Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males
Abstract
1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Participants
2.3. Overview of Study Design
2.4. Experimental Visits
2.5. Measurements
2.6. Statistics
3. Results
3.1. Participant Characteristics
3.2. Plasma NO3− and NO2−
3.3. Blood Pressure, Pulse Wave Velocity, and Augmentation Index
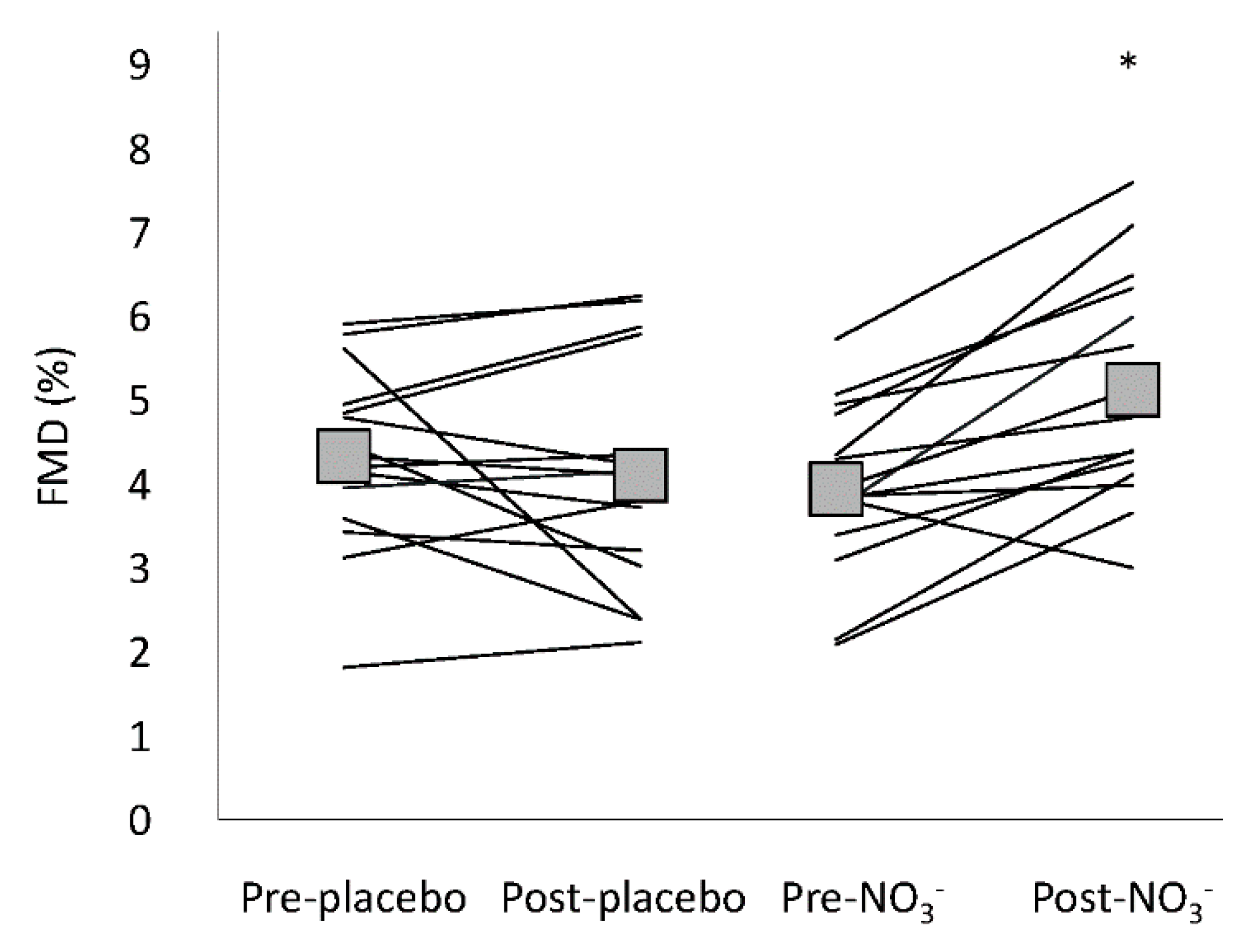
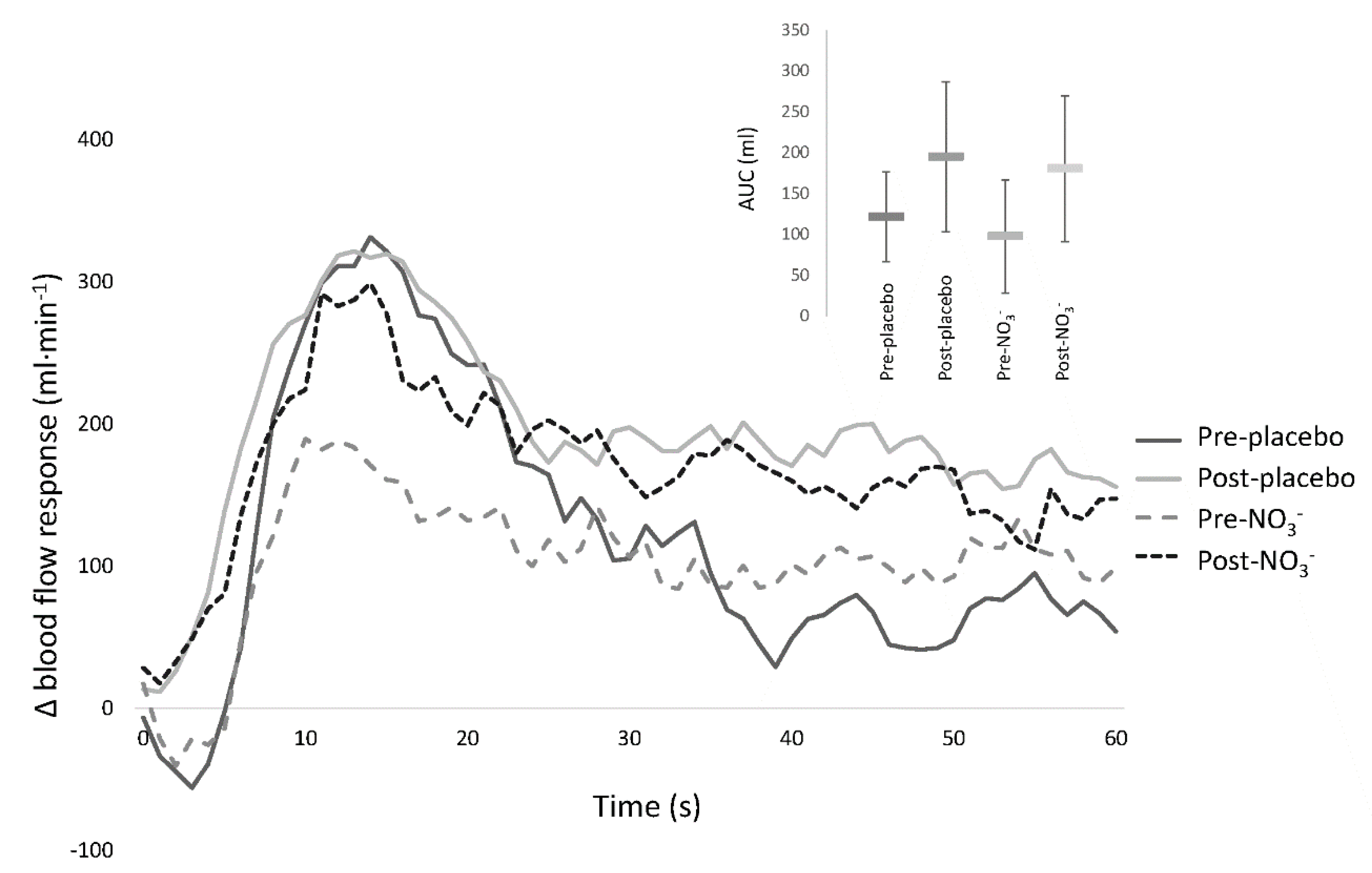
3.4. Flow Mediated Dilatation
3.5. Passive Leg Movement
4. Discussion
Limitations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Naghavi, M. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch-Eur. J. Physiol. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Seals, D.R.; DeSouza, C.A.; Donato, A.J.; Tanaka, H. Habitual exercise and arterial aging. J. Appl. Physiol. 2008, 105, 1323–1332. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chavoshan, B.; Sander, M.; Sybert, T.E.; Hansen, J.; Victor, R.G.; Thomas, G.D. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J. Physiol. 2002, 540, 377–386. [Google Scholar] [CrossRef]
- Green, D.J.; Dawson, E.A.; Groenewoud, H.M.M.; Jones, H.; Thijssen, D.H.J. Is Flow-Mediated Dilation Nitric Oxide Mediated? A Meta-Analysis. Hypertension 2014, 63, 376–382. [Google Scholar] [CrossRef]
- Kooijman, M.; Thijssen, D.H.J.; de Groot, P.C.E.; Bleeker, M.W.P.; van Kuppevelt, H.J.M.; Green, D.J.; Rongen, G.A.; Smits, P.; Hopman, M.T.E. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J. Physiol.-Lond. 2008, 586, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Uberoi, A.; Wray, D.W.; Nishiyama, S.; Lawrenson, L.; Richardson, R.S. Differential effects of aging on limb blood flow in humans. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H272–H278. [Google Scholar] [CrossRef]
- Wray, D.W.; Nishiyama, S.K.; Donato, A.J.; Richardson, R.S. Human Vascular Aging: Limb-Specific Lessons. Exerc. Sport Sci. Rev. 2010, 38, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Hoier, B.; Walker, P.J.; Schulze, K.; Bangsbo, J.; Hellsten, Y.; Askew, C.D. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 2015, 246, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Coats, A.J.S. The ‘skeletal muscle hypothesis in heart failure’ revised. Eur. Heart J. 2013, 34, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.; Smoliga, J.M.; Tarzia, B.; Stabler, T.; Van Bruggen, M.; Allen, J.D. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide-Biol. Chem. 2016, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, M.C.; Liddle, L.; Monaghan, C.; Muggeridge, D.J.; Sculthorpe, N.; Butcher, J.P.; Henriquez, F.L.; Allen, J.D.; Easton, C. