Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract (AM-Ex)
2.2. Animals and Treatment
2.3. Biochemical Analyses of Blood Samples
2.4. Histological Analysis
2.5. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
2.6. Statistical Analyses
3. Results
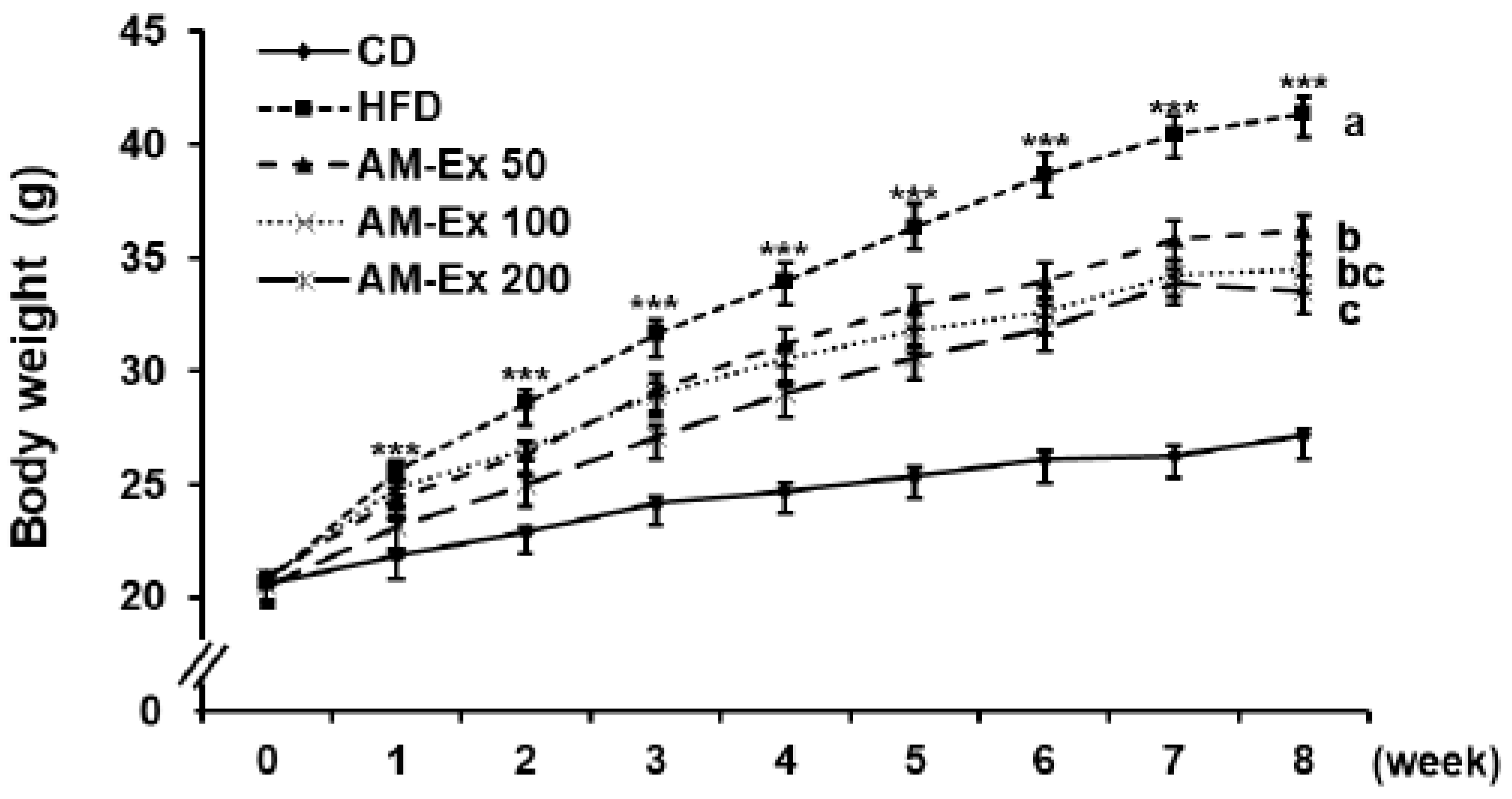
3.1. AM-Ex Reduces Body Weight Gain and Body Fat Mass in HFD-Induced Obese C57BL/6 Mice
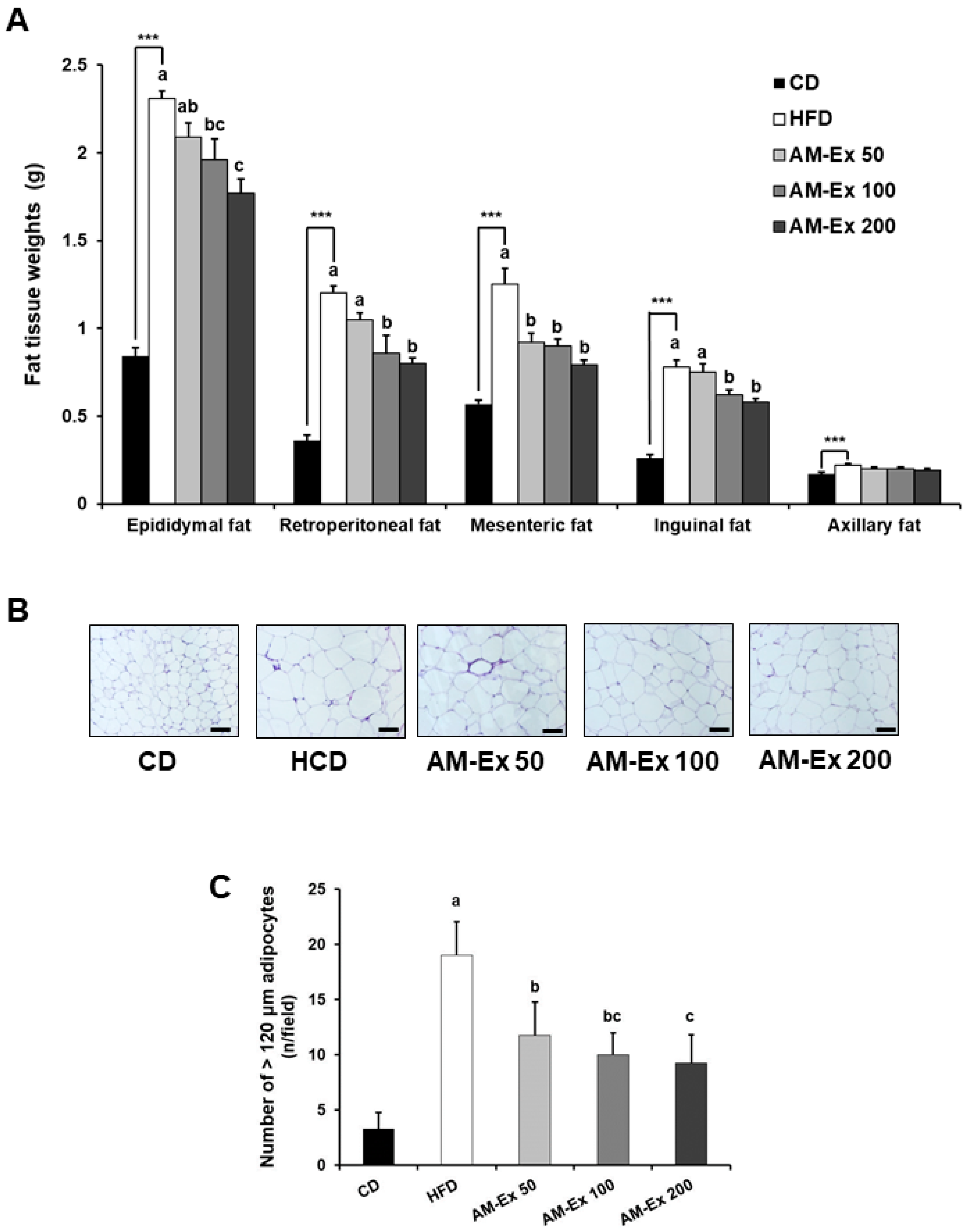
3.2. AM-Ex Suppresses White Adipose Tissue Accumulation in HFD-Induced Obese C57BL/6 Mice
3.3. AM-Ex Improves Insulin Resistance and Serum Lipid Profiles in HFD-Induced Obese C57BL/6 Mice
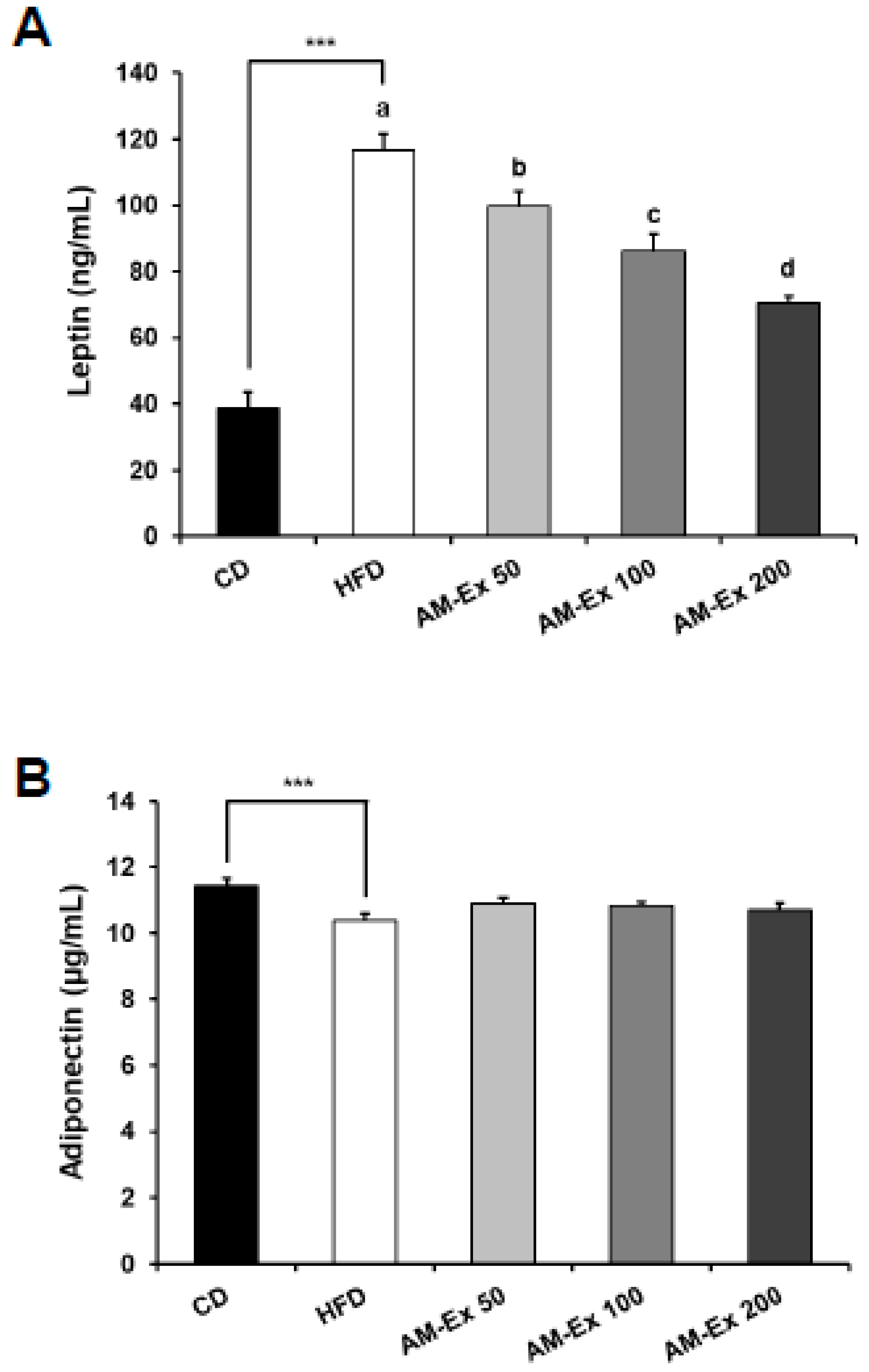
3.4. AM-Ex Reduces Leptin Levels in HFD-Induced Obese C57BL/6 Mice
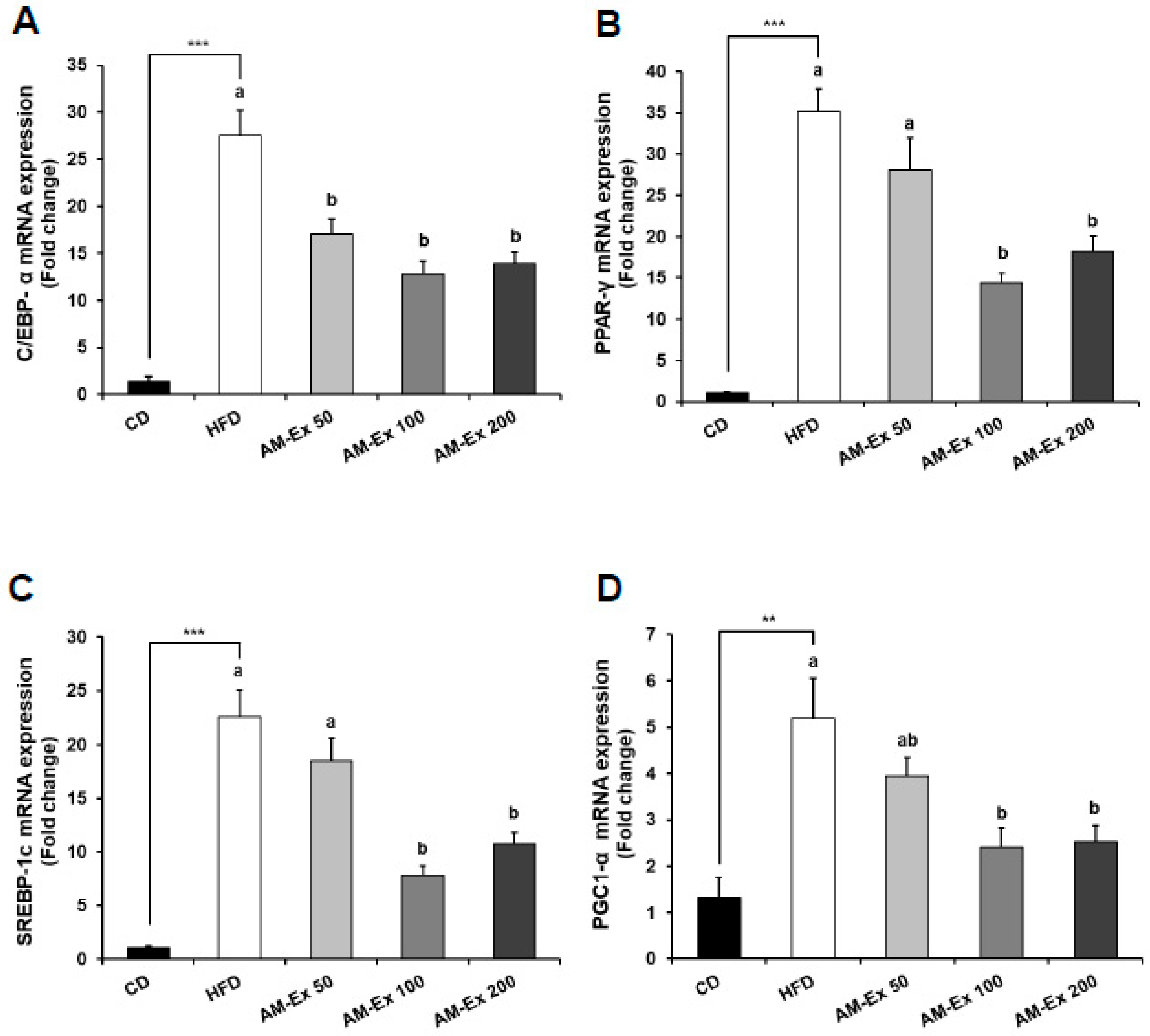
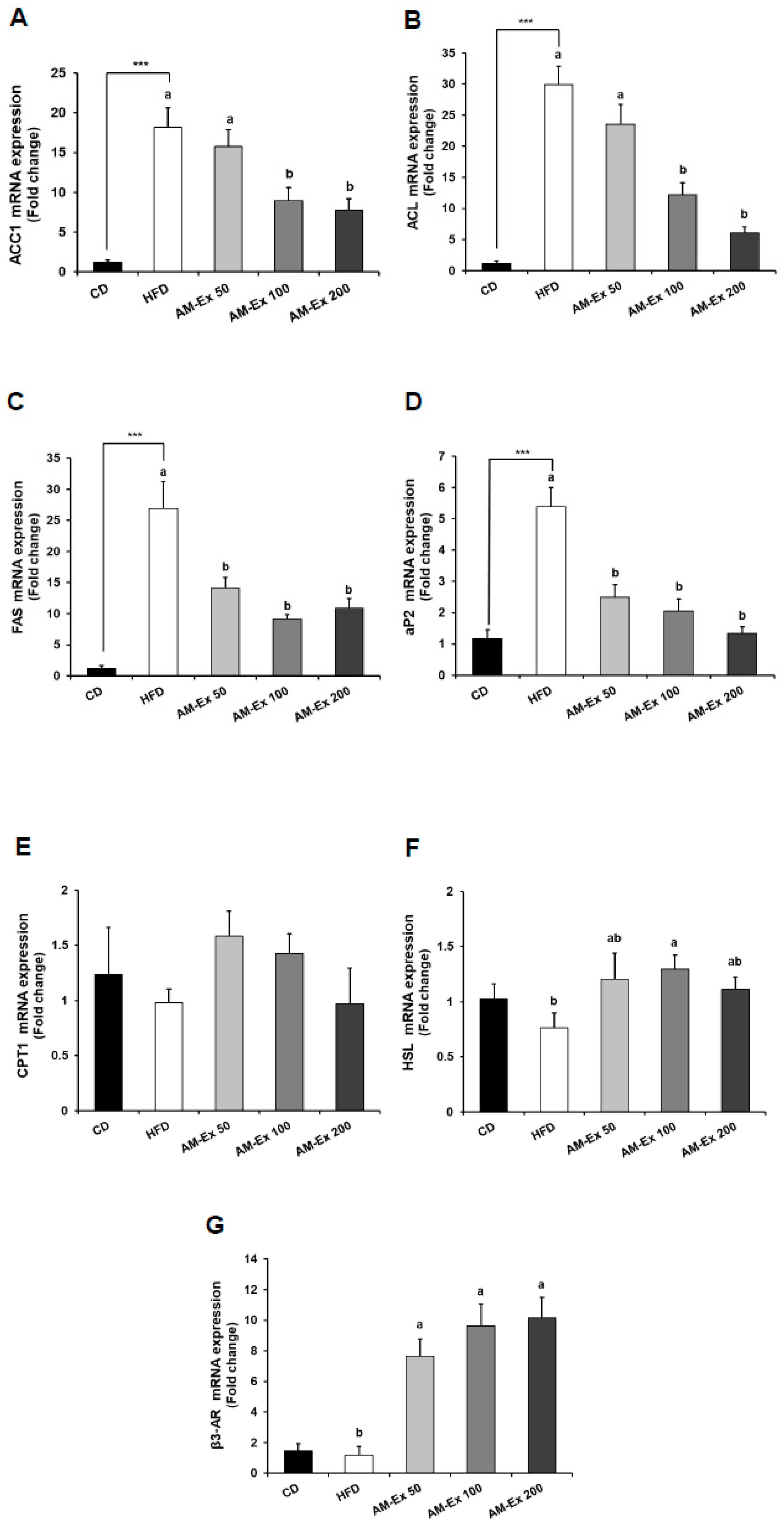
3.5. AM-Ex Modulates the Expression of Adipogenic Transcription Factors and their Target Genes in the Epididymal Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. World Obesity Federation Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.; Milani, R.V. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-Y.; Kim, H.-L.; Park, J.; An, H.-J.; Kim, S.-H.; Kim, S.-J.; So, H.-S.; Park, R.; Um, J.-Y.; Hong, S.-H. Rubi Fructus (Rubus coreanus) inhibits differentiation to adipocytes in 3T3-L1 cells. Evid. Based Complement. Altern. Med. 2013, 2013, 475386. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.; Wang, J.; Flint, C.; Ribnicky, D.; Kuhn, P.; De Mejia, E.G.; Raskin, I.; Lila, M.A. Alaskan wild berry resources and human health under the cloud of climate change. J. Agric. Food Chem. 2010, 58, 3884–3900. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015, 185, 383–388. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Suzuki, R.; Tanaka, M.; Takanashi, M.; Hussain, A.; Yuan, B.; Toyoda, H.; Kuroda, M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr. Metab. 2011, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Baretić, M. Obesity drug therapy. Minerva Endocrinol. 2013, 38, 245–254. [Google Scholar] [PubMed]
- Halford, J.C.G. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs 2006, 7, 312–318. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Borissova, P.; Valcheva, S.; Belcheva, A. Antiinflammatory effect of flavonoids in the natural juice from Aronia melanocarpa, rutin and rutin-magnesium complex on an experimental model of inflammation induced by histamine and serotonin. Acta Physiol Pharm. Bulg 1994, 20, 25–30. [Google Scholar]
- Matsumoto, M.; Hara, H.; Chiji, H.; Kasai, T. Gastroprotective effect of red pigments in black chokeberry fruit (Aronia melanocarpa Elliot) on acute gastric hemorrhagic lesions in rats. J. Agric. Food Chem. 2004, 52, 2226–2229. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef]
