Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
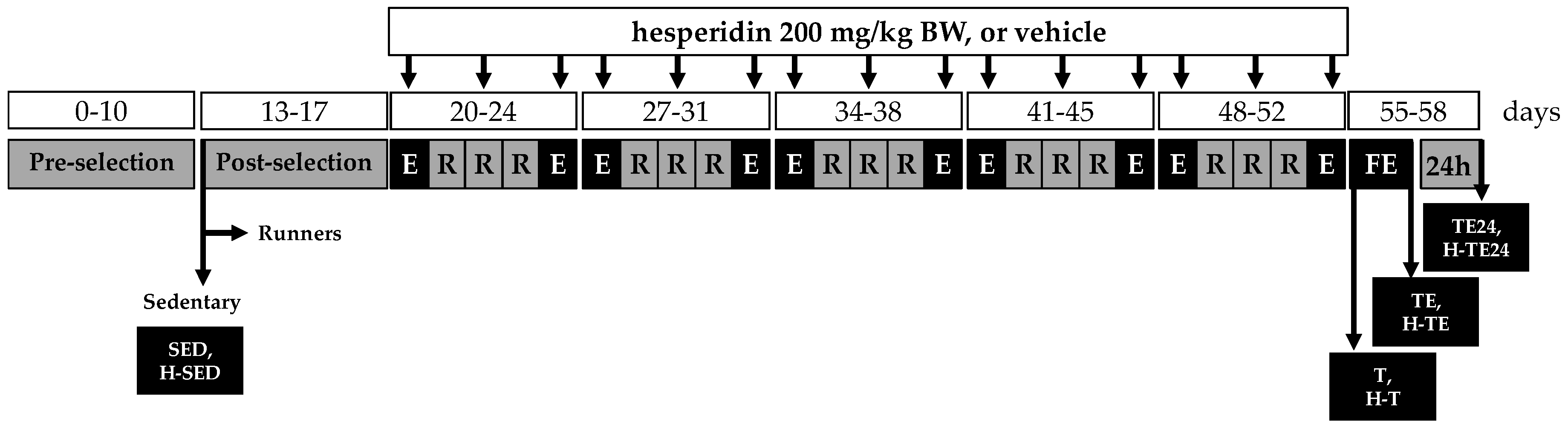
2.2. Training Program
2.3. Hesperidin Supplement
2.4. Animal Groups and Sample Collection
2.5. Peritoneal Macrophage Isolation and ROS Production
2.6. Catalase Activity in Spleen, Thymus and Liver
2.7. SOD Activity in Spleen, Thymus and Liver
2.8. Glutathione Peroxidase Activity in Liver
2.9. Statistical Analysis
3. Results
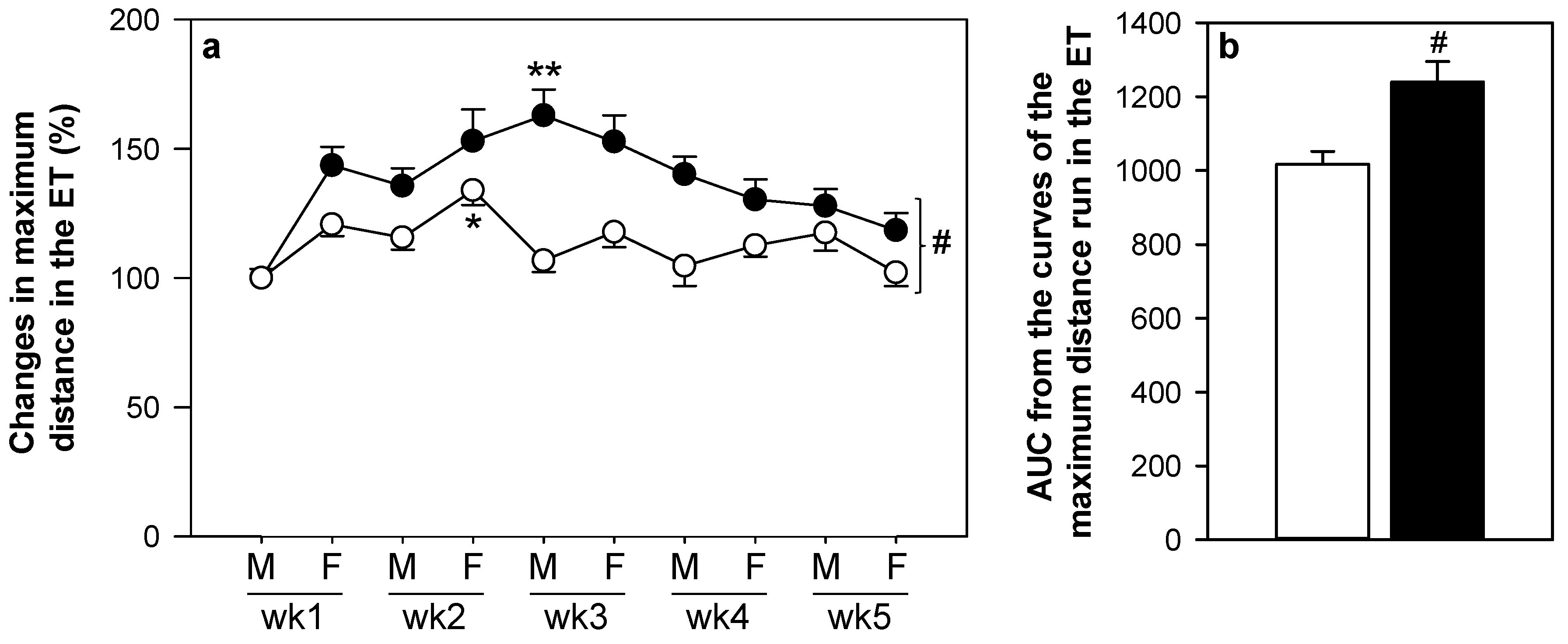
3.1. Training Program
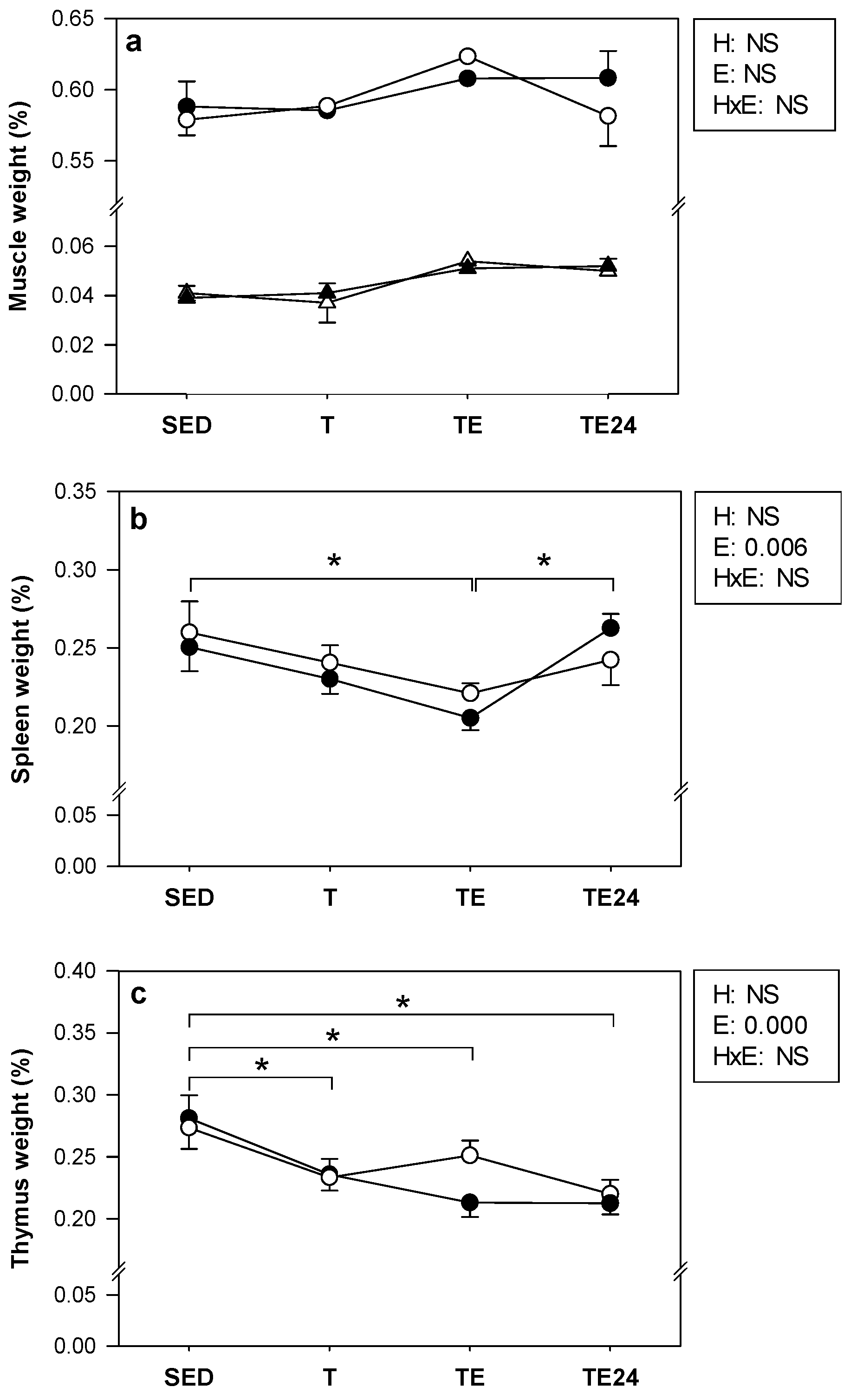
3.2. Body Weight, Food Intake and Organ Weight
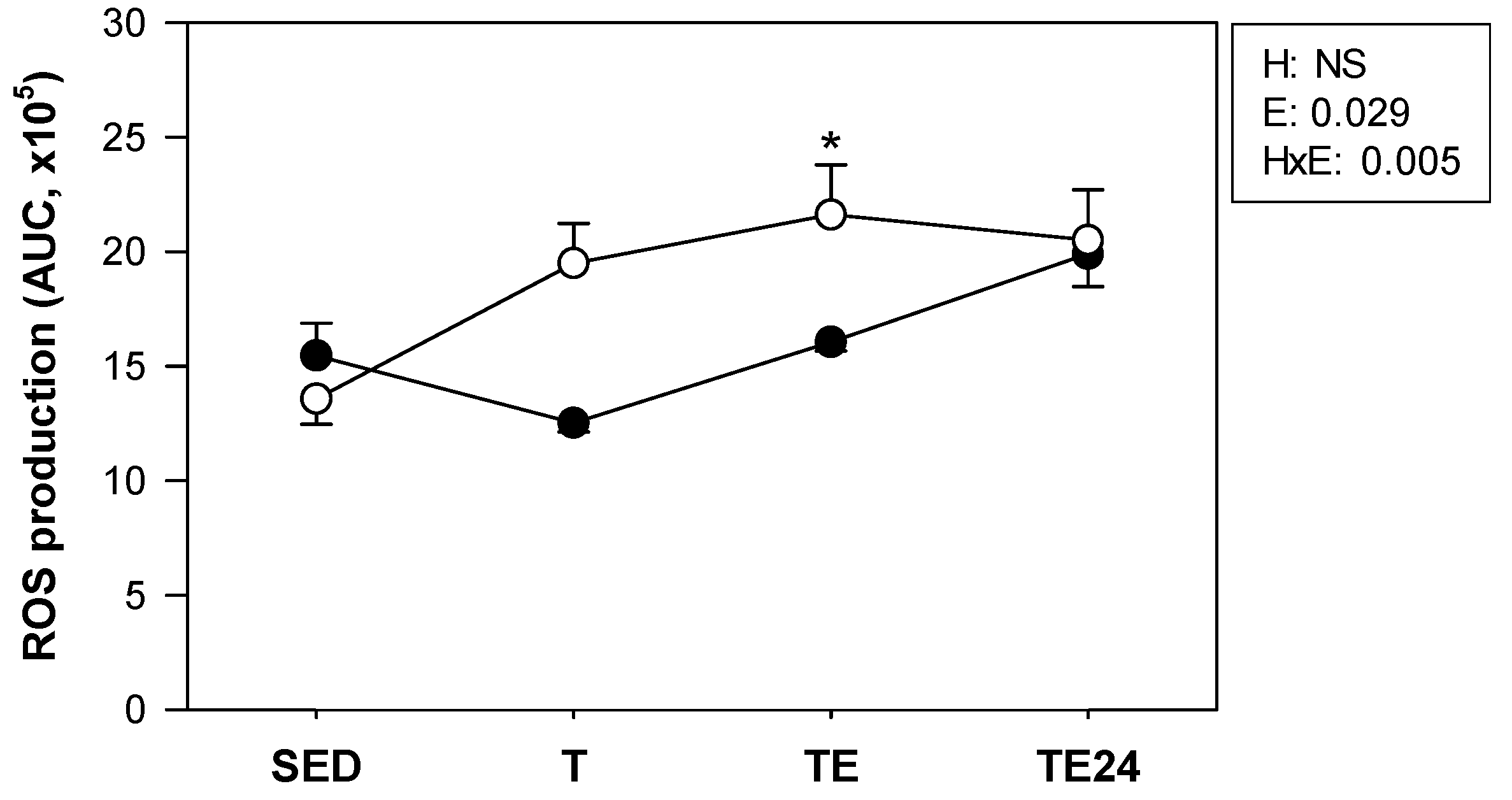
3.3. ROS Production by Peritoneal Macrophages
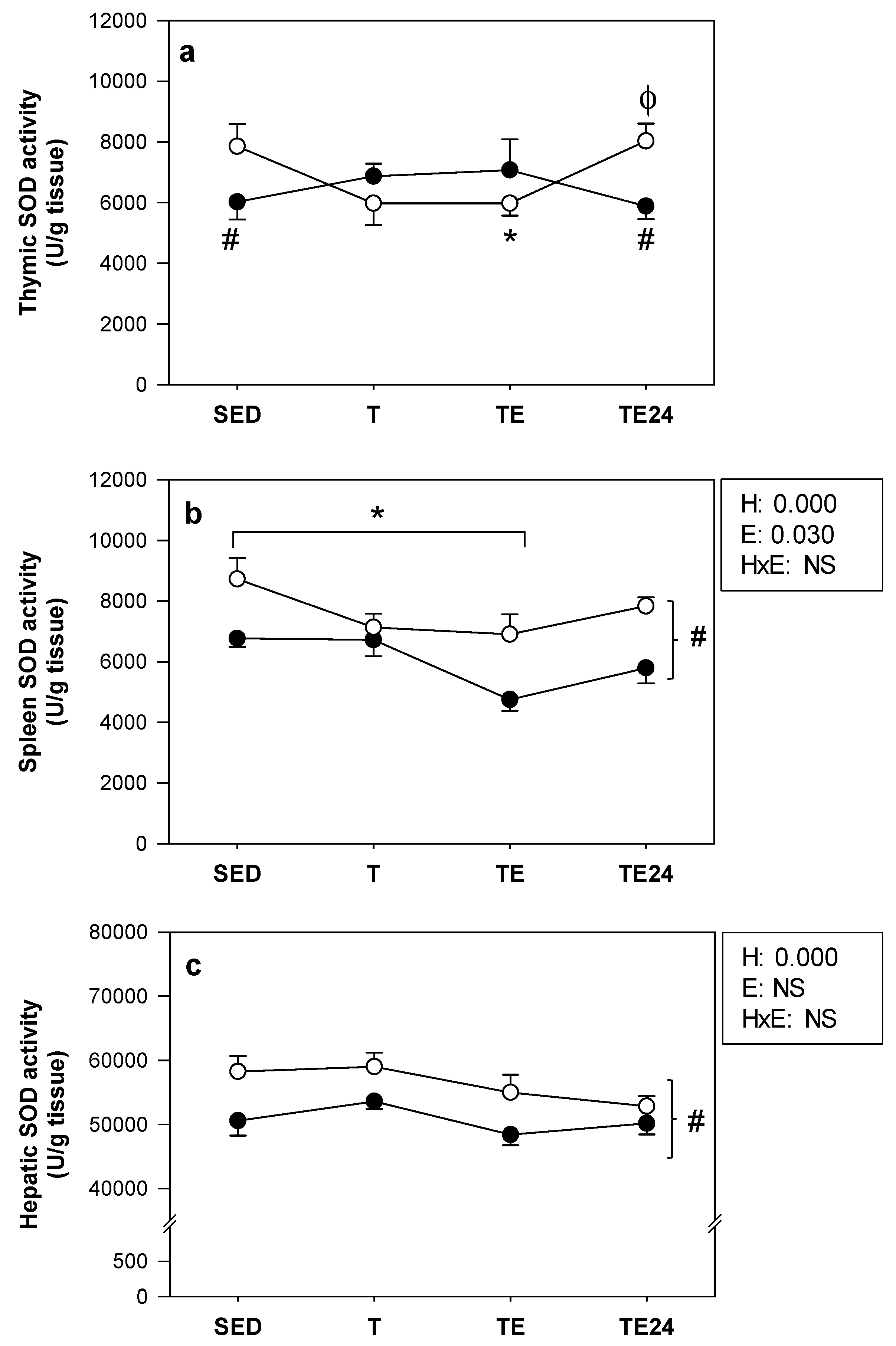
3.4. SOD Activity
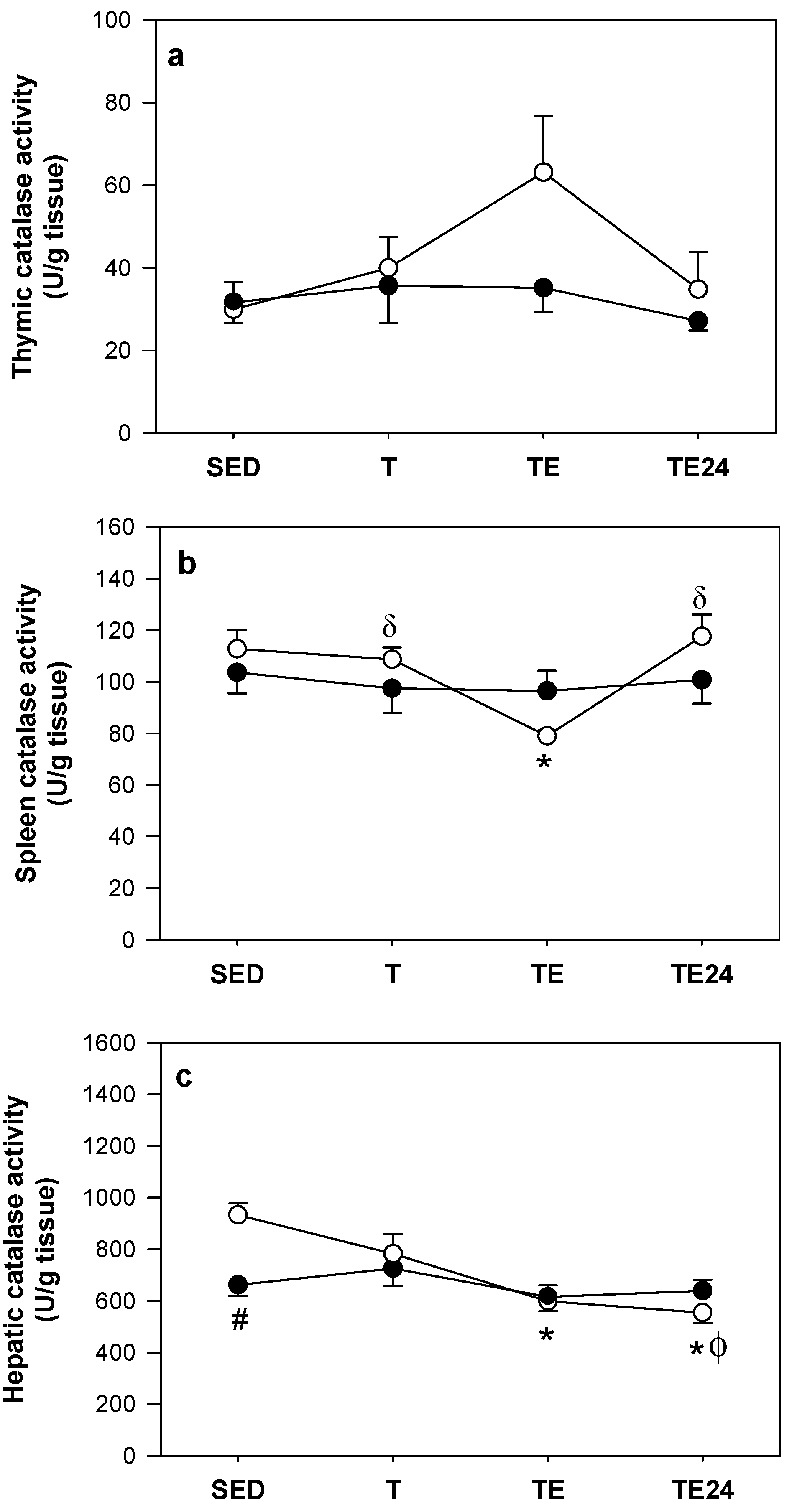
3.5. Catalase Activity
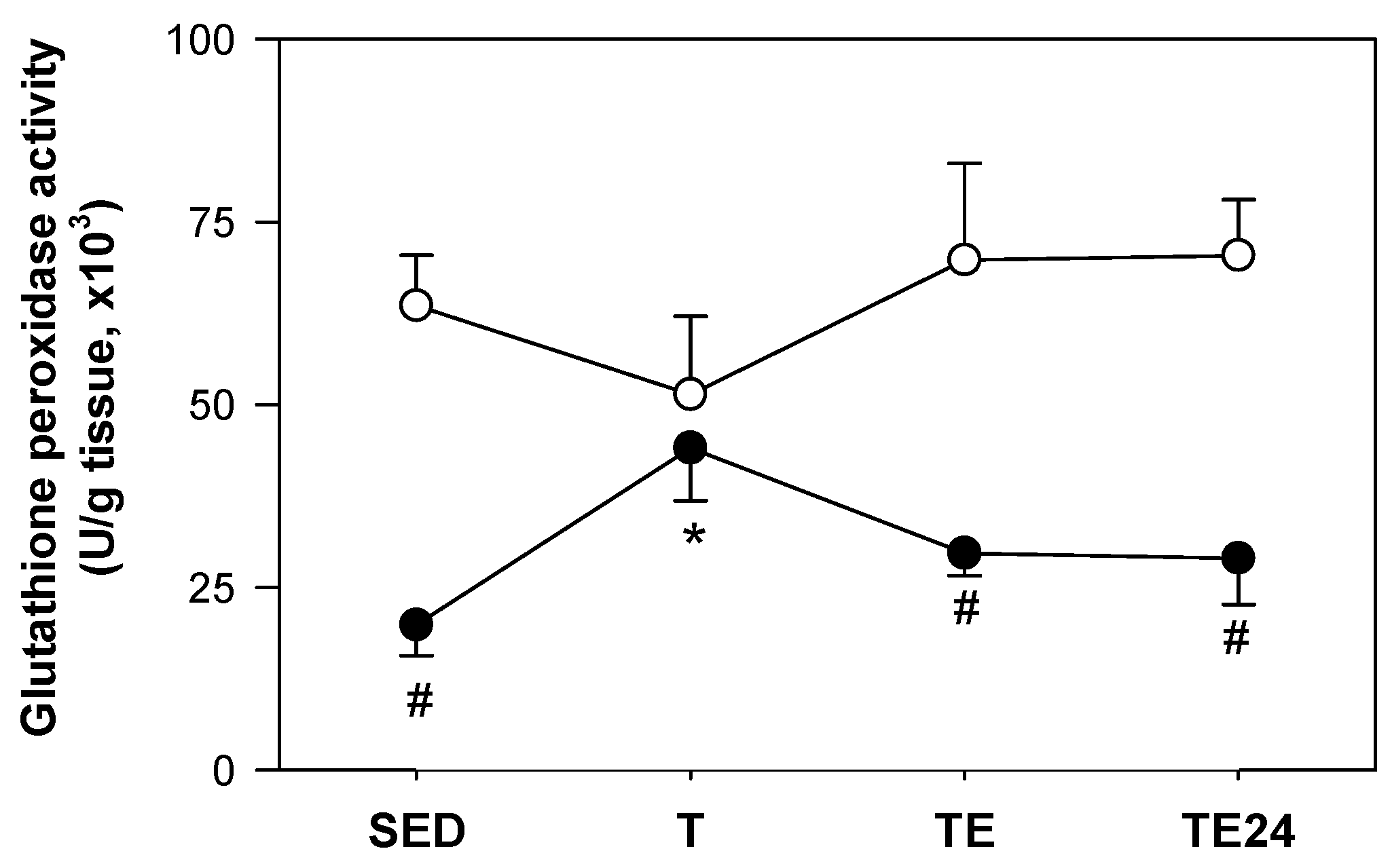
3.6. Hepatic Glutathione Peroxidase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A. Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Pace, G.; Lima, P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Griess, B.; Tom, E.; Domann, F.; Teoh-Fitzgerald, M. Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 2017, 112, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Knight, J.A. Review: Free radicals, antioxidants, and the immune system. Ann. Clin. Lab. Sci. 2000, 30, 145–158. [Google Scholar]
- de la Fuente, M.; Hernanz, A.; Vallejo, M.C. The immune system in the oxidative stress conditions of aging and hypertension: Favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005, 7, 1356–1366. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Freitas-Lima, L.C. The association between physical exercise and reactive oxygen species (ROS) production. J. Sports Med. Doping Stud. 2015, 5, 152. [Google Scholar] [CrossRef]
