, S.R.; Mignini, F. Micronized palmitoylethanolamide/trans-polydatin treatment of endometriosis-related pain: A meta-analysis. Ann. Ist. Super. Sanita 2017, 53, 125–134. [Google Scholar]
- Murina, F.; Graziottin, A.; Felice, R.; Radici, G.; Tognocchi, C. Vestibulodynia: Synergy between palmitoylethanolamide + transpolydatin and transcutaneous electrical nerve stimulation. J. Low Genit. Tract Dis. 2013, 17, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, E.; Armentano, M.; Giugliano, B.; Sena, T.; Giuliano, P.; Loffredo, C.; Mastrantonio, P. Effectiveness of the association n-palmitoylethanolamine and transpolydatin in the treatment of primary dysmenorrhea. J. Pediatr. Adolesc. Gynecol. 2015, 28, 447–450. [Google Scholar] [CrossRef]
- Esposito, E.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Gugliandolo, E.; Crupi, R.; Cuzzocrea, S. A new co-micronized composite containing palmitoylethanolamide and polydatin shows superior oral efficacy compared to their association in a rat paw model of carrageenan-induced inflammation. Eur. J. Pharmacol. 2016, 782, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Inferrera, A.; Esposito, E.; Di Paola, R.; Cuzzocrea, S. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol. Appl. Pharmacol. 2017, 329, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; Biundo, F.; D’Amico, R.; Benedetto, F.; Di Paola, R.; Cuzzocrea, S. Palmitoylethanolamide and polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacol. Res. 2017, 123, 83–92. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-ras expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef]
- Wang, H.K. The therapeutic potential of flavonoids. Expert Opin. Investig. Drugs 2000, 9, 2103–2119. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Dirsch, V.M. Modulation of endothelial nitric oxide by plant-derived products. Nitric Oxide Biol. Chem. 2009, 21, 77–91. [Google Scholar] [CrossRef]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Kren, V.; Walterova, D. Silybin and silymarin-new effects and applications. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech. Repub. 2005, 149, 29–41. [Google Scholar] [CrossRef]
- Saller, R.; Melzer, J.; Reichling, J.; Brignoli, R.; Meier, R. An updated systematic review of the pharmacology of silymarin. Complement. Med. Res. 2007, 14, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Agarwal, R. Silymarin and epithelial cancer chemoprevention: How close we are to bedside? Toxicol. Appl. Pharmacol. 2007, 224, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Basiglio, C.L.; Sanchez Pozzi, E.J.; Mottino, A.D.; Roma, M.G. Differential effects of silymarin and its active component silibinin on plasma membrane stability and hepatocellular lysis. Chem. Biol. Interact. 2009, 179, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin-new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Bruschetta, G.; Ahmad, A.; Crupi, R.; Siracusa, R.; Di Paola, R.; Paterniti, I.; Prosdocimi, M.; Esposito, E.; Cuzzocrea, S. Effects of palmitoylethanolamide and silymarin combination treatment in an animal model of kidney ischemia and reperfusion. Eur. J. Pharmacol. 2015, 762, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of scutellaria baicalensis georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Wang, C.Z.; Mehendale, S.R.; Yuan, C.S. Commonly used antioxidant botanicals: Active constituents and their potential role in cardiovascular illness. Am. J. Chin. Med. 2007, 35, 543–558. [Google Scholar] [CrossRef]
- Huang, Y.; Tsang, S.Y.; Yao, X.; Chen, Z.Y. Biological properties of baicalein in cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 177–184. [Google Scholar] [CrossRef]
- Chi, Y.S.; Lim, H.; Park, H.; Kim, H.P. Effects of wogonin, a plant flavone from scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003, 66, 1271–1278. [Google Scholar] [CrossRef]
