Prevention and Treatment of Sarcopenic Obesity in Women
Abstract
1. Introduction
1.1. Age-Related and Obesity-Related Changes in Muscle Composition, Structure and Function in Women
1.2. Risk Factors for Sarcopenic Obesity and Related Disability
2. Methods of Narrative Review
3. Definition of Sarcopenic Obesity
- -
- Gait speed ≤ 0.8 m/s
- -
- Short Physical Performance Battery (SPPB) ≤ 8-point score
- -
- Timed-Up and Go test (TUG) ≥ 20 s
- -
- Non-completion or ≥ 6 min for completion of the 400-m walk test.
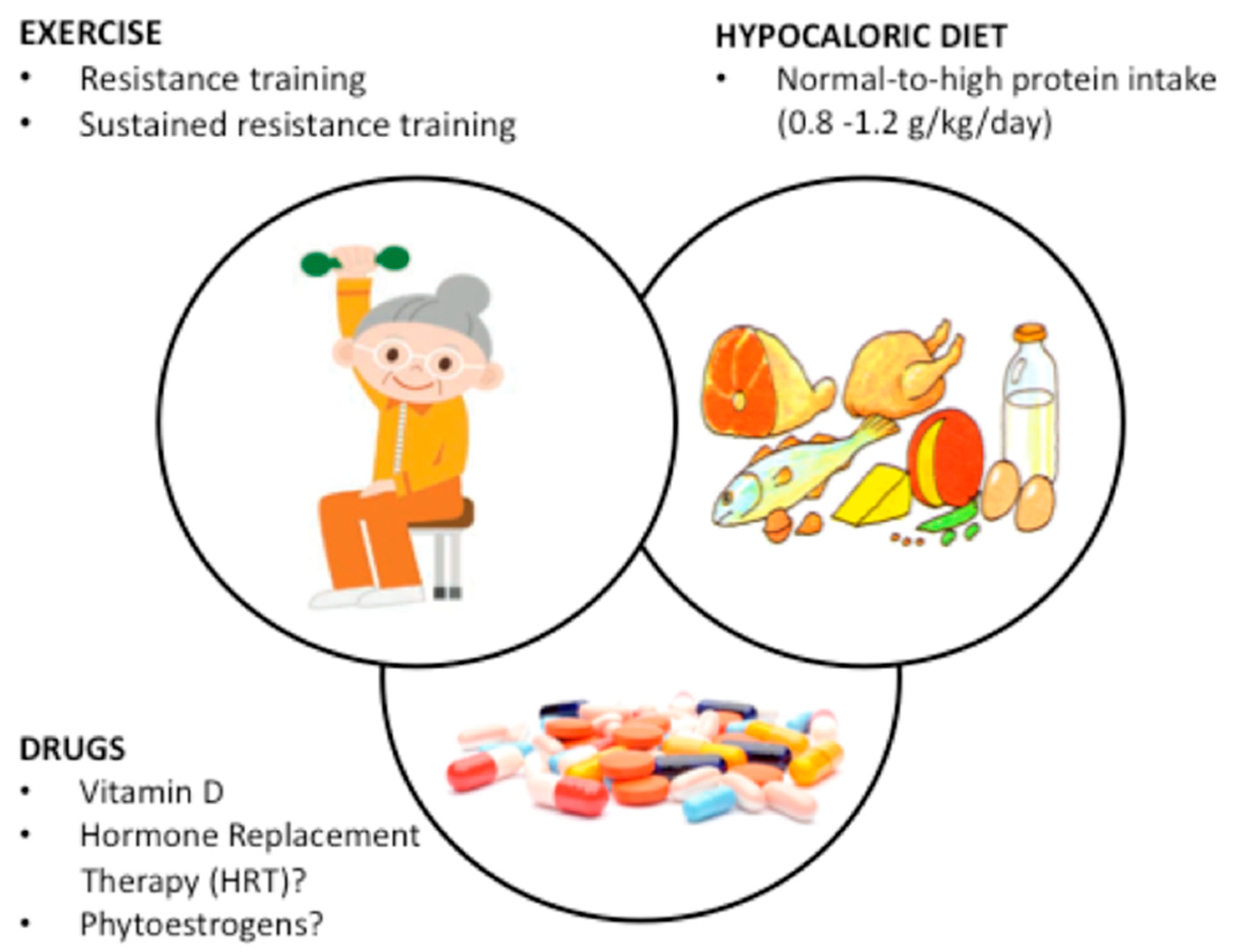
4. Prevention of Sarcopenic Obesity
4.1. Single Interventions
4.1.1. Nutrition
4.1.2. Pharmacotherapy
4.1.3. Exercise
4.2. Combined Interventions
4.2.1. Exercise Plus Nutritional Therapy
4.2.2. Exercise Plus Pharmacotherapy
5. Treatment of Sarcopenic Obesity
5.1. Single Treatment
5.1.1. Nutrition
5.1.2. Pharmacotherapy
5.1.3. Exercise and Physical Therapy
5.2. Combined Treatments
6. Discussion
6.1. Prevention of Sarcopenic Obesity
6.2. Treatment of Sarcopenic Obesity
6.3. Sex-Related Aspects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6MWT | 6-min walking test |
| AE | Aerobic exercise |
| ASMM | Appendicular skeletal muscle mass |
| BIA | Bioelectrical impedance analysis |
| CT | Computerized tomography |
| DEXA | Dual-energy x-ray absorptiometry |
| EAA | Essential amino acids |
| EWGSOP1 | European Working Group on Sarcopenia in Older People 1 (2010 criteria) |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 (2019 criteria) |
| FFM | Fat-free mass |
| HGS | Handgrip strength |
| HRT | Hormone replacement treatment |
| IADL | Instrumental activities of daily living |
| MRI | Magnetic resonance imaging |
| RCT | Randomized controlled trial |
| RT | Resistance training |
| SBBP | Short physical performance battery |
| SF-36 | Short form-36 questionnaire |
| SMI | Skeletal muscle index |
| SO | Sarcopenic obesity |
| WB-EMS | Whole-body electromyostimulation |
References
- Peralta, M.; Ramos, M.; Lipert, A.; Martins, J.; Marques, A. Prevalence and trends of overweight and obesity in older adults from 10 European countries from 2005 to 2013. Scand. J. Public Health 2018, 46, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Samper-Ternent, R.; Al Snih, S. Obesity in Older Adults: Epidemiology and implications for Disability and Disease. Rev. Clin. Gerontol. 2012, 22, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Calugi, S.; Dalle Grave, R. Sarcopenic obesity: Definition, health consequences and clinical management. Open Nutr. J. 2018, 12, 70–73. [Google Scholar] [CrossRef]
- Martínez-Amat, A.; Aibar-Almazán, A.; Fábrega-Cuadros, R.; Cruz-Díaz, D.; Jiménez-García, J.D.; Pérez-López, F.R.; Achalandabaso, A.; Barranco-Zafra, R.; Hita-Contreras, F. Exercise alone or combined with dietary supplements for sarcopenic obesity in community-dwelling older people: A systematic review of randomized controlled trials. Maturitas 2018, 110, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Chumlea, W.C.; Guo, S.S.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Heymsfield, S.B.; Lukaski, H.C.; Friedl, K.; Hubbard, V.S. Body composition estimates from NHANES III bioelectrical impedance data. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1596–1609. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.W.; Sowers, M.R. The interrelationship between body topology and body composition varies with age among women. J. Nutr. 2000, 130, 2371–2377. [Google Scholar] [CrossRef]