- Jeon, Y.-D.; Kang, S.-H.; Moon, K.-H.; Lee, J.-H.; Kim, D.-G.; Kim, W.; Kim, J.-S.; Ahn, B.-Y.; Jin, J.-S. The Effect of Aronia Berry on Type 1 Diabetes In Vivo and In Vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given Aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Kim, J.-H.; Lee, E.B.; Hur, W.; Kwon, O.-J.; Park, H.-J.; Yoon, S.K. Aronia melanocarpa Extract Ameliorates Hepatic Lipid Metabolism through PPARγ2 Downregulation. PLoS ONE 2017, 12, e0169685. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant. Foods Hum. Nutr. 2007, 62, 19–24. [Google Scholar] [CrossRef]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Cebova, M.; Klimentova, J.; Janega, P.; Pechanova, O. Effect of Bioactive Compound of Aronia melanocarpa on Cardiovascular System in Experimental Hypertension. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Lim, S.-M.; Jung, J.I.; Kim, N.Y.; Bae, J.-S.; Lee, H.S.; Kim, E.J. Cyanidine-3-O-Galactoside Enriched Aronia melanocarpa Extract Inhibits Adipogenesis and Lipogenesis via Down-Regulation of Adipogenic Transcription Factors and Their Target Genes in 3T3-L1 Cells. Food Nutr. Sci. 2019, 10, 128–147. [Google Scholar] [CrossRef][Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric. Conspec. Sci. 2007, 72, 301–306. [Google Scholar]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Oszmianski, J.; Sapis, J.C. Anthocyanins in Fruits of Aronia Melanocarpa (Chokeberry). J. Food Sci. 1988, 53, 1241–1242. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich Phytochemicals from Aronia Fruits Inhibit Visceral Fat Accumulation and Hyperglycemia in High-fat Diet-induced Dietary Obese Rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef]
- Kardum, N.; Petrović-Oggiano, G.; Takic, M.; Glibetić, N.; Zec, M.; Debeljak-Martacic, J.; Konić-Ristić, A. Effects of glucomannan-enriched, Aronia juice-based supplement on cellular antioxidant enzymes and membrane lipid status in subjects with abdominal obesity. Sci. World J. 2014, 2014, 869250. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Kong, W.-H.; Oh, S.-H.; Ahn, Y.-R.; Kim, K.-W.; Kim, J.-H.; Seo, S.-W. Antiobesity effects and improvement of insulin sensitivity by 1-deoxynojirimycin in animal models. J. Agric. Food Chem. 2008, 56, 2613–2619. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Carr, D.B.; Barsness, S.M.; Kahn, S.E.; Schwartz, R.S. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J. Clin. Endocrinol. Metab. 2004, 89, 2704–2710. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef]
- Skoczyñska, A.; Jêdrychowska, I.; Affelska, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 6, 177–182. [Google Scholar]
- Kim, J.B.; Sarraf, P.; Wright, M.; Yao, K.M.; Mueller, E.; Solanes, G.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ortuño Sahagún, D.; Márquez-Aguirre, A.L.; Quintero-Fabián, S.; López-Roa, R.I.; Rojas-Mayorquín, A.E. Modulation of PPAR-γ by Nutraceutics as Complementary Treatment for Obesity-Related Disorders and Inflammatory Diseases. Ppar Res. 2012, 2012, 318613. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: The diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Tuncman, G.; Görgün, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef]
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005, 1, 107–119. [Google Scholar] [CrossRef]
- Makowski, L.; Boord, J.B.; Maeda, K.; Babaev, V.R.; Uysal, K.T.; Morgan, M.A.; Parker, R.A.; Suttles, J.; Fazio, S.; Hotamisligil, G.S.; et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001, 7, 699–705. [Google Scholar] [CrossRef]
- Kim, T.S.; Leahy, P.; Freake, H.C. Promoter usage determines tissue specific responsiveness of the rat acetyl-CoA carboxylase gene. Biochem. Biophys. Res. Commun. 1996, 225, 647–653. [Google Scholar] [CrossRef]
- Guay, C.; Madiraju, S.R.M.; Aumais, A.; Joly, E.; Prentki, M. A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J. Biol. Chem. 2007, 282, 35657–35665. [Google Scholar] [CrossRef]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef]
- Guo, H.; Liu, G.; Zhong, R.; Wang, Y.; Wang, D.; Xia, M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis 2012, 11, 10. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Kawano, J.; Arora, R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J. Cardiometab. Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef]