- Ma, Z.; Otsuyama, K.; Liu, S.; Abroun, S.; Ishikawa, H.; Tsuyama, N.; Obata, M.; Li, F.J.; Zheng, X.; Maki, Y.; et al. Baicalein, a component of scutellaria radix from huang-lian-jie-du-tang (hljdt), leads to suppression of proliferation and induction of apoptosis in human myeloma cells. Blood 2005, 105, 3312–3318. [Google Scholar] [CrossRef]
- Ma, S.C.; Du, J.; But, P.P.; Deng, X.L.; Zhang, Y.W.; Ooi, V.E.; Xu, H.X.; Lee, S.H.; Lee, S.F. Antiviral chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 2002, 79, 205–211. [Google Scholar] [CrossRef]
- Wu, J.A.; Attele, A.S.; Zhang, L.; Yuan, C.S. Anti-hiv activity of medicinal herbs: Usage and potential development. Am. J. Chin. Med. 2001, 29, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Motoo, Y.; Sawabu, N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett. 1994, 86, 91–95. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wu, J.; Ye, F.; Xue, L.; Jiang, S.; Yi, J.; Zhang, W.; Wei, H.; Sung, M.; Wang, W.; et al. Inhibition of cancer cell proliferation and prostaglandin e2 synthesis by scutellaria baicalensis. Cancer Res. 2003, 63, 4037–4043. [Google Scholar] [PubMed]
- Yu, J.; Liu, H.; Lei, J.; Tan, W.; Hu, X.; Zou, G. Antitumor activity of chloroform fraction of scutellaria barbata and its active constituents. Phytother. Res. Int. J. Devot. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 817–822. [Google Scholar]
- D’Amico, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; Di Paola, R. Effects of a new compound containing palmitoylethanolamide and baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomed. Int. J. Phytother. Phytopharm. 2019, 54, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharmacol. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Campolo, M.; Impellizzeri, D.; Paterniti, I.; Allara, M.; Gugliandolo, E.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Esposito, E.; et al. 2-pentadecyl-2-oxazoline, the oxazoline of pea, modulates carrageenan-induced acute inflammation. Front. Pharmacol. 2017, 8, 308. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. N-palmitoylethanolamine-oxazoline as a new therapeutic strategy to control neuroinflammation: Neuroprotective effects in experimental models of spinal cord and brain injury. J. Neurotrauma 2017, 34, 2609–2623. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline reduces neuroinflammatory environment in the mptp model of parkinson disease. Mol. Neurobiol. 2018, 55, 9251–9266. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of pea-oxa on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-palmitoylethanolamine-oxazoline (pea-oxa): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef] [PubMed]
| Type of Study | Molecular Targets | References |
|---|---|---|
| Mouse model of Anxiety/Depressive | BrdU ↑ | [70] |
| DCX ↑ | ||
| BDNF ↑ | ||
| Bax ↓ | ||
| Bcl-2 ↑ | ||
| Mouse model of Spinal Cord Injury | Cox-2 ↓ | [71,72,81] |
| iNOS ↓ | ||
| nNOS ↑ | ||
| PPAR ↑ | ||
| PPARβ/δ ↓ | ||
| PPARγ ↓ | ||
| Beclin-1 ↓ | ||
| p62 ↓ | ||
| MAP-LC3 ↓ | ||
| mTOR ↑ | ||
| p70S6K ↑ | ||
| p-AKT ↑ | ||
| BrdU ↑ | ||
| DCX ↑ | ||
| GFAP ↓ | ||
| MAP-2 ↑ | ||
| BDNF ↑ | ||
| GDNF ↑ | ||
| NGF ↑ | ||
| NT-3 ↑ | ||
| Mouse model of Rheumatoid Arthritis (CIA) | Chymase ↓ | [77] |
| Tryptase ↓ | ||
| Mast cells ↓ | ||
| MIP-1α ↓ | ||
| MIP-2 ↓ | ||
| IL-1β ↓ | ||
| IL-6 ↓ | ||
| TNF-α ↓ | ||
| MPO activity ↓ | ||
| Nitrotyrosine ↓ | ||
| MDA ↓ | ||
| In vitro model of Alzheimer’s disease | IκBa ↑ | [75] |
| NFkB p65 ↓ | ||
| BDNF ↑ | ||
| GDNF ↑ | ||
| GFAP ↓ | ||
| iNOS ↓ | ||
| nNOS ↑ | ||
| AIF ↓ | ||
| Caspase-3 ↓ | ||
| PARP-1 ↓ | ||
| Mouse and in vitro model of Parkinson’s disease | TH ↑ | [74] |
| DAT ↑ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| GFAP ↓ | ||
| TNF-α ↓ | ||
| iNOS ↓ | ||
| nNOS ↑ | ||
| Cox-2 ↓ | ||
| Bax ↓ | ||
| Bad ↓ | ||
| Bcl-2 ↑ | ||
| mTOR ↓ | ||
| p70S6K ↓ | ||
| Beclin-1 ↑ | ||
| p62 ↑ | ||
| MAP-LC3 ↑ | ||