- Tian, S.; Morio, B.; Denis, J.-B.; Mioche, L. Age-related changes in segmental body composition by ethnicity and history of weight change across the adult lifespan. Int. J. Environ. Res. Public Health 2016, 13, 821. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J.C. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic obesity: Time to meet the challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Tallis, J.; James, R.S.; Seebacher, F. The effects of obesity on skeletal muscle contractile function. J. Exp. Biol. 2018, 221 Pt 13. [Google Scholar] [CrossRef] [PubMed]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.L.; Muls, E. Assessment of isokinetic muscle strength in women who are obese. J. Orthop. Sports Phys. Ther. 2002, 32, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Winwood, K.; Morse, C.I.; Onambélé, G.L. The impact of obesity on skeletal muscle architecture in untrained young vs. old women. J. Anat. 2014, 225, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, F.; Capodaglio, P.; Orgiu, S.; Santovito, C.; Caramenti, M.; Cadioli, M.; Falini, A.; Rizzo, G.; Lafortuna, C.L. Effects of muscle composition and architecture on specific strength in obese older women. Exp. Physiol. 2015, 100, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef]
- Gretebeck, K.A.; Sabatini, L.M.; Black, D.R.; Gretebeck, R.J. Physical activity, functional ability, and obesity in older adults: A gender difference. J. Gerontol. Nurs. 2017, 43, 38–46. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gratteri, S.; Sarlo, F.; Cabibbo, A.; Colica, C.; De Lorenzo, A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Dis. Markers 2014, 743634. [Google Scholar] [CrossRef]
- Di Renzo, L.; Sarlo, F.; Petramala, L.; Iacopino, L.; Monteleone, G.; Colica, C.; De Lorenzo, A. Association between −308 G/A TNF-alpha polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis. Markers 2013, 35, 615–623. [Google Scholar] [CrossRef]
- Anagnostis, P.; Dimopoulou, C.; Karras, S.; Lambrinoudaki, I.; Goulis, D.G. Sarcopenia in post-menopausal women: Is there any role for vitamin D? Maturitas 2015, 82, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Jho, S.; No, J.-K.; Kim, H.-S. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr. Res. 2015, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Lee, J.S.; Visser, M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Nevitt, M.; Harris, T.B. Weight change and the conservation of lean mass in old age: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.P.; Rubele, S.; Caliari, C.; Pedelini, F.; Calugi, S.; Soave, F.; Chignola, E.; Bazzani, P.V.; Dalle Grave, R.; Zamboni, M. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity 2019. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic Obesity: Current Knowledge, Revised Identification Criteria and Treatment Principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Wells, J.C.; Smith, S.R.; Stephan, B.C.; Siervo, M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef] [PubMed]
- American Society of Bariatric Physicians (ASBP) Obesity Algorithm. 2015. Available online: http://www.asbp.org/obesityalgorithm.html (accessed on 26 May 2019).
