Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Portulaca Oleracea L. juice (PJ) Processing
2.3. Antioxidant In Vitro Assays
2.4. Vitamins Determination
2.5. Total Phenolics Determination
2.6. Caco-2 Cell Culture
2.7. Secretion of Pro-Inflammatory Mediators
2.8. Intracellular Reactive Oxygen Species (ROS)
2.9. Measurement of Intestinal Barrier Function
2.10. Statistical Analysis
3. Results
3.1. Portulaca Oleracea L. Juice (PJ) Processing
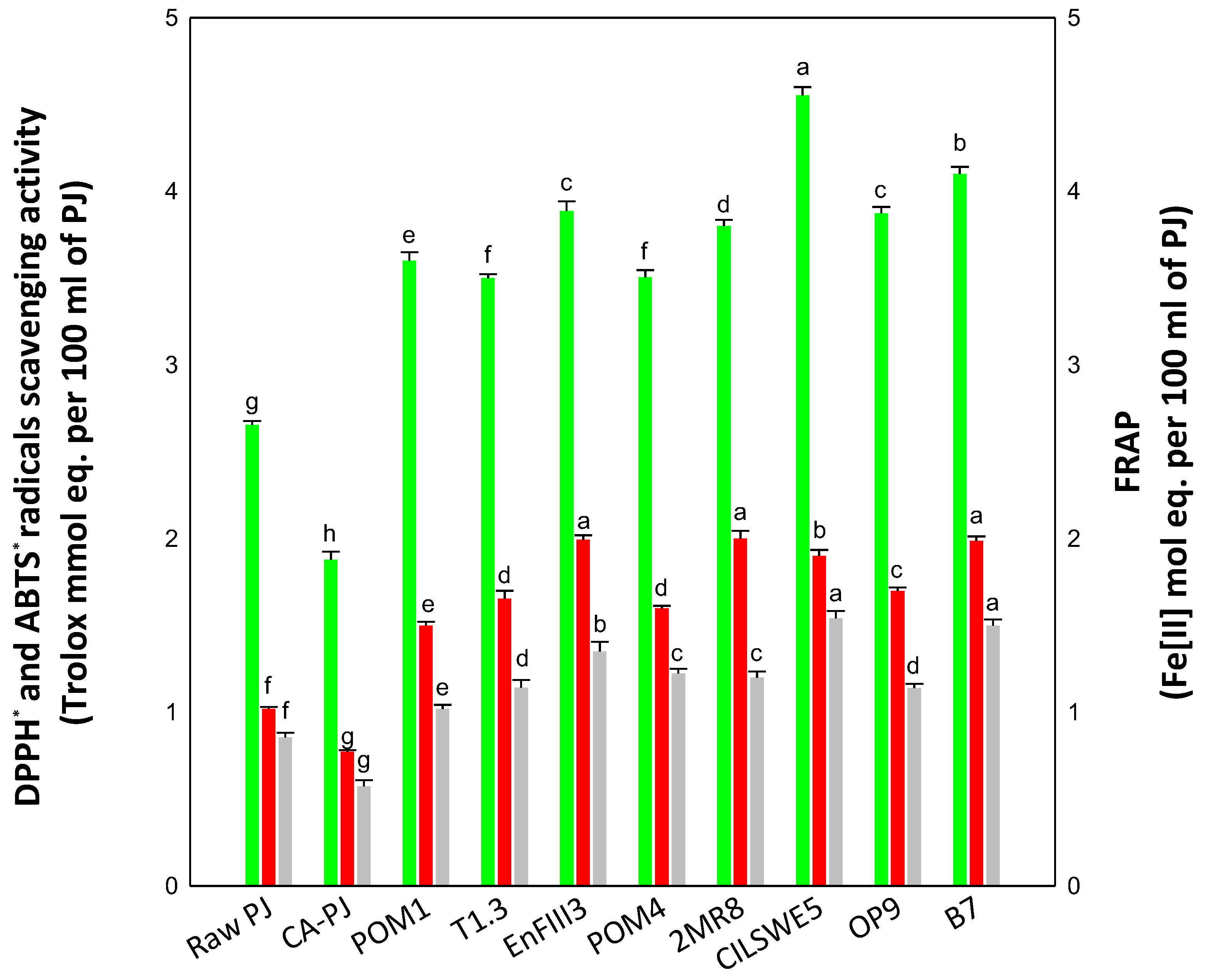
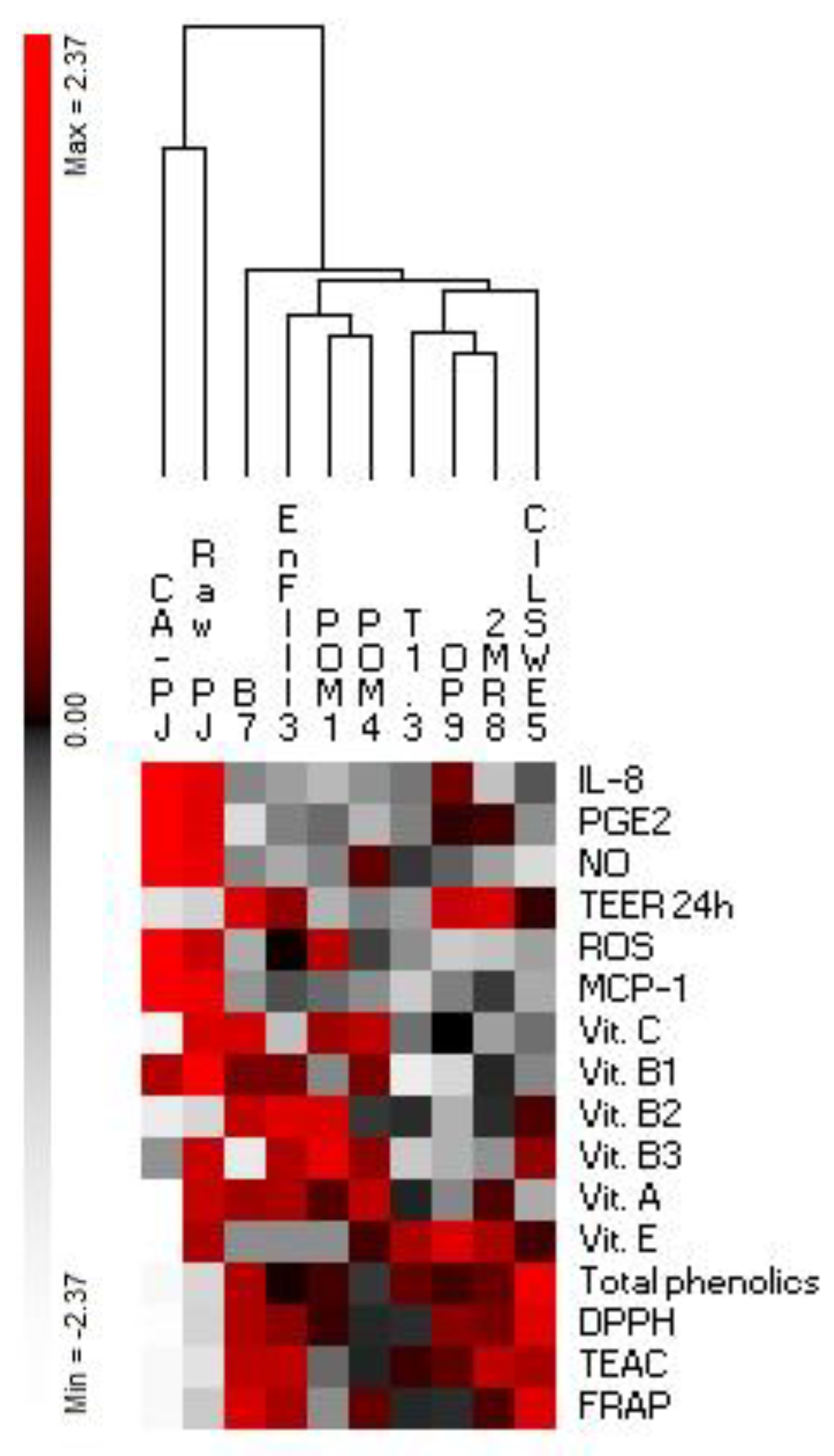
3.2. In Vitro Antioxidant Activity
3.3. Vitamins and Phenolics Determination
3.4. Viability of Caco-2 Cells
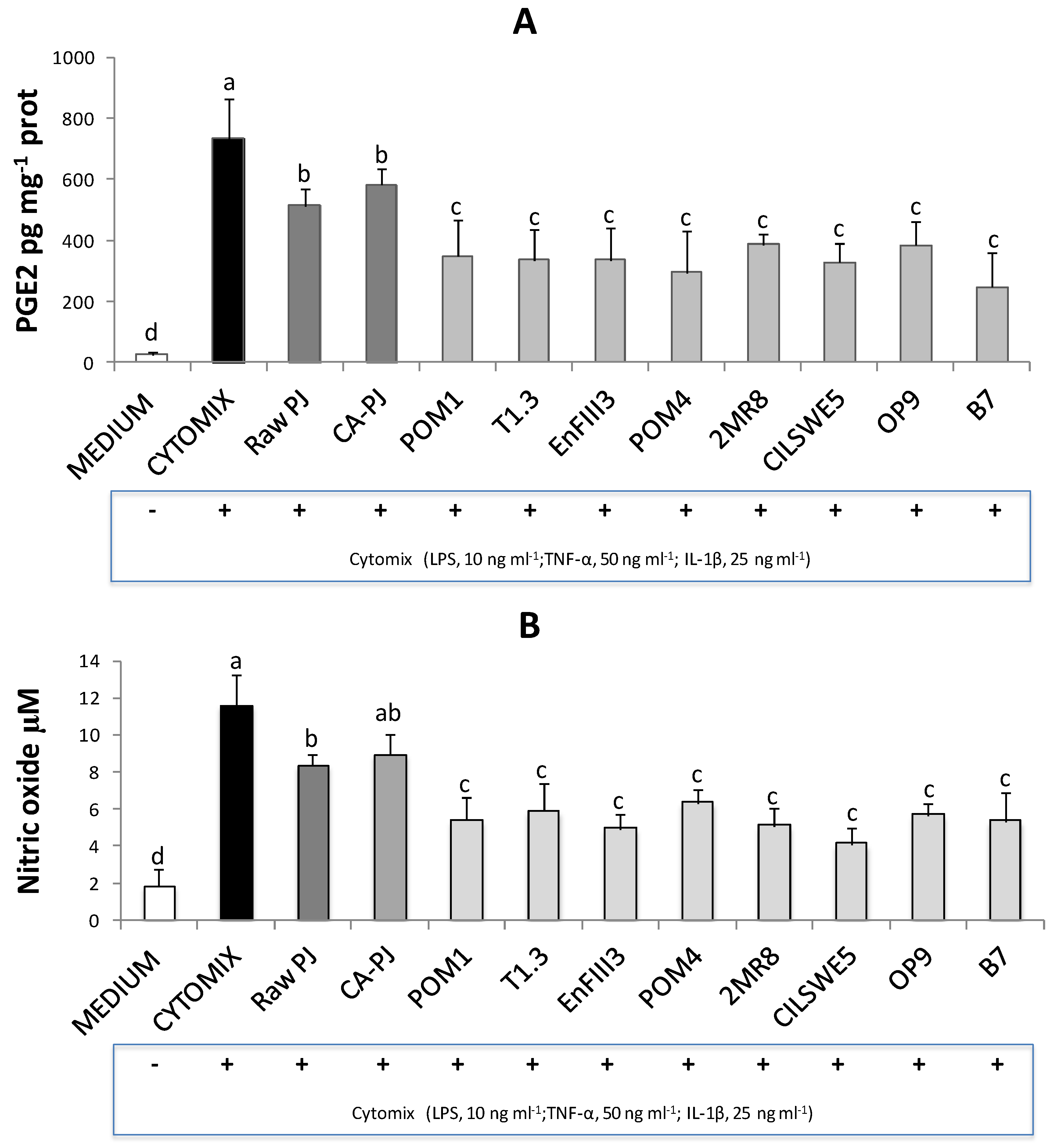
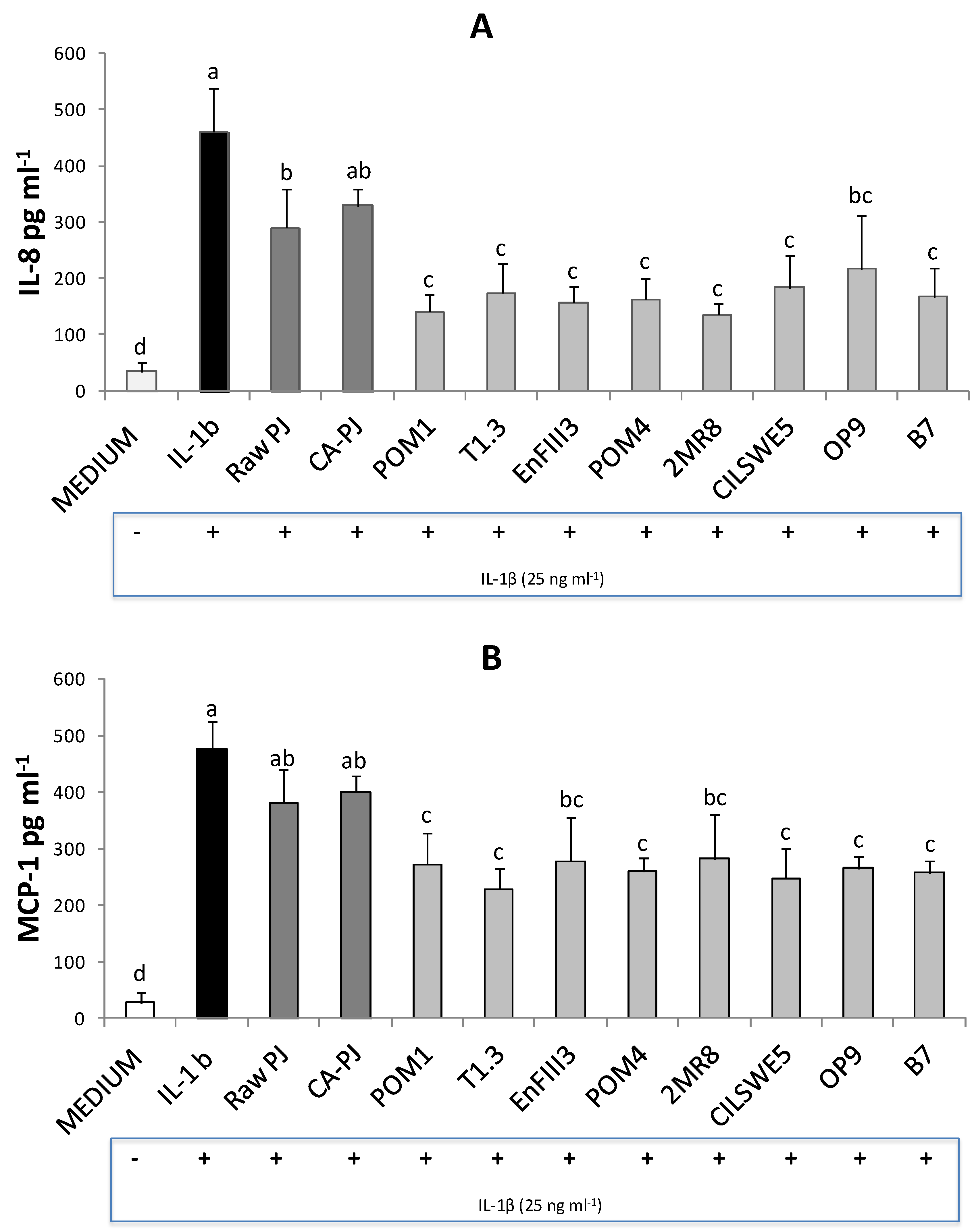
3.5. Secretion of Pro-Inflammatory Mediators
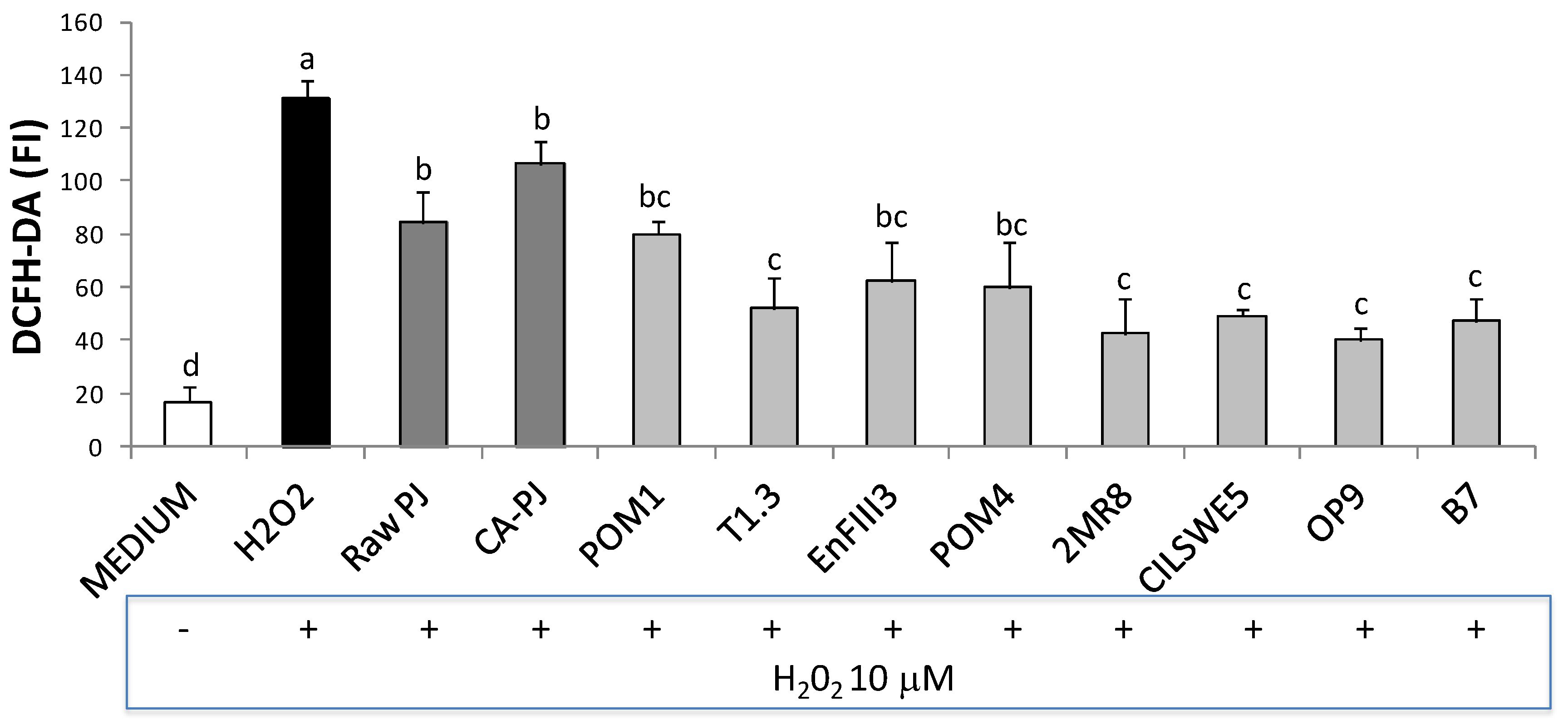
3.6. Intracellular Reactive Oxygen Species (ROS)
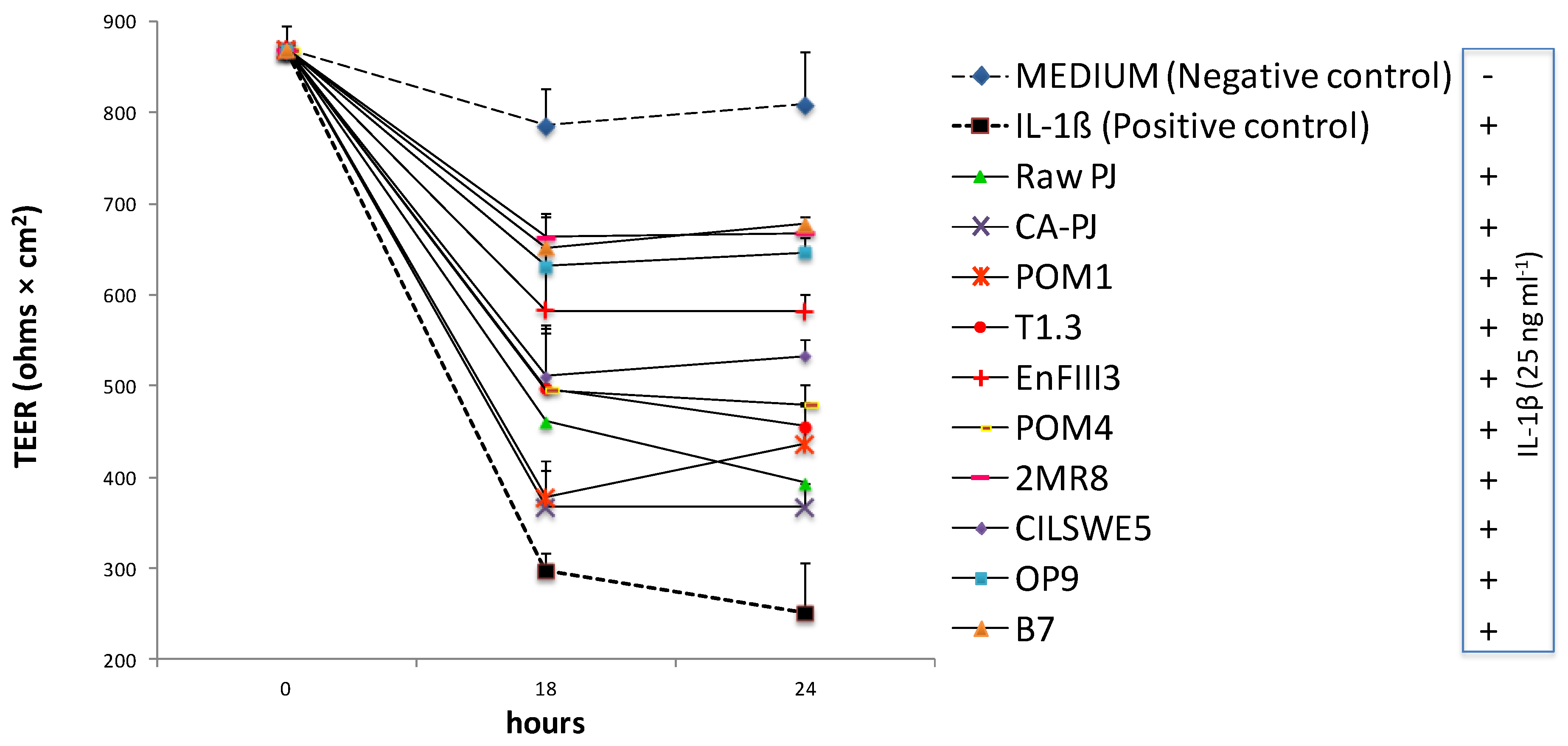
3.7. Measurement of Intestinal Barrier Function by Determination of Transepithelial Electrical Resistance (TEER)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Debasis, B., Sreejayan, N., Eds.; Academc Press: London, UK, 2017; pp. 29–45. [Google Scholar]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods: Commercial trends, research, and health implications. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: A review. Crit. Rev. Food Sci. 2015, 55, 503–520. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation of smoothies and juices. In Lactic Acid Fermentation of Fruits and Vegetables, 1st ed.; Paramithiotis, S., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 269–284. [Google Scholar]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Meng, Y.H.; Ying, Z.M.; Xu, N.; Hao, D.; Gao, M.Z.; Zhang, W.J.; Xu, L.; Gao, Y.C.; Ying, X.X. Three novel alkaloids from Portulaca oleracea L. and their anti-inflammatory effects. J. Agric. Food Chem. 2016, 64, 5837–5844. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.J.; Jang, H.J.; Lee, S.; Jung, K.; Lee, S.W.; Lee, S.J.; Rho, M.C. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res. Int. 2018, 106, 335–343. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Trani, A.; Cantatore, V.; Gambacorta, G.; Gobbetti, M. Lactic acid fermentation enriches the profile of biogenic compounds and enhances the functional features of Portulaca oleracea L. J. Funct. Foods 2017, 39, 175–185. [Google Scholar] [CrossRef]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulxs, E.; Lacrox, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Cilla, A.; Rodrigo, M.J.; Zacarías, L.; De