| Demyelinating diseases (Maturation of Oligodendrocyte Precursor Cells) | Cnr1 ↑ | [76,82,83] |
| Cnr2 ↑ | ||
| Cat ↑ | ||
| Cnp ↑ | ||
| Hmgcr ↑ | ||
| Idi1 ↑ | ||
| Mki67 ↑ | ||
| Mbp ↑ | ||
| Plp1 ↑ | ||
| Scd1 ↑ | ||
| Sod2 ↑ | ||
| Ugt8 ↑ | ||
| PDGFR-α ↑ | ||
| Mouse model of Traumatic Brain Injury | IκBa ↑ | [73] |
| NFkB p65 ↓ | ||
| TNF-α ↓ | ||
| IL-1β ↓ | ||
| GFAP ↓ | ||
| Iba1 ↓ | ||
| Chymase ↓ | ||
| Tryptase ↓ | ||
| GDNF ↑ | ||
| iNOS ↓ | ||
| pJNK ↓ | ||
| Bax ↓ | ||
| Caspase-3 ↓ | ||
| mTOR ↑ | ||
| p70S6K ↑ | ||
| Beclin-1 ↓ | ||
| p62 ↓ | ||
| MAP-LC3 ↓ | ||
| Rat model of Cerebral Ischemia (MCAO) | GFAP ↓ | [78] |
| BDNF ↑ | ||
| GDNF ↑ | ||
| Mast cells ↓ | ||
| Chymase ↓ | ||
| Tryptase ↓ | ||
| Bax ↓ | ||
| Bcl-2 ↑ | ||
| Mouse model of Autism | IκBa ↑ | [79] |
| NFkB p65 ↓ | ||
| iNOS ↓ | ||
| GFAP ↓ | ||
| TNF-α ↓ | ||
| IL-1β ↓ | ||
| Chymase ↓ | ||
| Tryptase ↓ | ||
| Bax ↓ | ||
| Bcl-2 ↑ | ||
| BrdU ↑ | ||
| DCX ↑ | ||
| Mouse model of Multiple Sclerosis (MS) | SAA1 ↓ | [84] |
| TNF-α ↓ | ||
| IL-1β ↓ | ||
| IFN-γ ↓ | ||
| TLR2 ↓ | ||
| Fpr2 ↓ | ||
| CD137 ↓ | ||
| CD3-γ ↓ | ||
| TCR-ζ chain ↓ | ||
| CB2 ↓ | ||
| Mouse model of Delirium | Bax ↓ | [80] |
| Bcl-2 ↑ | ||
| TNF- α ↓ | ||
| IL-1β ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| Nrf-2 ↑ | ||
| Mn-SOD ↑ | ||
| GDNF ↑ |
| Type of Study | Molecular Targets | References |
|---|---|---|
| Rat paw model of carrageenan-induced inflammation (in vivo and in vitro study) | TNF-α ↓ | [103] |
| IL-6 ↓ | ||
| IL-1β ↓ | ||
| MPO ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| COX-2 ↓ | ||
| iNOS ↓ | ||
| Mn-SOD ↑ | ||
| Mouse model of surgically-induced Endometriosis | MMP9 ↓ | [99] |
| Mast Cell ↓ | ||
| NGF ↓ | ||
| VEGF ↓ | ||
| ICAM-1 ↓ | ||
| MPO ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| Nitrotyrosine ↓ | ||
| PAR ↓ | ||
| Rat model of Benign Prostatic Hyperplasia | PGE2 ↓ | [104] |
| DHT ↓ | ||
| 5α-reductase 1 ↓ | ||
| 5α-reductase 2 ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| iNOS ↓ | ||
| COX-2 ↓ | ||
| Nrf-2 ↑ | ||
| HO-1 ↑ | ||
| Mn-SOD ↑ | ||
| Mouse model of Vascular Injury | ICAM-1 ↓ | [105] |
| V-CAM ↓ | ||
| TNF- α ↓ | ||
| IL-1β ↓ | ||
| iNOS ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| Bax ↓ | ||
| FAS-Ligand ↓ | ||
| α-sma ↑ | ||
| MCP-1 ↑ | ||
| BrdU ↑ |
| Type of Study | Molecular Targets | Reference |
|---|---|---|
| Rat paw model of carrageenan-induced inflammation and osteoarthritic pain model | TNF-α ↓ | [109] |
| IL-1β ↓ | ||
| MPO ↓ | ||
| NGF ↓ | ||
| MMP-1 ↓ | ||
| MMP-3 ↓ | ||
| MMP-9 ↓ |
| Type of Study | Molecular Targets | Reference |
|---|---|---|
| Mouse model of Kidney Ischemia and Reperfusion | MPO ↓ | [115] |
| TNF-α ↓ | ||
| IL-1β ↓ | ||
| Nitrite/Nitrate ↓ | ||
| Superoxide ↓ | ||
| CuZn SOD ↑ | ||
| Mn-SOD ↑ | ||
| Catalase ↑ | ||
| Nitrotyrosine ↓ | ||
| PAR ↓ | ||
| MDA ↓ | ||
| Chymase ↓ | ||
| ICAM-1 ↓ | ||
| p-selectin ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| Bax ↓ | ||
| Bcl-2 ↑ |
| Type of Study | Molecular Targets | Reference |
|---|---|---|
| Rat model of myocardial I/R injury | MPO ↓ | [126] |
| Mast Cell ↓ | ||
| Chymase ↓ | ||
| Tryptase ↓ | ||
| IκBa ↑ | ||
| NFkB p65 ↓ | ||
| TNF-α ↓ | ||
| IL-1β ↓ | ||
| Bax ↓ | ||
| Bcl-2 ↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. https://doi.org/10.3390/nu11092175
Peritore AF, Siracusa R, Crupi R, Cuzzocrea S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients. 2019; 11(9):2175. https://doi.org/10.3390/nu11092175
Chicago/Turabian StylePeritore, Alessio Filippo, Rosalba Siracusa, Rosalia Crupi, and Salvatore Cuzzocrea. 2019. "Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets" Nutrients 11, no. 9: 2175. https://doi.org/10.3390/nu11092175
APA StylePeritore, A. F., Siracusa, R., Crupi, R., & Cuzzocrea, S. (2019). Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients, 11(9), 2175. https://doi.org/10.3390/nu11092175