- Cesari, M.; Fielding, R.A.; Pahor, M.; Goodpaster, B.; Hellerstein, M.; Van Kan, G.A.; Anker, S.D.; Rutkove, S.; Vrijbloed, J.W.; Isaac, M.; et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 181–190. [Google Scholar] [CrossRef]
- Petroni, M.L.; Bertoli, S.; Maggioni, M.; Morini, P.; Battezzati, A.; Tagliaferri, M.A. Feasibility of air plethysmography (BOD POD) in morbid obesity: A pilot study. Acta Diabetol. 2003, 40, S59–S62. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E. Osteosarcopenic Obesity Syndrome: What Is It and How Can It Be Identified and Diagnosed? Curr. Gerontol. Geriatr. Res. 2016, 2016, 7325973. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef]
- Domiciano, D.S.; Figueiredo, C.P.; Lopes, J.B.; Caparbo, V.F.; Takayama, L.; Menezes, P.R.; Bonfa, E.; Pereira, R.M. Discriminating sarcopenia in community-dwelling older women with high frequency of overweight/obesity: The São Paulo Ageing & Health Study (SPAH). Osteoporos. Int. 2013, 24, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Brown, T.; Kiel, D.P.; et al. Establishing the link between lean mass and grip strength cut-points with mobility disability and other health outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J. Gerontol. A Biol. Sci. Med. Sci. 2019. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Rossi, A.P.; Calugi, S.; Rubele, S.; Soave, F.; Zamboni, M.; Chignola, E.; Mazzali, G.; Bazzani, P.V.; Dalle Grave, R. Physical performance measures in screening for reduced lean body mass in adult females with obesity. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Porter Starr, K.N.; Pieper, C.F.; Orenduff, M.C.; McDonald, S.R.; McClure, L.B.; Zhou, R.; Payne, M.E.; Bales, C.W. Improved function with enhanced protein intake per meal: A pilot study of weight reduction in frail, obese older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1369–1375. [Google Scholar] [CrossRef]
- Bales, C.W.; Porter Starr, K.N.; Orenduff, M.C.; McDonald, S.R.; Molnar, K.; Jarman, A.K.; Onyenwoke, A.; Mulder, H.; Payne, M.E.; Pieper, C.F. Influence of protein intake, race, and age on responses to a weight-reduction intervention in obese women. Curr. Dev. Nutr. 2017, 1, e000703. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Jakicic, J.M.; Lowery, J.B.; Hames, K.C.; Kelley, D.E.; Goodpaster, B.H. African American women exhibit similar adherence to intervention but lose less weight due to lower energy requirements. Int. J. Obes. 2014, 38, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Wingo, B.C.; Carson, T.L.; Ard, J. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obes. Rev. 2014, 15 (Suppl. 4), 46–61. [Google Scholar] [CrossRef]
- Sorensen, M.B.; Rosenfalck, A.M.; Hojgaard, L.; Ottesen, B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes. Res. 2001, 9, 622–626. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2019, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.M.; Ribeiro, A.S.; Tomeleri, C.M.; Schoenfeld, B.J.; Silva, A.M.; Souza, M.F.; Nascimento, M.A.; Sardinha, L.B.; Cyrino, E.S. The effects of resistance training volume on osteosarcopenic obesity in older women. J. Sports Sci. 2018, 36, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, A.; Dutra, M.; de Moraes, W.M.; Funghetto, S.; Lopes de Farias, D.; dos Santos, P.H.F.; Vieira, D.C.L.; da Cunha Nascimento, D.; Orsano, V.S.M.; Schoenfeld, B.J.; et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin. Interv. Aging 2018, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.E.; Hudson, R.; Kilpatrick, K.; Kuk, J.L.; McMillan, K.; Janiszewski, P.M.; Lee, S.; Lam, M.; Ross, R. Effects of exercise modality on insulin resistance and functional limitation in older adults: A randomized controlled trial. Arch. Intern. Med. 2009, 169, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Xiao, L.; Imayama, I.; Duggan, C.R.; Foster-Schubert, K.E.; Kong, A.; Neuhouser, M.L.; Alfano, C.M. Influence of diet, exercise, and serum vitamin d on sarcopenia in postmenopausal women. Med. Sci. Sports Exerc. 2013, 45, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Mojtahedi, M.C.; Thorpe, M.P.; Karampinos, D.C.; Johnson, C.L.; Layman, D.K.; Georgiadis, J.G.; Evans, E.M. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 1218–1225. [Google Scholar] [CrossRef]
- Galbreath, M.; Campbell, B.; LaBounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.; Levers, K.; Galvan, E.; et al. Effects of adherence to a higher protein diet on weight loss, markers of health, and functional capacity in older women participating in a resistance-based exercise program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef]