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018, 120, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Blackwell, J.R.; L'Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO-synthase independent NO generation in mammals. Biochem. Biophys. Res. Commun. 2010, 396, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kim-Shapiro, D.B.; Gladwin, M.T. Mechanisms of nitrite bioactivation. Nitric Oxide-Biol. Chem. 2014, 38, 58–68. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.P.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Casey, D.P.; Treichler, D.P.; Ganger, C.T.; Schneider, A.C.; Ueda, K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J. Appl. Physiol. 2015, 118, 178–186. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Kelly, J.; Winyard, P.G.; Fulford, J.; Jones, A.M. Dietary Nitrate Reduces Blood Pressure and Improves Walking Economy and Cognitive Function In Older People. Med. Sci. Sports Exerc. 2016, 48, 257. [Google Scholar] [CrossRef]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; VanBruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.N.; VanBruggen, M.D.; Pieper, C.F.; Sloane, R.; Kraus, W.E.; Gow, A.J.; Allen, J.D. Beet the Best? Dietary Inorganic Nitrate to Augment Exercise Training in Lower Extremity Peripheral Artery Disease with Intermittent Claudication. Circ. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cosby, K.; Partovi, K.S.; Crawford, J.H.; Patel, R.P.; Reiter, C.D.; Martyr, S.; Yang, B.K.; Waclawiw, M.A.; Zalos, G.; Xu, X.L.; et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003, 9, 1498–1505. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary Inorganic Nitrate Improves Mitochondrial Efficiency in Humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients A Randomized, Phase 2, Double-Blind, Placebo-Controlled Study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Rammos, C.; Hendgen-Cotta, U.B.; Sobierajski, J.; Bernard, A.; Kelm, M.; Rassaf, T. Dietary Nitrate Reverses Vascular Dysfunction in Older Adults with Moderately Increased Cardiovascular Risk. J. Am. Coll. Cardiol. 2014, 63, 1584–1585. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults—The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.B.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Aging affects vascular structure and function in a limb-specific manner. J. Appl. Physiol. 2008, 105, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Rowley, N.; Padilla, J.; Simmons, G.H.; Laughlin, M.H.; Whyte, G.; Cable, N.T.; Green, D.J. Relationship between upper and lower limb conduit artery vasodilator function in humans. J. Appl. Physiol. 2011, 111, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.J.M.; Miralles, J.D.; Aguilar, E.M.; Gonzalez, A.F.; Garcia, J.R.M.; Garcia, F.A. Relationship Between Noninvasively Measured Endothelial Function and Peripheral Arterial Disease. Angiology 2009, 60, 725–731. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Di Giacomo, S.; Bucur, R.; Di Donato, A.; Schiano, V.; Scopacasa, F. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J. Vasc. Surg. 2003, 38, 374–379. [Google Scholar] [CrossRef]