- Salim, S. Oxidative stress: A potential link between emotional wellbeing and immune response. Curr. Opin. Pharmacol. 2016, 29, 70–76. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Guan, J. Voluntary exercise training in mice increases the expression of antioxidant enzymes and decreases the expression of TNF-α in intestinal lymphocytes. Brain Behav. Immun. 2009, 23, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Kudo, K.Y.; Marcelino, T.B.; August, P.M.; Matté, C. Swimming exercise enhances the hippocampal antioxidant status of female Wistar rats. Redox Rep. 2015, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, O.; Reichhold, S.; Nics, L.; Hoelzl, C.; Valentini, J.; Stadlmayr, B.; Knasmüller, S.; Wagner, K.-H. Antioxidant responses to an acute ultra-endurance exercise: Impact on DNA stability and indications for an increased need for nutritive antioxidants in the early recovery phase. Br. J. Nutr. 2010, 104, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R.; Mcanulty, S. Oxidative stress assessment in response to ultraendurance exercise: Thiols redox status and ROS production according to duration of a competitive race. Oxid. Med. Cell. Longev. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.; Lim, W.; Kim, T.; Kang, C. Strenuous exercise induces mitochondrial damage in skeletal muscle of old mice. Biochem. Biophys. Res. Commun. 2015, 461, 354–360. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Duclos, M.; Gouarne, C.; Bonnemaison, A.D. Acute and chronic effects of exercise on tissue sensitivity to glucocorticoids. J. Appl. Physiol. 2003, 94, 869–875. [Google Scholar] [CrossRef]