- Figueroa, A.; Going, S.B.; Milliken, L.A.; Blew, R.M.; Sharp, S.; Teixeira, P.J.; Lohman, T.G. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 266–270. [Google Scholar] [CrossRef]
- Shea, M.K.; Nicklas, B.J.; Marsh, A.P.; Houston, D.K.; Miller, G.D.; Isom, S.; Miller, M.E.; Carr, J.J.; Lyles, M.F.; Harris, T.B.; et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity 2011, 19, 1636–1646. [Google Scholar] [CrossRef]
- Corica, F.; Bianchi, G.; Corsonello, A.; Mazzella, N.; Lattanzio, F.; Marchesini, G. Obesity in the context of aging: Quality of lfe considerations. Pharmacoeconomics 2015, 33, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Aleman-Mateo, H.; Macias, L.; Esparza-Romero, J.; Astiazaran-Garcia, H.; Blancas, A.L. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: Evidence from a randomized clinical trial using a protein-rich food. Clin. Interv. Aging 2012, 7, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, E.; Nasti, G.; Siervo, M.; Di Maro, M.; Lapi, D.; D’Addio, G.; Colantuoni, A. Dietary protein intake in sarcopenic obese older women. Clin. Interv. Aging 2016, 11, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, R.; Marra, M.; Di Guglielmo, M.L.; Naccarato, M.; Contaldo, F.; Poggiogalle, E.; Donini, L.M.; Pasanisi, F. Evaluation of hypocaloric diet with protein supplementation in middle-aged sarcopenic obese women: A pilot study. Obes. Facts 2017, 10, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Six months of isoflavone supplement increases fat-free mass in obese–sarcopenic postmenopausal women: A randomized double-blind controlled trial. Eur. J. Clin. Nutr. 2007, 61, 1442–1444. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Paiva, F.M.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Chung, Y.-C.; Chen, Y.-J.; Ho, S.-Y.; Wu, H.-J. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Park, J.; Kwon, Y.; Park, H. Effects of 24-week aerobic and resistance training on carotid artery intima-media thickness and flow velocity in elderly women with sarcopenic obesity. J. Atheroscler. Thromb. 2017, 24, 1117–1124. [Google Scholar] [CrossRef]
- Liao, C.-D.; Tsauo, J.-Y.; Huang, S.-W.; Ku, J.-W.; Hsiao, D.-J.; Liou, T.-H. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci. Rep. 2018, 8, 2317. [Google Scholar] [CrossRef]
- Huang, S.-W.; Ku, J.-W.; Lin, L.-F.; Liao, C.-D.; Chou, L.-C.; Liou, T.-H. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 556–563. [Google Scholar] [CrossRef]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs. hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Teschler, M.; Weissenfels, A.; Bebenek, M.; von Stengel, S.; Kohl, M.; Freiberger, E.; Goisser, S.; Jakob, F.; Sieber, C.; et al. Whole-body electromyostimulation to fight sarcopenic obesity in community-dwelling older women at risk. Results of the randomized controlled FORMOsA-sarcopenic obesity study. Osteoporos. Int. 2016, 27, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Kojima, N.; Fujino, K.; Hosoi, E.; Kobayashi, H.; Somekawa, S.; Niki, Y.; Yamashiro, Y.; Yoshida, H. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Chmelo, E.; Delbono, O.; Carr, J.J.; Lyles, M.F.; Marsh, A.P. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: A randomized controlled trial. Am. J. Clin. Nutr. 20151, 101, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Migliaccio, S.; Lenzi, A.; Donini, L.M. Treatment of body composition changes in obese and overweight older adults: Insight into the phenotype of sarcopenic obesity. Endocrine 2014, 47, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving muscle mass during weight loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef]
- Bopp, M.J.; Houston, D.K.; Lenchik, L.; Easter, L.; Kritchevsky, S.B.; Nicklas, B.J. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J. Am. Diet. Assoc. 2008, 108, 1216–1220. [Google Scholar] [CrossRef]
- Backx, E.M.; Tieland, M.; Borgonjen-van den Berg, K.J.; Claessen, P.R.; van Loon, L.J.; de Groot, L.C. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int. J. Obes. 2016, 40, 299–304. [Google Scholar] [CrossRef]