- You, M.; Rogers, C.Q. Adiponectin: A key adipokine in alcoholic fatty liver. Exp. Biol. Med. 2009, 234, 850–859. [Google Scholar] [CrossRef]
- Bhaswant, M.; Fanning, K.; Netzel, M.; Mathai, M.L.; Panchal, S.K.; Brown, L. Cyanidin 3-glucoside improves diet-induced metabolic syndrome in rats. Pharmacol. Res. 2015, 102, 208–217. [Google Scholar] [CrossRef]
- Havel, P.J. Role of adipose tissue in body-weight regulation: Mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000, 59, 359–371. [Google Scholar] [CrossRef]
- Qin, B.; Polansky, M.M.; Anderson, R.A. Cinnamon extract regulates plasma levels of adipose-derived factors and expression of multiple genes related to carbohydrate metabolism and lipogenesis in adipose tissue of fructose-fed rats. Horm. Metab. Res. 2010, 42, 187–193. [Google Scholar] [CrossRef]
| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| ACC1 | GGAGATGTACGCTGACCGAGAA | ACCCGACGCATGGTTTTCA |
| ACL | TGGATGCCACAGCTGACTAC | GGTTCAGCAAGGTCAGCTTC |
| aP2 | GGATTTGGTCACCATCCGGT | TTCACCTTCCTGTCGTCTGC |
| β3-AR | CCTAGCTGTCACCAACCCTTT | GACGAAGAGCATCACAAGGAG |
| C/EBPα | TGGACAAGAACAGCAACGAGTAC | TGGACAAGAACAGCAACGAGTAC |
| CPT1 | GTGCTGGAGGTGGCTTTGGT | TGCTTGACGGATGTGGTTCC |