- Azenabor, A.A.; Hoffman-Goetz, L. Intrathymic and intrasplenic oxidative stress mediates thymocyte and splenocyte damage in acutely exercised mice. J. Appl. Physiol. 1999, 86, 1823–1827. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, G.F.; Zhu, W.B.; Wang, Y.H. Effects of high intensity exhaustive exercise on SOD, MDA, and NO levels in rats with knee osteoarthritis. Genet. Mol. Res. 2015, 14, 12367–12376. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Gomez-Cabrera, M.-C.; Vina, J. Exercise and hormesis. Activation of cellular antioxidant signaling pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y. Effect of different methods of hypoxic exercise training on free radical oxidation and antioxidant enzyme activity in the rat brain. Biomed. Rep. 2013, 1, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Terblanche, S.E. The effects of exhaustive exercise on the activity levels of catalase in various tissues of male and female rats. Cell Biol. Int. 1999, 23, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.M.; Hyatt, H.W.; Smuder, A.J.; Sollanek, K.J.; Morton, A.B.; Roberts, M.D.; Kavazis, A.N. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res. Notes 2015, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Mastella, M.H.; Ribeiro, E.E.; do Prado-Lima, P.A.S.; de Souza Praia, R.; Medeiros Frescura Duarte, M.M.; da Cruz, I.B.M. Xanthine-catechin mixture enhances lithium-induced anti-inflammatory response in activated macrophages in vitro. BioMed Res. Int. 2017, 2017, 4151594. [Google Scholar] [CrossRef]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.; Cho, S.-G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Kwon, Y. Food-derived polyphenols inhibit the growth of ovarian cancer cells irrespective of their ability to induce antioxidant responses. Heliyon 2018, 4, e00753. [Google Scholar] [CrossRef]