- Beavers, K.M.; Nesbit, B.A.; Kiel, J.R.; Sheedy, J.L.; Arterburn, L.M.; Collins, A.E.; Ford, S.A.; Henderson, R.M.; Coleman, C.D.; Beavers, D.P. Effect of an energy-restricted, nutritionally complete, higher protein meal plan on body composition and mobility in older adults with obesity: A randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Frimel, T.N.; Sinacore, D.R.; Villareal, D.T. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med. Sci. Sports Exerc. 2008, 40, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.K.; Miller, M.E.; Kitzman, D.W.; Rejeski, W.J.; Messier, S.P.; Lyles, M.F.; Kritchevsky, S.B.; Nicklas, B.J. Long-term effects of randomization to a weight loss intervention in older adults: A pilot study. J. Nutr. Gerontol. Geriatr. 2019, 38, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Mauvais-Jarvis, F., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 153–197. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Callahan, D.M.; McGlory, C.; Toth, M.J.; Phillips, S.M. Maintenance of skeletal muscle function following reduced daily physical activity in healthy older adults: A pilot trial. Appl. Physiol. Nutr. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
| Ref. | No. Subjects Age (years) | Inclusion Criteria | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [38] | 67 (80% women) Mean age, 68 | BMI ≥ 30 kg/m2, functionally impaired (SPPB score of 4–10 out of 12) | Parallel group (n = 2) RCT Duration 6 months | Normal protein (NP, 0.8 g/kg) or high protein (HP, 1.2 g/kg), moderately hypocaloric diet No exercise | WL (kg): −7.5 ± 6.2 (NP); −8.7 ± 7.4 (HP), both p < 0.001 vs. BL Lean mass (kg): −1.8 (NP) −1.1 (HP), both p < 0.01 vs. BL Total SPPB score: +0.9 (NP), p < 0.01 vs. BL; +2.4 (HP), p = 0.02 vs. NP, p < 0.001 vs. BL HGS (kg): +1.3 (NP) +1.1 (HP), both p < 0.01 vs. BL | Mean BMI = 37.1 kg/m2 Body composition measured by air displacement plethysmography |
| [39] | 80 women ≥ 45 years (48.8% white) Mean age, 60 | BMI ≥ 30 kg/m2 | Parallel group (n = 2) RCT Duration 6 months | Normal protein (NP, 0.8 g/kg) or high protein (HP, 1.2 g/kg) moderately hypocaloric diet No exercise | WL (kg): −6.2 (NP), −6.4 (HP), both p < 0.001 vs. BL. WL greater in white (–7.2) than in black women (−4.0), p < 0.04 Lean mass (kg): −1.0 (NP) −0.6 (HP), both p < 0.01 vs. BL Total SPPB score: +1.2 (NP); +1.0 (HP), p < 0.001 vs. BL 6MWT (m): +46.8 (NP) +46.9 (HP), both p < 0.001 vs. BL | Mean BMI = 37.8 kg/m2 Body composition measured by air displacement plethysmography |
| Ref. | No. Subjects Age (years) | Inclusion Criteria | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [42] | 16 post-menopausal women Mean age, 55 | BMI ≥ 25kg/m2 | Cross-over PL-controlled RCT 3-month washout in-between | HRT (12 weeks) Placebo (PL) (12 weeks) No diet or exercise advice | FFM (kg): HRT + 0.35, p < 0.05 vs. PL; PL: −1.0, p < 0.05 vs. pre-treatment Total bone mineral density (g/cm2): HRT +8.6, p < 0.05 vs. PL; PL−3.9 p < 0.05 vs. BL Abdominal fat mass (kg): HRT −0.19, p < 0.05 vs. BL; PL +0.25 p < 0.05 vs. BL | Mean BMI = 27 kg/m2 Mean BF = 43% Body composition measured by DEXA |
| [43] | Subjects (62 men, 66 women) pre-sarcopenic and deficient in vitamin D w/wo associated obesity Mean age, 73 | Presarcopenia as skeletal muscle mass/height2 <5.45 kg/m2 for women Serum level of 25(OH)D < 20 ng/mL Obesity defined as BMI ≥ 30 kg/m2 | Parallel group (n = 2) controlled RCT Duration, 6 months | 10,000 IU cholecalciferol 3/week (vitamin D) Placebo (PL) | HS: no difference in vitamin D vs. PL Appendicular skeletal muscle mass (ASMM): increased in vitamin D group (p < 0.001 vs. PL); (two-way ANOVA) effect of vitamin D on ASMM much higher in non-obese vs. obese subjects. (1.57 vs. 1.32, p < 0.001). No sex-related effect was observed in the presarcopenic obese group | Obesity in 49% of study population Body composition measured by DEXA |
| Ref. | No. Subjects | Inclusion Criteria | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [44] | 62 sedentary women aged ≥60 Mean age, 67 | Physical independency Obesity not mentioned | Parallel groups (n = 3) RCT Duration,12 weeks | RT 1 set (30 min) 3/week (GS1) RT 3 sets (50 min) 3/week (GS3) Control (no exercise − C) | Strength (%): GS1 + 18.5, GS3 + 25, both p < 0.05 vs. C (−7.2); GS3 p < 0.05 vs. GS1 SMM (kg): GS1 +0.9, GS3 +1.1, both p < 0.05 vs. C (+0.2) Body fat (%): GS1: −0.4, GS3 −2.5, both p < 0.05 vs. C (+0.6); GS3 p < 0.05 vs. GS1 | Mean BMI = 27 kg/m2 Body composition measured by DEXA |
| [45] | 41 sedentary obese non- sarcopenic women aged ≥ 60 years Mean age, 66 | Body fat > 32% AFFM above a population specific cut-off | Parallel groups (n = 2) RCT comparing non-sarcopenic with SO Duration 16 weeks | RT (2 sessions of 40–50 min/week) All subjects advised not to change usual diet | In the subgroup of non-sarcopenic obese: BF: −0,6 kg, p = 0,03 vs. BL. No changes in AFFM vs. BL 30 s chair stand-up and timed-up-and-go: improved vs. BL (p = 0.000) Strength parameters: improved (moderate effect size) vs. BL (p ≤ 0.01) | Mean BMI 28 kg/m2 Body composition measured by DEXA |