Ancos, B.; Sánchez-Moreno, C.; Barberá, R.; Alegría, A. Protective effect of bioaccessible fractions of citrus fruit pulps against H2O2-induced oxidative stress in Caco-2 cells. Food Res. Int. 2018, 103, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Ramírez, D.; Cilla, A.; Contreras-Calderón, J.; Alegría-Torán, A. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon Caco-2 cells of commercial Colombian coffee. Food Chem. 2017, 219, 364–372. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Paradiso, A.; De Angelis, M.; Salmon, J.C.; Buchin, S.; De Gara, L.; Gobbetti, M. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food microbiol. 2009, 128, 473–483. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cavoski, I.; Lanera, A.; Mamdouh, B.M.; Gobbetti, M. Bioprocessing technology to exploit organic palm date (Phoenix dactylifera L. cultivar Siwi) fruit as a functional dietary supplement. J. Funct. Foods 2017, 31, 9–19. [Google Scholar] [CrossRef]
- Pontonio, E.; Di Cagno, R.; Tarraf, W.; Filannino, P.; De Mastro, G.; Gobbetti, M. Dynamic and Assembly of Epiphyte and Endophyte Lactic Acid Bacteria During the Life Cycle of Origanum vulgare L. Front. Microbiol. 2018, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Cardinali, G.; Minervini, G.; Antonielli, L.; Rizzello, C.G.; Ricciuti, P.; Gobbetti, M. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 2010, 27, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Surico, R.F.; Minervini, G.; Rizzello, C.G.; Lovino, R.; Servili, M.; Taticchi, A.; Urbani, S.; Gobbetti, M. Exploitation of sweet cherry (Prunus avium L.) puree added of stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol. 2011, 28, 900–909. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Lanera, A.; Cavoski, I.; Gobbetti, M. Exploitation of Leuconostoc mesenteroides strains to improve shelf life, rheological, sensory and functional features of prickly pear (Opuntia ficus-indica L.) fruit puree. Food Microbiol. 2016, 59, 176–189. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Addante, R.; Pontonio, E.; Gobbetti, M. Metabolism of fructophilic lactic acid bacteria isolated from Apis mellifera L. bee-gut: A focus on the phenolic acids as external electron acceptors. Appl. Environ. Microbiol. 2016, 82, 6899–6911. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- EN 14130:2003. Foodstuffs—Determination of Vitamin C by HPLC—Measurement of L(+) Ascorbic Acid and Dehydro L(+) Ascorbic Acid; European Committee for Standardization: Bruxelles, Belgium, 2003. [Google Scholar]