- Kouka, P.; Chatzieffraimidi, G.-A.; Raftis, G.; Stagos, D.; Angelis, A.; Stathopoulos, P.; Xynos, N.; Skaltsounis, A.-L.; Tsatsakis, A.M.; Kouretas, D. Antioxidant effects of an olive oil total polyphenolic fraction from a Greek Olea europaea variety in different cell cultures. Phytomedicine 2018, 47, 135–142. [Google Scholar] [CrossRef]
- Jia, M.; Ren, D.; Nie, Y.; Yang, X. Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct. 2017, 8, 1282–1292. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Urpí-Sardà, M.; Pérez-Cano, F.J.; Franch, A.; Castellote, C.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Castell, M. Cocoa-enriched diet enhances antioxidant enzyme activity and modulates lymphocyte composition in thymus from young rats. J. Agric. Food Chem. 2007, 55, 6431–6438. [Google Scholar] [CrossRef] [PubMed]
- Haramizu, S.; Ota, N.; Hase, T.; Murase, T. Catechins attenuate eccentric exercise-induced inflammation and loss of force production in muscle in senescence-accelerated mice. J. Appl. Physiol. 2011, 111, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Muroyama, K.; Yamamoto, Y.; Murosaki, S. A combination of glucosyl hesperidin and caffeine exhibits an anti-obesity effect by inhibition of hepatic lipogenesis in mice. Phyther. Res. 2015, 29, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Saidullah, B.; Sultana, S. Anti-carcinogenic effect of hesperidin against renal cell carcinoma by targeting COX-2/PGE2 pathway in Wistar rats. Environ. Toxicol. 