| [46] | 74 women out of 136 abdominally obese adults aged 60–80 Mean age, 67 | WC ≥ 88 cm in women No conditions incompatible with exercise engagement | Parallel groups (n = 2) RCT Duration 6 months | Control, no exercise AE (150 min/week) RT (60 min/week) AE + RT (150 min/week) All on isocaloric diet | Combined z-score of tests for functional limitation improved AE, RT, RT + AE p < 0.05 vs. control; RT + AE p < 0.05 vs. AE and RT Oxygen consumption (peak VO2) increased in AE and RT + AE vs. RT (p < 0.05) and vs. control (p < 0.05) Skeletal muscle increased in RT and RT + AE vs. AE (p < 0.05) and vs. control (p < 0.05) | Mean BMI = 30 kg/m2 Women-specific data not provided (responses not different between sexes within treatment groups) Body composition measured by MRI |
| Ref. | No. Subjects Age (years) | Inclusion Criteria | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [47] | 439 overweight or obese post- menopausal sedentary women Mean age, 58 | BMI ≥ 25.0 kg/m2 (≥23.0 if Asian American) Sarcopenia as SMI ≤ 5.67 kg/m2 | Parallel groups (n = 4) RCT Duration 12 months | Moderately hypocaloric diet (D) Exercise (AE − 225 min/week) Combined (D + AE) Control (C-no intervention) | Total FFM (kg): D: −1.1 vs. −0.1 C, p < 0.01; AE: no significant changes; D + AE: −0.6 kg, p > 0.01 vs. AE Appendicular FFM (kg): D −0.5, p = 0.02 vs. control; AE 0.0 kg (not significant vs. C), D + AE − 0.2, (p < 0.01 vs. D) No differences in sarcopenia incidence among non-sarcopenic women | 17% at BL (mean BMI 31 kg/m2) had sarcopenia Body composition measured by DEXA |
| [48] | 31 overweight or obese, postmenopausal women Mean age, 65 | BMI ≥ 28 kg/m2 | parallel group (n = 2) RCT Duration 6 months | Hypocaloric diet + whey protein (2 × 25 g/day) (PRO) Hypocaloric diet supplemented with maltodextrine (CARB) Mild exercise (flexibility + aerobic 40′–50′ sessions 2–3/week) in both groups | Whole body mass (kg): CARB −3.6, PRO −7.7; (p = 0.051 vs. CARB) Thigh muscle mass (%): CHO +4.5, PRO +10.3 (p = 0.049 vs. CARB) Intermuscular adipose tissue (cm2): CARB −1.0, PRO −9.2; (p = 0.03 vs. CARB) No differences in changes in strength, balance, or physical performance measures between PRO and CARB | Mean BMI = 33.4 kg/m2 Body composition measured by DEXA and MRI |
| [49] | 54 overweight and obese sedentary women aged 60–75 Mean age, 66 | BMI ≥ 27 kg/m2 and/or body fat percentage above 35% | Parallel groups (n = 3) RCT Duration 14 weeks | Exercise (RT, 3/week), no diet (Ex) Ex + low-calorie high-CHO diet (ExHC) Ex + low-calorie high-protein diet (ExHP) | No reduction in FFM in all groups Percent BF: Ex −2.0%; ExHC −4.3; ExHP −6.3%; p = 0.002 vs. Ex and HP Strength increased in all groups with no group interaction | Mean BMI 30 kg/m2 Target HC diet: 55% HC, 15% P, 30% fat Target HP diet: P 1.2 g/kg, 30% fat Body composition by DEXA |
| [50] | 94 post-menopausal sedentary women Age range 40–65 | Being either on HRT (n = 39) or not HRT (n = 55) | Parallel groups (n = 4) RCT (2 × 2 factorial design) Duration, 12 months | Exercise (RT + weight bearing training) 3/week + HRT Exercise, no HRT No exercise, HRT No exercise, no HRT | Exercise groups: FFM total (+12%), arm (+15%), leg (+11%; strength (+9–20%); % BF (−1.9%) vs. BL (p < 0.001); No significant differences in no-exercise groups No interaction effects of HRT | Mean BF = 38% at BL Body composition measured by DEXA |
| [51] | 40 women and 48 men nondiabetic overweight/obese aged 65–79 Mean age, 70 | SPPB 3–10 (values < 10 predictive of mobility and disability risk) | Parallel groups RCT (n = 4) with 2 × 2 factorial design Duration 16 weeks | Hypocaloric diet (D) + Resistance Training (RT) D + RT + pioglitazone 30 mg (PIO) D + PIO D only | Women overall: WL −6.5%; FM −9.7%; LM –4.1% (all p < 0.05 vs. BL) Thigh muscle volume (cm3): RT −34 vs. no−RT 59, p = 0.040 Thigh subcutaneous fat (cm3): PIO −104 vs. no-PIO−298; mean difference 194, p = 0.002 | Women BMI = 33 kg/m2 Unlike women, PIO significantly reduced abdominal fat in men Body composition by DEXA and CT |
| Ref. | No. Subjects Age (years) | SO Definition | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [53] | Analysis by sex of 23 women and 17 men Mean age, 76 | Sarcopenia diagnosed by the residual method Overweight or obesity were not inclusion criteria High prevalence with cases with elevated %BF | Parallel group (n = 2) RCT Duration 12 weeks | Intervention: Protein supplements (210 g/day of ricotta cheese) plus the habitual diet Control: habitual diet | No significant effect of protein supplementation on ASMM or strength in both sexes | Mean BF in women 41% Body composition measured by DEXA |