- EN 14152:2003. Foodstuffs—Determination of Vitamin B2 by HPLC—Measurement of Riboflavin; European Committee for Standardization: Bruxelles, Belgium, 2003. [Google Scholar]
- EN 14122:2003. Foodstuffs—Determination of Vitamin B1 by HPLC—Measurement of Total Thiamin Including Its Phosphorylated Derivatives; European Committee for Standardization: Bruxelles, Belgium, 2003. [Google Scholar]
- EN 15652:2009. Foodstuffs—Determination of Niacin by HPLC—HPLC Measurement by Three Different Ways of Hydrolysis, Acid Hydrolysis, Enzymatic Hydrolysis or Acid/Alkaline Hydrolysis; European Committee for Standardization: Bruxelles, Belgium, 2009. [Google Scholar]
- EN 12823-2:2000. Foodstuffs—Determination of Vitamin A by High Performance Liquid Chromatography—Part 2: Measurements of Beta-Carotene; European Committee for Standardization: Bruxelles, Belgium, 2000. [Google Scholar]
- EN 12822:2000. Foodstuffs—Determination of Vitamin E by High Performance Liquid Chromatography—Measurement of Alpha-, Beta-, Gamma-, and Delta-Tocopherols; European Committee for Standardization: Bruxelles, Belgium, 2000. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–153. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999, 29, 152–178. [Google Scholar]
- Engvall, E.; Perlmann, P. Enzyme-Linked Immunosorbent Assay, Elisa III. Quantitation of Specific Antibodies by Enzyme-Labeled Anti-Immunoglobulin in Antigen-Coated Tubes. J. Immunol. 1972, 109, 129–135. [Google Scholar] [PubMed]
- Lin, A.V. Direct ELISA. In The ELISA Guidebook, 2nd ed.; Hnasko, R., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1318. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Lebel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Srinivasan, B.; Reddy Kolli, A.; Esch, M.B.; Erbil Abaci, H.; Shuler, M.L.; Hickman, J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafi, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 1–11. [Google Scholar]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 12845. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Giacomin, P.; Navarro, S.; Miller, C.; Loukas, A.; Wangchuk, P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J. Ethnopharmacol. 2018, 211, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Hu, X.; Mei, R.; Shu, Y.; Gan, D.; Cai, L.; Ding, Z. Four new diterpenoid alkaloids with anti-inflammatory activities from Aconitum taronense Fletcher et Lauener. Phytochem. Lett. 2018, 25, 152–155. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Wang, B.; Zhang, L.; Zhang, Q.; Li, D.; Zhang, S.; Gao, H.; Wang, X. Protective role of liriodendrin in mice with dextran sulphate sodium-induced ulcerative colitis. Int. Immunopharmacol. 2017, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef]
- Kang, G.D.; Lim, S.; Kim, D.H. Oleanolic acid ameliorates dextran sodium sulfateinduced colitis in mice by restoring the balance of Th17/Treg cells and inhibiting NF-κB signaling pathway. Int. Immunopharmacol. 2015, 29, 393–400. [Google Scholar] [CrossRef]
- Cho, J.H.; Abraham, C. Inflammatory bowel disease genetics: Nod2. N. Engl. J. Med. 2009, 361, 401–416. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.; Panchal, S.K.; Brown, L. Nutraceuticals in rodent models as potential treatments for human Inflammatory Bowel Disease. Pharmacol. Res. 2018, 132, 99–107. [Google Scholar] [CrossRef]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Ahuja, V.; Tandon, R.K. Inflammatory bowel disease in the Asia-Pacific area: A comparison with developed countries and regional differences. J. Dig. Dis. 2010, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Jurjus, A.; Eid, A.; Al Kattar, S.; Zeenny, M.N.; Gerges-Geagea, A.; Haydar, H.; Hilal, A.; Oueidat, D.; Matar, M.; Tawilah, J.; et al. Inflammatory bowel disease, colorectal cancer and type 2 diabetes mellitus: The links. BBA Clin. 2016, 5, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.C.; Durães, C.; Coelho, R.; Grácio, D.; Silva, M.; Peixoto, A.; Lago, P.; Pereira, M.; Catarino, T.; Pinho, S.; et al. Association between polymorphisms in antioxidant genes and inflammatory bowel disease. PLoS ONE 2017, 12, e0169102. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Zamyatnin, A.A., Jr.; Werner, J.; Bazhin, A.V. Reactive oxygen species and colorectal cancer. J. Cell. Physiol. 2018, 233, 5119–5132. [Google Scholar] [CrossRef]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2020, 33, 819–830. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Holub, M.C.; Mako, E.; Devay, T.; Dank, M.; Szalai, C.; Fenyvesi, A.; Falus, A. Increased interleukin-6 levels, interleukin-6 receptor and gp130 expression in peripheral lymphocytes of patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1998, 228, 47–50. [Google Scholar]