2018, 33, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Influence of hesperidin on the systemic and intestinal rat immune response. Nutrients 2017, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Bitencourt, D.; Leal, R.; Olabiyi, A.A.; Oboh, G.; Castilhos, L.G. Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep. 2017, 22, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Oreščanin-Dušić, Z.; Milenković, D.; Konić-Ristić, A.; Blagojević, D.; Milošević, V.; Šošić-Jurjević, B. Citrus flavanones naringenin and hesperetin improve antioxidant status and membrane lipid compositions in the liver of old-aged Wistar rats. Exp. Gerontol. 2016, 84, 49–60. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Dourado, G.K.Z.S.; Cesar, T.B. Hesperidin associated with continuous and interval swimming improved biochemical and oxidative biomarkers in rats. J. Int. Soc. Sports Nutr. 2013, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Batatinha, H.A.P.; Lima, E.A.; Teixeira, A.A.S.; Souza, C.O.; Biondo, L.A.; Silveira, L.S.; Lira, F.S.; Jos, A.; Neto, E.C.R. Association between aerobic exercise and rosiglitazone avoided the NAFLD and liver inflammation exacerbated in PPAR-α knockout mice. J. Cell. Physiol. 2017, 232, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Pérez-Cano, F.J.; Ramiro-Puig, E.; Franch, A.; Castell, M. Cocoa intake attenuates oxidative stress associated with rat adjuvant arthritis. Pharmacol. Res. 2012, 66, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Perche, O.; Vergnaud-Gauduchon, J.; Morand, C.; Dubray, C.; Mazur, A.; Vasson, M.P. Orange juice and its major polyphenol hesperidin consumption do not induce immunomodulation in healthy well-nourished humans. Clin. Nutr. 2014, 33, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R.; McCabe, G.P.; McCabe, L.D.; Jackson, G.S.; Horcajada, M.N.; Offord-Cavin, E.; Peacock, M.; Weaver, C.M. Effect of hesperidin with and without a calcium (Calcilock) supplement on bone health in postmenopausal women. J. Clin. Endocrinol. Metab. 2016, 101, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Williams, A.S.; Shanely, R.A.; Jin, F.; McAnulty, S.R.; Triplett, N.T.; Austin, M.D.; Henson, D.A. Quercetin’s influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010, 42, 338–345. [Google Scholar] [CrossRef]
- MacRae, S.H.; Mefferd, K.M. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef]
- Overdevest, E.; Wouters, J.A.; Wolfs, K.H.M.; Van Leeuwen, J.J.M.; Possemiers, S. Citrus flavonoid supplementation improves exercise performance in trained athletes. J. Sports Sci. Med. 2018, 17, 24–30. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.J.; Choi, J.S.; Chung, H.Y. Hesperetin: A potent antioxidant against peroxynitrite. Free Radic. Res. 2004, 38, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant supplementation during exercise training. Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Živković, I.P.; Rakin, A.K.; Petrović-Djergović, D.M.; Kosec, D.J.; Mićić, M.V. Exposure to forced swim stress alters morphofunctional characteristics of the rat thymus. J. Neuroimmunol. 2005, 160, 77–86. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Savino, W.; Dardenne, M. Neuroendocrine control of thymus physiology. Endocr. Rev. 2000, 21, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.F. Normal structure, function, and histology of the spleen. Toxicol. Pathol. 