- Hoier, B.; Rufener, N.; Bojsen-Moller, J.; Bangsbo, J.; Hellsten, Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J. Physiol. 2010, 588, 3833–3845. [Google Scholar] [CrossRef]
- Mortensen, S.P.; Askew, C.D.; Walker, M.; Nyberg, M.; Hellsten, Y. The hyperaemic response to passive leg movement is dependent on nitric oxide; a new tool to evaluate endothelial nitric oxide function. J. Physiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Trinity, J.D.; Groot, H.J.; Layec, G.; Rossman, M.J.; Ives, S.J.; Runnels, S.; Gmelch, B.; Bledsoe, A.; Richardson, R.S. Nitric oxide and passive limb movement: A new approach to assess vascular function. J. Physiol.-Lond. 2012, 590, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Trinity, J.D.; Groot, H.J.; Layec, G.; Rossman, M.J.; Ives, S.J.; Morgan, D.E.; Gmelch, B.S.; Bledsoe, A.D.; Richardson, R.S. Passive leg movement and nitric oxide-mediated vascular function: The impact of age. Am. J. Physiol. Heart Circ. Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.U.; Miedlar, J.A.; Parker, B.A.; Proctor, D.N. Relation of Femoral Diameter, Shear Rate, and Dilatory Response to Knee Extensor Exercise. Med. Sci. Sports Exerc. 2010, 42, 1870–1875. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Eggebeen, J.; Kim-Shapiro, D.B.; Haykowsky, M.; Morgan, T.M.; Basu, S.; Brubaker, P.; Rejeski, J.; Kitzman, D.W. One Week of Daily Dosing with Beetroot Juice Improves Submaximal Endurance and Blood Pressure in Older Patients with Heart Failure and Preserved Ejection Fraction. JACC-Heart Fail. 2016, 4, 428–437. [Google Scholar] [CrossRef]
- Butlin, M.; Qasem, A.; Avolio, A.P. Estimation of central aortic pressure waveform features derived from the brachial cuff volume displacement waveform. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; IEEE: New York, NY, USA, 2012; pp. 2591–2594. [Google Scholar]
- Wilkinson, I.B.; Fuchs, S.A.; Jansen, I.M.; Spratt, J.C.; Murray, G.D.; Cockcroft, J.R.; Webb, D.J. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J. Hypertens. 1998, 16, 2079–2084. [Google Scholar] [CrossRef]
- Hwang, M.H.; Yoo, J.K.; Kim, H.K.; Hwang, C.L.; Mackay, K.; Hemstreet, O.; Nichols, W.W.; Christou, D.D. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J. Hum. Hypertens. 2014, 28, 475–481. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Black, M.A.; Cable, N.T.; Thijssen, D.H.J.; Green, D.J. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 2008, 51, 203–210. [Google Scholar] [CrossRef]