- Komatsu, M.; Kobayashi, D.; Saito, K.; Feruya, D.; Yagihashi, A.; Araake, H.; Tsuji, N.; Sakamaki, S.; Niitsu, Y.; Watanabe, N. Tumor necrosis factor-α in serum of patients with inflammatory bowel disease as measured by ba highly sensitive immuno-PCR. Clin. Chem. 2001, 47, 1297–1301. [Google Scholar]
- Louis, E.; Belaiche, J.; van Kemseke, C.; Franchimont, D.; de Groote, D.; Green, V.; Mary, J.Y. A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn’s disease. Eur. J. Gastroenterol. 1997, 9, 939–944. [Google Scholar] [CrossRef]
- Al-Sadi, R.M.; Ma, T.Y. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 2007, 178, 4641–4649. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Lu, Y.C.; Ou, C.C.; Lin, S.L.; Tsai, C.C.; Huang, C.T.; Lin, M.Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wiercińska-Drapało, A.; Flisiak, R.; Prokopowicz, D. Effects of ulcerative colitis activity on plasma and mucosal prostaglandin E2 concentration. Prostaglandins Other Lipid Mediat. 1999, 58, 159–165. [Google Scholar] [CrossRef]
- Yamashita, S. Studies on changes of colonic mucosal PGE2 levels and tissue localization in experimental colitis. Gastroenterol. Jpn. 1993, 28, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Zifroni, A.; Treves, A.J.; Sachar, D.B.; Rachmilewitz, D. Prostanoid synthesis by cultured intestinal epithelial and mononuclear cells in inflammatory bowel disease. Gut 1983, 24, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.E.; Waters, W.R.; Rudolph, K.M.; Drew, M.L. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis Canadensis and Ovis aries. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karimian, M.S.; Pirro, M.; Majeed, M.; Sahebkar, A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017, 33, 55–63. [Google Scholar] [CrossRef]
- Selby, W.; Janossy, G.; Bofil, M.; Jewell, D. Intestinal lymphocyte subpopulations in inflammatory bowel disease: An analysis by immunohistochemical and cell isolation techniques. Gut 1984, 25, 32–40. [Google Scholar] [CrossRef]
- Reinecker, H.C.; Loh, E.Y.; Ringler, D.J.; Mehta, A.; Ronbeau, J.L.; MacDermott, R.P. MCP-1 gene expression in intestinal epithelial cells and IBD mucosa. Gastroenterology 1995, 108, 40–50. [Google Scholar] [CrossRef]
- Mazzucchelli, L.; Hauser, C.; Zgraggen, K.; Wagner, H.E.; Hess, M.W.; Laissue, J.A.; Mueller, C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J. Pathol. 1996, 178, 201–206. [Google Scholar] [CrossRef]