2006, 34, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B. Lymphocyte responses to maximal exercise. Sports Med. 2003, 33, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Gerpe, L.; Rey-Méndez, M. Alterations induced by chronic stress in lymphocyte subsets of blood and primary and secondary immune organs of mice. BMC Immunol. 2001, 2. [Google Scholar] [CrossRef]
- Hackney, A.C.; Lane, A.R. Exercise and the regulation of endocrine hormones. Prog. Mol. Biol. Transl. Sci. 2015, 135, 293–311. [Google Scholar] [CrossRef]
- Pereira, B.; Rosa, L.F.; Safi, D.A.; Bechara, E.J.; Curi, R. Superoxide dismutase, catalase and glutathione peroxidase activities in the lymphoid organs of diabetic rats. J. Endocrinol. 1994, 142, 161–165. [Google Scholar] [CrossRef]
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J. Appl. Physiol. 2011, 110, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Okudan, N.; Belviranli, M. Effects of exercise training on hepatic oxidative stress and antioxidant status in aged rats. Arch. Physiol. Biochem. 2016, 122, 180–185. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Xiong, Z.; Wang, S. The effect of sedi herba on the metabolism of free radicals in liver tissues of rats after exercise. Acta Med. Mediterr. 2016, 32, 1881–1884. [Google Scholar] [CrossRef]
- Taysi, S.; Oztasan, N.; Efe, H.; Polat, M.F.; Gumustekin, K.; Siktar, E.; Canakci, E.; Akcay, F.; Dane, S.; Gul, M. Endurance training attenuates the oxidative stress due to acute exhaustive exercise in rat liver. Acta Physiol. Hung. 2008, 95, 337–347. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Souza, M.A.; Amaral, G.P.; Stefanello, S.T.; Bresciani, G.; Fighera, M.R.; Soares, F.A.A.; Barbosa, N.V. Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci. 2014, 96, 40–45. [Google Scholar] [CrossRef]