| FAS | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| HSL | CCGTTCCTGCAGACTCTCTC | CCACGCAACTCTGGGTCTAT |
| PGC-1α | CCCTGCCATTGTTAAGACC | TGCTGCTGTTCCTGTTTTC |
| PPARγ | CAAAACACCAGTGTGAATTA | ACCATGGTAATTTCTTGTGA |
| SREBP-1c | CACTTCTGGAGACATCGCAAAC | ATGGTAGACAACAGCCGCATC |
| GAPDH | AGGTTGTCTCCTGCGACT | TGCTGTAGCCGTATTCATTGTCA |
| Groups | CD | HFD | AM-Ex 50 | AM-Ex 100 | AM-Ex 200 | |
|---|---|---|---|---|---|---|
| Variables | ||||||
| Initial body weight (g) | 20.5 ± 0.2 | 20.6 ± 0.4 | 20.9 ± 0.2 | 20.9 ± 0.1 | 20.5 ± 0.3 | |
| Final body weight (g) | 27.1 ± 0.3 | 41.3 ± 0.8 ***,a | 36.2 ± 0.7 b | 34.4 ± 0.7 bc | 33.5 ± 0.6 c | |
| Body weight gain (g) | 6.6 ± 0.4 | 20.7 ± 0.9 ***,a | 15.3 ± 0.7 b | 13.6 ± 0.7 bc | 13.0 ± 0.6 c | |
| Fat mass percentage (%) | 27.8 ± 0.6 | 44.2 ± 0.6 ***,a | 41.5 ± 0.6 b | 39.1 ± 0.8 c | 36.9 ± 0.6 d | |
| Food intake (g/day) | 2.32 ± 0.01 | 2.30 ± 0.02 a | 2.06 ± 0.03 b | 2.00 ± 0.02 bc | 1.96 ± 0.02 c | |
| Food efficiency ratio 1) | 0.051 ± 0.004 | 0.161 ± 0.007 ***,a | 0.133 ± 0.005 b | 0.121 ± 0.005 b | 0.118 ± 0.005 b | |
| Groups | CD | HFD | AM-Ex 50 | AM-Ex 100 | AM-Ex 200 | |
|---|---|---|---|---|---|---|
| Variables | ||||||
| Glucose (mg/dL) | 163.6 ± 6.7 | 201.6 ± 3.8 *** | 206.6 ± 4.6 | 190.8 ± 4.3 | 189.4 ± 8.7 | |
| Insulin (ng/mL) | 4.16 ± 0.90 | 9.21 ± 1.49 **,a | 7.47 ± 0.78 ab | 7.12 ± 0.47 ab | 5.99 ± 0.51 b | |
| HOMA-IR 1) | 39.4 ± 8.3 | 109.2 ± 17.4 **,a | 90.8 ± 9.3 ab | 80.4 ± 5.4 ab | 66.7 ± 5.9 b | |