- Banks, C.; Bateman, A.; Payne, R.; Johnson, P.; Sheron, N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 2003, 199, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Toyonaga, A.; Sasaki, E.; Watanabe, K.; Tateishi, H.; Nishiyama, T.; Saiki, T.; Ikeda, H.; Tsuruta, O.; Tanikawa, K. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 1994, 96, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Parkos, C.A.; Delp, C.; Arnaout, M.A.; Madara, J.L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J. Clin. Investig. 1991, 88, 1605–1612. [Google Scholar] [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]
- Blake, K.M.; Carrigan, S.O.; Issekutz, A.C.; Stadnyk, A.W. Neutrophils migrate across intestinal epithelium using β2 integrin (CD11b/CD18)-independent mechanisms. Clin. Exp. Immunol. 2004, 136, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef]
- Zhou, G.X.; Liu, Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- McElroy, S.J.; Hobbs, S.; Kallen, M.; Tejera, N.; Rosen, M.J.; Grishin, A.; Matta, P.; Schneider, C.; Upperman, J.; Ford, H.; et al. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PLoS ONE 2012, 7, e38373. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.; Li, H.; Kortagere, S.; Sun, K.; Ding, L.; Ren, G.; Wang, Z.; Mani, S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J. Nutr. Biochem. 2014, 25, 923–933. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Mallouchos, A.; Kimbaris, A.; Alexopoulos, A.; Bezirtzoglou, E.; Plessas, S. Assessment of Volatile Compounds Evolution, Antioxidant Activity, and Total Phenolics Content during Cold Storage of Pomegranate Beverage Fermented by Lactobacillus paracasei K5. Fermentation 2018, 4, 95. [Google Scholar] [CrossRef]
- Vitali, B.; Minervini, G.; Rizzello, C.G.; Spisni, E.; Maccaferri, S.; Brigidi, P.; Gobetti, M.; Di Cagno, R. Novel probiotic candidates for humans isolated from raw fruits and vegetables. Food Microbiol. 2012, 31, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo, Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT-Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Dilna, S.V.; Surya, H.; Aswathy, R.G.; Varsha, K.K.; Sakthikumar, D.N.; Pandey, A.; Nampoothiri, K.M. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT-Food Sci. Technol. 2015, 64, 1179–1186. [Google Scholar] [CrossRef]