- Pari, L.; Karthikeyan, A.; Karthika, P.; Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol. Rep. 2015, 2, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ilankeswaran, M.; Senthil Kumar, G.; Rajadurai, M.; Stephan, R. Hepatoprotective effect of hesperidin on normal and streptozotocin-induced diabetic rats: Biochemical and histological evidences. Int. J. Environ. Sci. Ecotechnol. 2011, 1, 55–66. [Google Scholar]
| Group | Body Weight (g) | Food Intake (g/day/rat) | |||
|---|---|---|---|---|---|
| Day 0 | Day 20 | Day 52 | Day 20 | Day 52 | |
| SED | 67.67 ± 3.41 | 144.80 ± 3.62 | 206.57 ± 8.22 | 15.76 ± 0.29 | 15.68 ± 0.10 |
| H-SED | 205.00 ± 4.46 | 15.77 ± 0.19 | |||
| Run | 71.85 ± 1.91 | 154.33 ± 1.59 | 222.17 ± 3.09 * | 15.27 ± 0.21 | 14.86 ± 0.49 |
| H-Run | 221.65 ± 3.61 * | 15.59 ± 0.45 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. https://doi.org/10.3390/nu11040783
Estruel-Amades S, Massot-Cladera M, Garcia-Cerdà P, Pérez-Cano FJ, Franch À, Castell M, Camps-Bossacoma M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients. 2019; 11(4):783. https://doi.org/10.3390/nu11040783
Chicago/Turabian StyleEstruel-Amades, Sheila, Malén Massot-Cladera, Pau Garcia-Cerdà, Francisco J. Pérez-Cano, Àngels Franch, Margarida Castell, and Mariona Camps-Bossacoma. 2019. "Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats" Nutrients 11, no. 4: 783. https://doi.org/10.3390/nu11040783
APA StyleEstruel-Amades, S., Massot-Cladera, M., Garcia-Cerdà, P., Pérez-Cano, F. J., Franch, À., Castell, M., & Camps-Bossacoma, M. (2019). Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients, 11(4), 783. https://doi.org/10.3390/nu11040783