- Woodman, R.J.; Playford, D.A.; Watts, G.F.; Cheetham, C.; Reed, C.; Taylor, R.R.; Puddey, I.B.; Beilin, L.J.; Burke, V.; Mori, T.A.; et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J. Appl. Physiol. 2001, 91, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.R.; Richardson, R.S. CORP: Ultrasound Assessment of Vascular Function with the Passive Leg Movement Technique. J. Appl. Physiol. (Bethesda, Md. 1985) 2017. [Google Scholar] [CrossRef] [PubMed]
- Pinder, A.G.; Rogers, S.C.; Khalatbari, A.; Ingram, T.E.; James, P.E. The Measurement of Nitric Oxide and Its Metabolites in Biological Samples by Ozone-Based Chemiluminescence. In Redox-Mediated Signal Transduction: Methods and Protocols; Hancock, J.T., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 10–27. [Google Scholar] [CrossRef]
- Sergeant, E. Epitools Epidemiological Calculators. Available online: http://epitools.ausvet.com.au (accessed on 7 June 2016).
- Atkinson, G. The dependence of FMD% on baseline diameter: A problem solved by allometric scaling. Clin. Sci. 2013, 125, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Dawson, E.A.; Black, M.A.; Hopman, M.T.E.; Cable, N.T.; Green, D.J. Heterogeneity in conduit artery function in humans: Impact of arterial size. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H1927–H1934. [Google Scholar] [CrossRef] [PubMed]
- Pyke, K.E.; Tschakovsky, M.E. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J. Physiol.-Lond. 2005, 568, 357–369. [Google Scholar] [CrossRef]
- Gibbs, B.B.; Dobrosielski, D.A.; Lima, M.; Bonekamp, S.; Stewart, K.J.; Clark, J.M. The association of arterial shear and flow-mediated dilation in diabetes. Vasc. Med. 2011, 16, 267–274. [Google Scholar] [CrossRef]
- Jackson, J.K.; Patterson, A.J.; MacDonald-Wicks, L.K.; Oldmeadow, C.; McEvoy, M.A. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta-analysis of human evidence. Nutr. Rev. 2018, 76, 348–371. [Google Scholar] [CrossRef]
- Schwarz, K.; Singh, S.; Parasuraman, S.K.; Rudd, A.; Shepstone, L.; Feelisch, M.; Minnion, M.; Ahmad, S.; Madhani, M.; Horowitz, J.; et al. Inorganic Nitrate in Angina Study: A Randomized Double-Blind Placebo-Controlled Trial. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- James, P.E.; Willis, G.R.; Allen, J.D.; Winyard, P.G.; Jones, A.M. Nitrate pharmacokinetics: Taking note of the difference. Nitric Oxide-Biol. Chem. 2015, 48, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Woessner, M.N.; McIlvenna, L.C.; de Zevallos, J.O.; Neil, C.J.; Allen, J.D. Dietary nitrate supplementation in cardiovascular health: An ergogenic aid or exercise therapeutic? Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H195–H212. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.D.; Marsh, A.P.; Dove, R.W.; Beavers, D.; Presley, T.; Helms, C.; Bechtold, E.; King, S.B.; Kim-Shapiro, D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutr. Res. 2012, 32, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Cable, N.T.; Green, D.J. Impact of exercise training on arterial wall thickness in humans. Clin. Sci. 2012, 122, 311–322. [Google Scholar] [CrossRef]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.V.; Morgado, M.; Pierucci, A.P.; Alvares, T.S. A single dose of a beetroot-based nutritional gel improves endothelial function in the elderly with cardiovascular risk factors. J. Funct. Food. 2016, 26, 301–308. [Google Scholar] [CrossRef]
- Virdis, A.; Ghiadoni, L.; Taddei, S. Effects of Antihypertensive Treatment on Endothelial Function. Curr. Hypertens. Rep. 2011, 13, 276–281. [Google Scholar] [CrossRef]
- Bock, J.M.; Treichler, D.P.; Norton, S.L.; Ueda, K.; Hughes, W.E.; Casey, D.P. Inorganic nitrate supplementation enhances functional capacity and lower-limb microvascular reactivity in patients with peripheral artery disease. Nitric Oxide 2018. [Google Scholar] [CrossRef]
- Carlstrom, M.; Montenegro, M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Intern. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Asgary, S.; Afshani, M.R.; Sahebkar, A.; Keshvari, M.; Taheri, M.; Jahanian, E.; Rafieian-Kopaei, M.; Malekian, F.; Sarrafzadegan, N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: A randomized crossover pilot study. J. Hum. Hypertens. 2016, 30, 627–632. [Google Scholar] [CrossRef]
- Carlstrom, M.; Persson, A.E.G.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011, 89, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Pei, R.S.; Guo, Y.; Ballar, K.D.; Barker, T.; Rogers, V.E.; Parker, B.A.; Taylor, A.W.; Traber, M.G.; Volek, J.S.; et al. gamma-Tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013, 65, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Effects of Supplementation with the Fat-Soluble Vitamins E and D on Fasting Flow-Mediated Vasodilation in Adults: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2015, 7, 1728–1743. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, R.E.; Gano, L.B.; Seals, D.R. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J. Appl. Physiol. 2014, 116, 156–163. [Google Scholar] [CrossRef]