| Triglyceride (mg/dL) | 51.8 ± 3.0 | 71.6 ± 4.6 **,a | 62.9 ± 4.4 ab | 55.2 ± 4.1 bc | 44.5 ± 1.5 c | |
| Total cholesterol (mg/dL) | 129.6 ± 4.7 | 170.6 ± 5.5 ***,a | 153.4 ± 3.9 bc | 161.8 ± 5.3 ab | 147.1 ± 3.9 c | |
| LDL-cholesterol (mg/dL) | 23.7 ± 1.9 | 38.8 ± 3.7 ***,a | 30.1 ± 2.1 b | 32.1 ± 1.2 ab | 31.5 ± 1.5 b | |
| HDL-cholesterol (mg/dL) | 90.1 ± 3.4 | 119.6 ± 6.0 *** | 113.5 ± 3.1 | 123.7 ± 3.4 | 111.9 ± 4.1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.-M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.-S.; Kim, E.J. Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice. Nutrients 2019, 11, 1190. https://doi.org/10.3390/nu11051190
Lim S-M, Lee HS, Jung JI, Kim SM, Kim NY, Seo TS, Bae J-S, Kim EJ. Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice. Nutrients. 2019; 11(5):1190. https://doi.org/10.3390/nu11051190
Chicago/Turabian StyleLim, Su-Min, Hyun Sook Lee, Jae In Jung, So Mi Kim, Nam Young Kim, Tae Su Seo, Jung-Shik Bae, and Eun Ji Kim. 2019. "Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice" Nutrients 11, no. 5: 1190. https://doi.org/10.3390/nu11051190
APA StyleLim, S.-M., Lee, H. S., Jung, J. I., Kim, S. M., Kim, N. Y., Seo, T. S., Bae, J.-S., & Kim, E. J. (2019). Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice. Nutrients, 11(5), 1190. https://doi.org/10.3390/nu11051190