| [54] | 104 women aged > 65 years with SO Mean age, 66 | BMI ≥ 30.0 kg/m2, or WC > 88.0 cm or FM% ≥ 35.0%, or FM index ≥ 9.5 kg/m2 Sarcopenia defined by MM index, MM/height2 (kg/m2), as <2SD the obesity-derived cut-off score (7.3 kg/m2-class 2) | Parallel group (n = 2) RCT Duration 12 weeks | High protein (1.2 g/kg) low-calorie diet (HP) Normal protein (0.8 g/kg bw reference) low-calorie diet (NP) | BMI (kg/m2): NP −1.3; HP −0.8, both p < 0.001 vs. BL MM index (kg/m2): NP −0.2, p < 0.01 vs. BL; HP +0.2, p < 0.01 vs. BL Arm-muscle area (cm2): NP −5.7, p < 0.001 vs. BL; HP −0.5, n.s. No significant difference in HGS vs. BL | Mean BMI = 31.5 kg/m2 Body composition measured by BIA and anthropometry |
| [55] | 18 women aged 41–74 years with SO Mean age, 55 | Obesity defined FM >34.8%; Sarcopenia defined by lean body mass <90% of the subject’s ideal FFM | Parallel group (n = 2) RCT (pilot) | Low-calorie high-protein diet (1.2–1.4 g/ kg bw reference/day) (HP) Low-calorie diet plus placebo (control) | WL: HP −3.9 kg (p = 0.01 vs. BL); control −3.8 kg (p = 0.05 vs. BL) FFM: HP +2.3 kg (p = 0.05 vs. control); control +0.6 kg (n.s) FM: HP −9.7 kg (p = 0.01 vs. BL); control −7.3 kg (p = 0.03 vs. BL) HGS: HP +1.6 kg (p = 0.01 vs. BL); control: n.s. No significant change in SPPB for both groups | Body composition measured by BIA |
| Ref. | No. Subjects Age (years) | SO Definition | Design | Type of Intervention | Main Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [56] | 18 post- menopausal women with SO aged 50–70 Mean age, 58 | Muscle mass (MM) index <6.87 kg Appendicular FFM/m2 FM > 40% | Parallel group (n = 2) PL-controlled RCT Duration 6 months | Isoflavones 70 mg (ISO) (n = 12) Placebo (PL) (n = 6) | Leg FFM (kg): ISO +0.29 vs. PL −0.62, p = 0.034 Appendicular FFM (kg): ISO +0.53, PL −0.78, p = 0.016 MM index: ISO +0.26, PL−0.27, p = 0.037 | BMI = 29 kg/m2 Body composition by DEXA |
| Ref. | No. Subjects Age (years) | SO Definition | Design | Type of Intervention | Intervention Effect (Main Findings) | Notes |
|---|---|---|---|---|---|---|
| [57] | 113 overweight and obese elderly women Mean age, 67 | BMI ≥ 25 kg/m2 appendicular FFM by residual values method including height and FM | Parallel groups (n = 2) RCT | Resistance exercise (RE) 3/week Control (C − no exercise) Duration 24 weeks | Total FFM (kg): RE: +0.6; p < 0.01 vs. BL and vs. C Appendicular FFM (kg): RE: +0.29; p < 0.01 vs. BL and vs. control Strength (Isokinetic relative peak torque 60°) (Nm/kg × 100) RE: + 20.6; p < 0.01 vs. BL and vs. C | BMI (27.1–29.1 kg/m2) Body composition measured by DEXA |
| [58] | 60 sarcopenic overweight and obese elderly (83% women) Mean age, 69 | BMI ≥ 25 kg/m2 and visceral fat area ≥ 100 cm plus skeletal MM ≤ 25.7% b.w. | Parallel groups (n = 4) RCT | Resistance/Aerobic Exercise (RT or AE) Combination (AE + RT) Control (C − no exercise) All sessions 2/week Duration 8 weeks | HGS (kg): RT: +3.5, p < 0.05 vs. all other groups, no changes in AE and RT + AE Skeletal MM (kg): RT: +0.1, AE: +0.1, RT+AE: +0.2 (in all p < 0.05 vs. C) FM (kg): RT: −1., AE: −0.7, RT + AE: −1.1 (in all p < 0.05 vs. C) Back extensor (kg): RT: +9.0, AE: +7.9, RT + AE: + 10.0 (in all p < 0.05 vs. C) | BMI (26.8–29.0kg/m2) Body composition measured by BIA Effect persisted 4 weeks after end of intervention |
| [45] | 8 sedentary women with obesity aged ≥ 60 years Mean age, 66 | body fat % > 32 Appendicular fat-free mass less than population- specific cut-off | Parallel group (n = 2) RCT of women w/wo SO Duration 16 weeks | RT (2 sessions of 40–50 min/week) | In the subgroup of women with SO: no difference in %BF, 30 s chair stand-up, timed-up-and-go vs. BL Improved strength vs. BL (p ≤ 0.01) with trivial effect sizes | Mean BMI = 28 kg/m2 Body composition measured by DEXA |
| [59] | 50 women aged ≥ 65 years with SO Mean age, 74 | BMI ≥ 25.0 kg/m2 + ASMM/weight < 25.1 % | Parallel groups (n = 2) RCT Duration 24 weeks | Combined RT and AE 5/week (Ex) Control (C − no exercise) | BF (%): Ex −2.0, p < 0.01 vs. BL; C: n.s. No effect on appendicular lean mass. HGS (kg): Ex +2.5, p < 0.001 vs. BL and vs. C; C −0.5, p < 0.05 vs. BL Maximum walking speed (m/s): Ex +0.15, p < 0.01 vs. BL and vs. C; C −0.04, p < 0.01 vs. BL | Body composition by BIA Improvement in carotid artery IMT and flow velocity |