- Groot, H.J.; Trinity, J.D.; Layec, G.; Rossman, M.J.; Ives, S.J.; Morgan, D.E.; Bledsoe, A.; Richardson, R.S. The role of nitric oxide in passive leg movement-induced vasodilatation with age: Insight from alterations in femoral perfusion pressure. J. Physiol.-Lond. 2015, 593, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.R.; Milsom, A.B.; Gunaruwan, P.; Abozguia, K.; Ahmed, I.; Weaver, R.A.; Thomas, P.; Ashrafian, H.; Born, G.V.R.; James, P.E.; et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 2008, 117, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Vanhatalo, A.; Bailey, S.J.; Wylie, L.J.; Tucker, C.; List, S.; Winyard, P.G.; Jones, A.M. Dietary nitrate supplementation: Effects on plasma nitrite and pulmonary O-2 uptake dynamics during exercise in hypoxia and normoxia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R920–R930. [Google Scholar] [CrossRef]
- Hellsten, Y.; Rufener, N.; Nielsen, J.J.; Hoier, B.; Krustrup, P.; Bangsbo, J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R975–R982. [Google Scholar] [CrossRef]
- Ter Woerds, W.; De Groot, P.C.E.; van Kuppevelt, D.; Hopman, M.T.E. Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Phys. Ther. 2006, 86, 636–645. [Google Scholar]
- Mortensen, S.P.; Gonzalez-Alonso, J.; Damsgaard, R.; Saltin, B.; Hellsten, Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007, 581, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, L.; Schattenkerk, D.W.E.; Venema, P.; Best, H.J.; van den Bogaard, B.; Stok, W.J.; Westerhof, B.E.; van den Born, B.J.H. Myocardial preload alters central pressure augmentation through changes in the forward wave. J. Hypertens. 2018, 36, 544–551. [Google Scholar] [CrossRef]
- Franklin, S.S.; Khan, S.A.; Wong, N.D.; Larson, M.G.; Levy, D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation 1999, 100, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Munir, S.; Guilcher, A.; Kamalesh, T.; Clapp, B.; Redwood, S.; Marber, M.; Chowienczyk, P. Peripheral Augmentation Index Defines the Relationship Between Central and Peripheral Pulse Pressure. Hypertension 2008, 51, 112–118. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Adji, A.; Safar, M.E. Structure and Function of Systemic Arteries: Reflections on the Arterial Pulse. Am. J. Hypertens. 2018, 31, 934–940. [Google Scholar] [CrossRef]
- McEniery, C.M.; Wallace, S.; Mackenzie, I.S.; McDonnell, B.; Yasmin; Newby, D.E.; Cockcroft, J.R.; Wilkinson, I.B. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006, 48, 602–608. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.B.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-term effects of nitrate-rich green leafy vegetables on blood pressure and arterial stiffness in individuals with high-normal blood pressure. Free Radic. Biol. Med. 2014, 77, 353–362. [Google Scholar] [CrossRef]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef]