- Asama, T.; Arima, T.H.; Gomi, T.; Keishi, T.; Tani, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K. Lactobacillus kunkeei YB38 from honeybee products enhances IgA production in healthy adults. J. Appl. Microbiol. 2015, 119, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Asama, T.; Kimura, Y.; Kono, T.; Tatefuji, T.; Hashimoto, K.; Benno, Y. Effects of heat-killed Lactobacillus kunkeei YB38 on human intestinal environment and bowel movement: A pilot study. Benef. Microbes 2016, 7, 337–344. [Google Scholar] [CrossRef]
- Asama, T.; Uematsu, T.; Kobayashi, N.; Tatefuji, T.; Hashimoto, K. Oral administration of heatkilled Lactobacillus kunkeei YB38 improves murine influenza pneumonia by enhancing IgA production. Biosci. Microbiota Food Health 2017, 36, 1–9. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rönkä, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Uroić, K.; Novak, J.; Hynönen, U.; Pietilä, T.E.; Pavunc, A.L.; Kant, R.; Kos, B.; Palva, A.; Šušković, J. The role of S-layer in adhesive and immunomodulating properties of probiotic starter culture Lactobacillus brevis D6 isolated from artisanal smoked fresh cheese. LWT-Food Sci. Technol. 2016, 69, 623–632. [Google Scholar] [CrossRef]
- Vidhyasagar, V.; Jeevaratnam, K. Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J. Funct. Food 2013, 5, 235–243. [Google Scholar] [CrossRef]
- Osmanagaoglu, O.; Kiran, F.; Ataoglu, H. Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrob Proteins 2010, 2, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Food 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Diana, C.R.; Humberto, H.S.; Jorge, Y.F. Probiotic properties of Leuconostoc mesenteroides isolated from aguamiel of Agave salmiana. Probiotics Antimicrob Proteins 2015, 7, 107–117. [Google Scholar] [CrossRef]
- Beganović, J.; Pavunc, A.L.; Gjuračić, K.; Špoljarec, M.; Šušković, J.; Kos, B. Improved sauerkraut production with probiotic strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 2011, 76, M124–M129. [Google Scholar] [CrossRef]
- Arena, M.P.; Russo, P.; Capozzi, V.; Rascon, A.; Felis, G.E.; Spano, G.; Fiocco, D. Combinations of cereal β-glucans and probiotics can enhance the anti-inflammatory activity on host cells by a synergistic effect. J. Funct. Foods 2016, 23, 12–23. [Google Scholar] [CrossRef]
| Strain | Accession Number * | Reference |
|---|---|---|
| Lactobacillus plantarum POM1 | MF967220 | [17] |
| L. plantarum T1.3 | MF967221 | [18] |
| L. plantarum EnFIII3 | n.a. | [19] |
| Lactobacillus brevis POM4 | MF967222 | [17] |
| Lactobacillus rossiae 2MR8 | MF967223 | [20] |
| Pediococcus pentosaceus CILSWE5 | MF967224 | [21] |