| [60] | 35 women aged 60–80 years with SO Mean age, 67 | BF > 30% SMI <7.15 kg/m2 | Parallel groups (n = 2) RCT Duration 12 weeks (intervention) + follow- up at 9 months | Elastic band resistance training (RT) 3 times/week Control (C-no exercise) | Results are reported at 9-mo follow-up. Absolute muscle mass: RT +0.72 kg, p < 0.01 vs. C); similar results for appendicular lean mass and SMI Global physical capacity score: RT + 4.22, p < 0.001 vs. C). Clinically significant improvement in all functional tests. Physical component score (SF-36): RT +15.06, p < 0.001 vs. C) | Mean BMI = 28 kg/m2 Body composition measured by BIA |
| [61] | 35 women aged ≥ 60 years with SO Mean age, 69 | BF > 30% SMI <27.6% | Parallel groups (n = 2) RCT Duration 12 weeks | Elastic band resistance training (RT) 3 times/week Control (home exercise) | Total BF: RT −0.58 kg, p = 0.03; control: n.s. Total bone density: RT +0.06 g/cm2, p = 0.026; control: n.s. No effect on lean appendicular mass | Mean BMI = 28 kg/m2 Body composition measured by BIA (screening) and DEXA (treatment) |
| [62] | 17 SO subjects (95% women) aged ≥ 60 years Mean age, 71 | BMI > 30 kg/m2 plus EWGSOP1 criteria | Parallel groups (n = 2) RCT | High-speed power training circuit (HSC) Standard strength hypertrophy training (ST) 2-week adaptation before treatment Duration 15 weeks | HSC improved physical function (SPPB) by 20% (adjmean difference 1.1; p = 0.02, effect size g = 0.6 with no changes in ST group No change for SMI, BF %, 6MWT, HGS vs. BL in both groups. Few IADL tasks with negligible to small changes for either HSC or ST | Adherence rates > 80% Lower ratings of perceived exertion in HSC vs. ST Subjects in ST with mild to moderate acute joint pain. |
| Ref. | No. Subjects Age (years) | SO Definition | Design | Type of Intervention | Intervention Effect | Notes |
|---|---|---|---|---|---|---|
| [47] see also Table 4 | Subgroup of 76 post menopausal sedentary women with SO Mean age, 58 | BMI ≥ 25.0 (or ≥23.0 kg/m2 if Asian American) Sarcopenia defined as SMI ≤ 5.67 kg/m2 | Parallel groups (n = 4) RCT Duration 12 months | Moderately hypocaloric diet (D) Aerobic exercise (AE) Combined D +AE Control (C-no intervention) | 14% in C, 8% in D, 50% in AE, 35% in the D + AE no longer met the sarcopenia criteria by 12 months. No subgroup-specific statistical analysis was provided. | 17% with sarcopenia (mean BMI = 31 kg/m2) Body composition measured by DEXA |
| [63] | 75 women aged ≥ 60 years with SO Mean age, 77 | Obesity as > 35% BF Sarcopenia as SMI < 5.75 kg/m2 | Parallel groups (n = 3) RCT Duration 26 weeks | Whole-body electro- myostimulation (WB-EMS, 1/week) WB-EMS + protein + vitamin D supplements (WB-EMS&P) Non-training controls (C) | SMI (kg/m2): WB-EMS +0.14, WB-EMS&P + 0.11; both p < 0.001 vs. C. Gait speed: WB-EMS 0.08 m/s, p = 0.026 vs. C; WB-EMS&P n.s. No significant changes in BF or HGS in all treatment groups | Mean body fat = 37% All groups were supplemented with vitamin D Body composition by DEXA |
| [64] | 139 women aged ≥ 70 years with SO Mean age, 81 | BF ≥32% and SMI < 5.67 kg/m2 or HGS < 17.0 kg or walking speed < 1.0 m/s. | Parallel groups (n = 4) RCT Duration 12 weeks | Exercise (RT + AE − 2/week) + EAA (3 g) + catechins + vitamin D) (ExNu) Exercise only (Ex) Nutritional intervention only (N) Control (health education) | Body FM decreased significantly in all groups vs. BL; ExNU −1.0 kg (p = 0.036 vs. N) Step length: ExNu +3.2 cm; Ex +3.5 cm (p = 0.007 vs. N); both significantly increased vs. BL No significant changes in SMI and HGS among groups | Body composition measured by DEXA (screening) and BIA (treatment) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petroni, M.L.; Caletti, M.T.; Dalle Grave, R.; Bazzocchi, A.; Aparisi Gómez, M.P.; Marchesini, G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients 2019, 11, 1302. https://doi.org/10.3390/nu11061302
Petroni ML, Caletti MT, Dalle Grave R, Bazzocchi A, Aparisi Gómez MP, Marchesini G. Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients. 2019; 11(6):1302. https://doi.org/10.3390/nu11061302
Chicago/Turabian StylePetroni, Maria L., Maria T. Caletti, Riccardo Dalle Grave, Alberto Bazzocchi, Maria P. Aparisi Gómez, and Giulio Marchesini. 2019. "Prevention and Treatment of Sarcopenic Obesity in Women" Nutrients 11, no. 6: 1302. https://doi.org/10.3390/nu11061302
APA StylePetroni, M. L., Caletti, M. T., Dalle Grave, R., Bazzocchi, A., Aparisi Gómez, M. P., & Marchesini, G. (2019). Prevention and Treatment of Sarcopenic Obesity in Women. Nutrients, 11(6), 1302. https://doi.org/10.3390/nu11061302