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide-Biol. Chem. 2013, 35, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Ali, A. Performance and Health Benefits of Dietary Nitrate Supplementation in Older Adults: A Systematic Review. Nutrients 2017, 9, 1171. [Google Scholar] [CrossRef]
- Virdis, A.; Bruno, R.M.; Neves, M.F.; Bernini, G.; Taddei, S.; Ghiadoni, L. Hypertension in the Elderly: An Evidence-based Review. Curr. Pharm. Des. 2011, 17, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.A.; Webb, A.J.; Lundberg, J.O.; Weitzberg, E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Intern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic Nitrate Supplementation Lowers Blood Pressure in Humans Role for Nitrite-Derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide-Biol. Chem. 2014, 38, 45–57. [Google Scholar] [CrossRef]
- Velmurugan, S.; Kapil, V.; Ghosh, S.M.; Davies, S.; McKnight, A.; Aboud, Z.; Khambata, R.S.; Webb, A.J.; Poole, A.; Ahluwalia, A. Antiplatelet effects of dietary nitrate in healthy volunteers: Involvement of cGMP and influence of sex. Free Radic. Biol. Med. 2013, 65, 1521–1532. [Google Scholar] [CrossRef]
- Bailey, J.C.; Feelisch, M.; Horowitz, J.D.; Frenneaux, M.P.; Madhani, M. Pharmacology and therapeutic role of inorganic nitrite and nitrate in vasodilatation. Pharmacol. Ther. 2014, 144, 303–320. [Google Scholar] [CrossRef]
- Lerner, D.J.; Kannel, W.B. Patterns of coronary heart disease morbidity and mortality in the sexes—A 26-year follow-up of the Framingham population. Am. Heart J. 1986, 111, 383–390. [Google Scholar] [CrossRef]
- Forte, P.; Kneale, B.J.; Milne, E.; Chowienczyk, P.J.; Johnston, A.; Benjamin, N.; Ritter, J.M. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension 1998, 32, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O.; et al. Nitrite infusion in humans and nonhuman primates—Endocrine effects, pharmacokinetics, and tolerance formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
| Participants (n) | 15 |
|---|---|
| Age (years) | 69 ± 4 |
| Weight (kg) | 83 ± 10 |
| Height (cm) | 177 ± 8 |
| BMI (kg·m2) | 26.6 ± 3.5 |
| ABI | 1.25 ± 0.13 |
| Systolic BP (mmHg) | 131 ± 13 |
| Diastolic BP (mmHg) | 75 ± 8 |
| Diuretics (%) | 0 |
| ARB/ACE inhibitors (%) | 0 |
| β-blockers (%) | 0 |
| Ca2+ channel blockers (%) | 0 |
| Statins (%) | 27 |
| Measure | Pre-Placebo | Post-Placebo | Pre-NO3− | Post-NO3− | Time × Condition (p-Value) |
|---|---|---|---|---|---|
| Plasma NO3− (μM) | 41 (31–51) | 43 (35–52) | 42 (34–50) | 617 * (572–663) | <0.001 |
| Plasma NO2− (nM) | 108 (42–174) | 87 (38–135) | 101 (66–136) | 832 * (526–1139) | <0.001 |