| Leuconostoc mesenteroides OP9 | MF967225 | [22] |
| Lactobacillus kunkeei B7 | KX833124 | [23] |
| mg per 100 mL of PJ | |||||||
|---|---|---|---|---|---|---|---|
| Ascorbic Acid (Vitamin C) | Thiamin (Vitamin B1) | Riboflavin (Vitamin B2) | Niacin (Vitamin B3) | β-Carotene (Vitamin A) | α-Tocopherol (Vitamin E) | Total Phenolics (Gallic Acid Equivalents) | |
| Raw–PJ | 22 ± 2 a | 0.047 ± 0.004 a | 0.11 ± 0.004 d | 0.48 ± 0.02 a | 2.00 ± 0.02 a | 2.5 ± 0.1 a | 85 ± 6 bc |
| CA–PJ | 12 ± 1 d | 0.044 ± 0.003 a | 0.10 ± 0.002 d | 0.42 ± 0.02 c | 1.50 ± 0.03 d | 2.0 ± 0.1 b | 70 ± 2 d |
| L. plantarum POM1 | 20 ± 2 a | 0.041 ± 0.002 a | 0.18 ± 0.004 a | 0.50 ± 0.01 a | 1.88 ± 0.02 b | 2.3 ± 0.1 a | 112 ± 4 b |
| L. plantarum T1.3 | 17 ± 2 b | 0.038 ± 0.002 a | 0.15 ± 0.006 b | 0.40 ± 0.03 c | 1.85 ± 0.04 b | 2.5 ± 0.3 a | 115 ± 6 b |
| L. plantarum EnFIII3 | 15 ± 2 c | 0.043 ± 0.005 a | 0.19 ± 0.005 a | 0.47 ± 0.02 a | 1.96 ± 0.04 a | 2.3 ± 0.1 a | 110 ± 3 b |
| L. brevis POM4 | 21 ± 3 a | 0.043 ± 0.005 a | 0.14 ± 0.003 b | 0.46 ± 0.02 b | 1.99 ± 0.03 a | 2.4 ± 0.2 a | 108 ± 4 b |
| L. rossiae 2MR8 | 16 ± 3 c | 0.042 ± 0.004 a | 0.15 ± 0.004 b | 0.42 ± 0.02 c | 1.88 ± 0.03 b | 2.5 ± 0.1 a | 115 ± 8 b |
| P. pentosaceus CILSWE5 | 17 ± 2 b | 0.041 ± 0.002 a | 0.15 ± 0.005 b | 0.46 ± 0.03 b | 1.75 ± 0.04 c | 2.4 ± 0.2 a | 145 ± 8 a |
| Leuc. mesenteroides OP9 | 18 ± 2 b | 0.039 ± 0.002 a | 0.12 ± 0.004 c | 0.41 ± 0.02 b | 1.79 ± 0.02 c | 2.6 ± 0.3 a | 112 ± 7 b |
| L. kunkeei B7 | 22 ± 2 a | 0.043 ± 0.002 a | 0.17 ± 0.002 a | 0.38 ± 0.01 c | 1.95 ± 0.02 a | 2.3 ± 0.1 a | 125 ± 6 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients 2019, 11, 248. https://doi.org/10.3390/nu11020248
Di Cagno R, Filannino P, Vincentini O, Cantatore V, Cavoski I, Gobbetti M. Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients. 2019; 11(2):248. https://doi.org/10.3390/nu11020248
Chicago/Turabian StyleDi Cagno, Raffaella, Pasquale Filannino, Olimpia Vincentini, Vincenzo Cantatore, Ivana Cavoski, and Marco Gobbetti. 2019. "Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury" Nutrients 11, no. 2: 248. https://doi.org/10.3390/nu11020248
APA StyleDi Cagno, R., Filannino, P., Vincentini, O., Cantatore, V., Cavoski, I., & Gobbetti, M. (2019). Fermented Portulaca oleracea L. Juice: A Novel Functional Beverage with Potential Ameliorating Effects on the Intestinal Inflammation and Epithelial Injury. Nutrients, 11(2), 248. https://doi.org/10.3390/nu11020248