| Measure | Pre-Placebo | Post-Placebo | Pre-NO3− | Post-NO3− | Time × Condition (p-Value) |
|---|---|---|---|---|---|
| Heart rate (b·min−1) | 62 (56–68) | 60 (54–67) | 60 (54–65) | 59 (54–63) | 0.545 |
| Systolic blood pressure (mmHg) | 128 (122–134 | 130 (124–135) | 126 (120–131) | 127 (121–133) | 0.992 |
| Diastolic blood pressure (mmHg) | 74 (69–78) | 75 (70–79) | 74 (70–77) | 72 (67–76) | 0.067 |
| Central systolic pressure (mmHg) | 116 (111–122) | 117 (113–122) | 114 (110–119) | 114 (109–119) | 0.609 |
| Central pulse pressure (mmHg) | 41 (37–46) | 42 (37–47) | 40 (34–45) | 41 (36–46) | 0.712 |
| Augmentation index (AIx75) | 19.4 (16.0–22.8) | 14.7 (11.0–18.4) | 17.3 (13.7–20.9) | 8.5 * (4.2–12.8) | 0.027 |
| PWV (m·s−1) | 11.7 (10.4–13.0) | 11.7 (10.8–12.5) | 12.2 (10.3–14.2) | 11.5 (10.4–12.7) | 0.143 |
| Measure | Pre-Placebo | Post-Placebo | Pre-NO3− | Post-NO3− | Time × Condition (p-Value) |
|---|---|---|---|---|---|
| Baseline SFA diameter (mm) | 6.93 (6.61–7.24) | 7.02 (6.57–7.46) | 6.95 (6.66–7.24) | 7.09 (6.82–7.36) | 0.783 |
| RH peak flow (mL·min−1) | 1093 (968–1218) | 1140 (1005–1275) | 1160 (1040–1279) | 1142 (972–1314) | 0.361 |
| RH AUC (mL) | 367 (306–428) | 417 (363–470) | 395 (352–437) | 409 (342–477) | 0.396 |
| Time to peak dilation (s) | 144 (121–168) | 130 (109–150) | 124 (108–150) | 140 (126–155) | 0.086 |
| Response shear (AUC) | 9997 (8014–11980) | 10904 (8870–12938) | 9038 (7787–10288) | 9199 (7545–10854) | 0.406 |
| Absolute FMD (mm) | 0.30 (0.26–0.35) | 0.29 (0.24–0.32) | 0.28 (0.24–0.32) | 0.37 * (0.31–0.42) | 0.002 |
| FMD·SRAUC−1 (103·s−1) | 5.31 (3.47–7.16) | 4.24 (2.60–5.90) | 4.99 (3.22–6.76) | 6.40 * (4.39–8.41) | <0.001 |
| Allometric scaled FMD (%) | 4.07 (3.38–4.76) | 3.91 (3.22–4.61) | 3.76 (3.07–4.46) | 4.75 * (4.06–5.45) | 0.010 # |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, M.A.; Bailey, T.G.; McIlvenna, L.; Allen, J.D.; Green, D.J.; Askew, C.D. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients 2019, 11, 954. https://doi.org/10.3390/nu11050954
Walker MA, Bailey TG, McIlvenna L, Allen JD, Green DJ, Askew CD. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients. 2019; 11(5):954. https://doi.org/10.3390/nu11050954
Chicago/Turabian StyleWalker, Meegan A., Tom G. Bailey, Luke McIlvenna, Jason D. Allen, Daniel J. Green, and Christopher D. Askew. 2019. "Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males" Nutrients 11, no. 5: 954. https://doi.org/10.3390/nu11050954
APA StyleWalker, M. A., Bailey, T. G., McIlvenna, L., Allen, J. D., Green, D. J., & Askew, C. D. (2019). Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients, 11(5), 954. https://doi.org/10.3390/nu11050954





