Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women
Abstract
1. Introduction
2. Materials and Methods
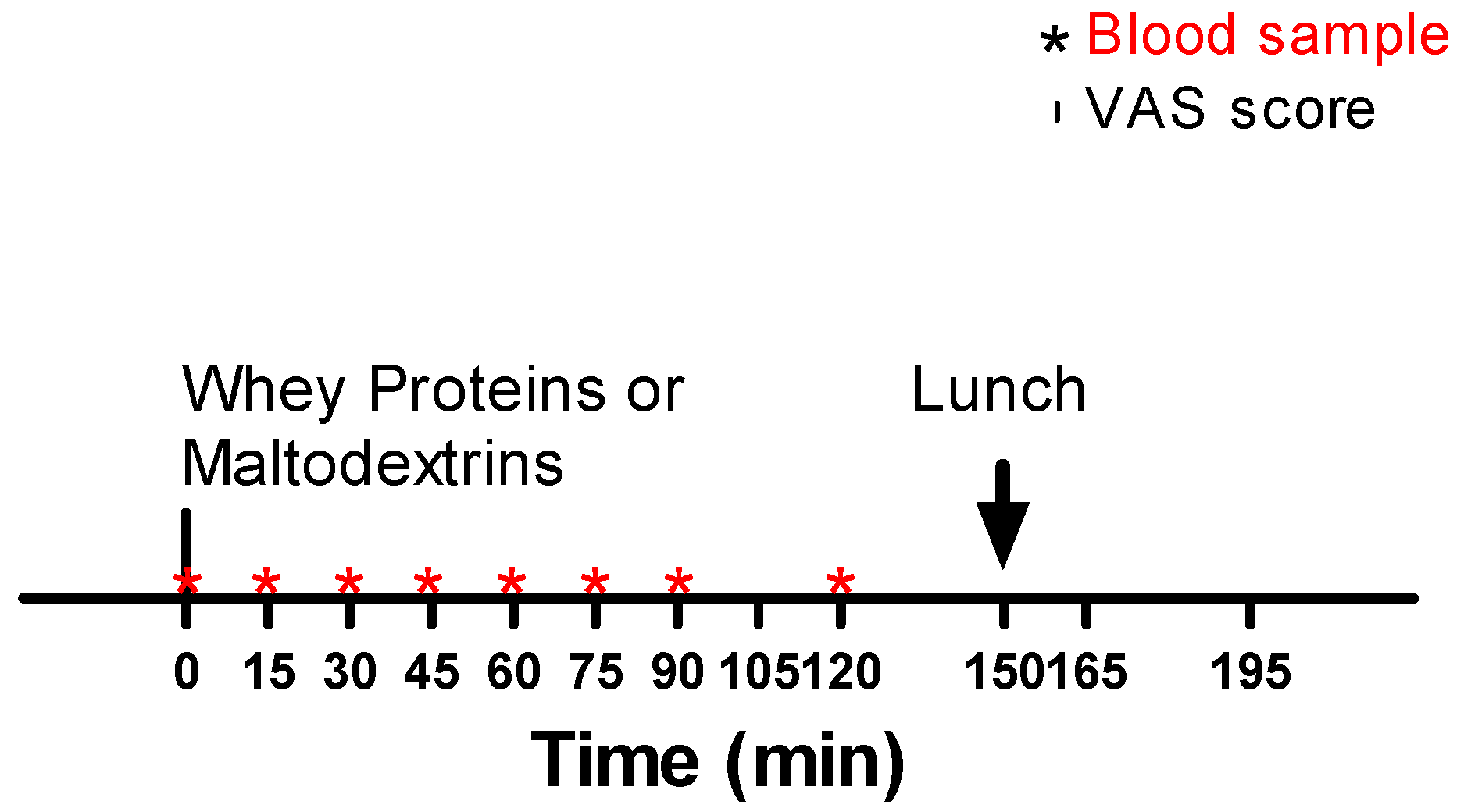
2.1. Patients and Experimental Protocol
2.2. Evaluation of Body Composition
2.3. Blood Sampling and Biochemical Measurements
2.4. Ethics Approval and Consent to Participate
2.5. Statistical Analyses
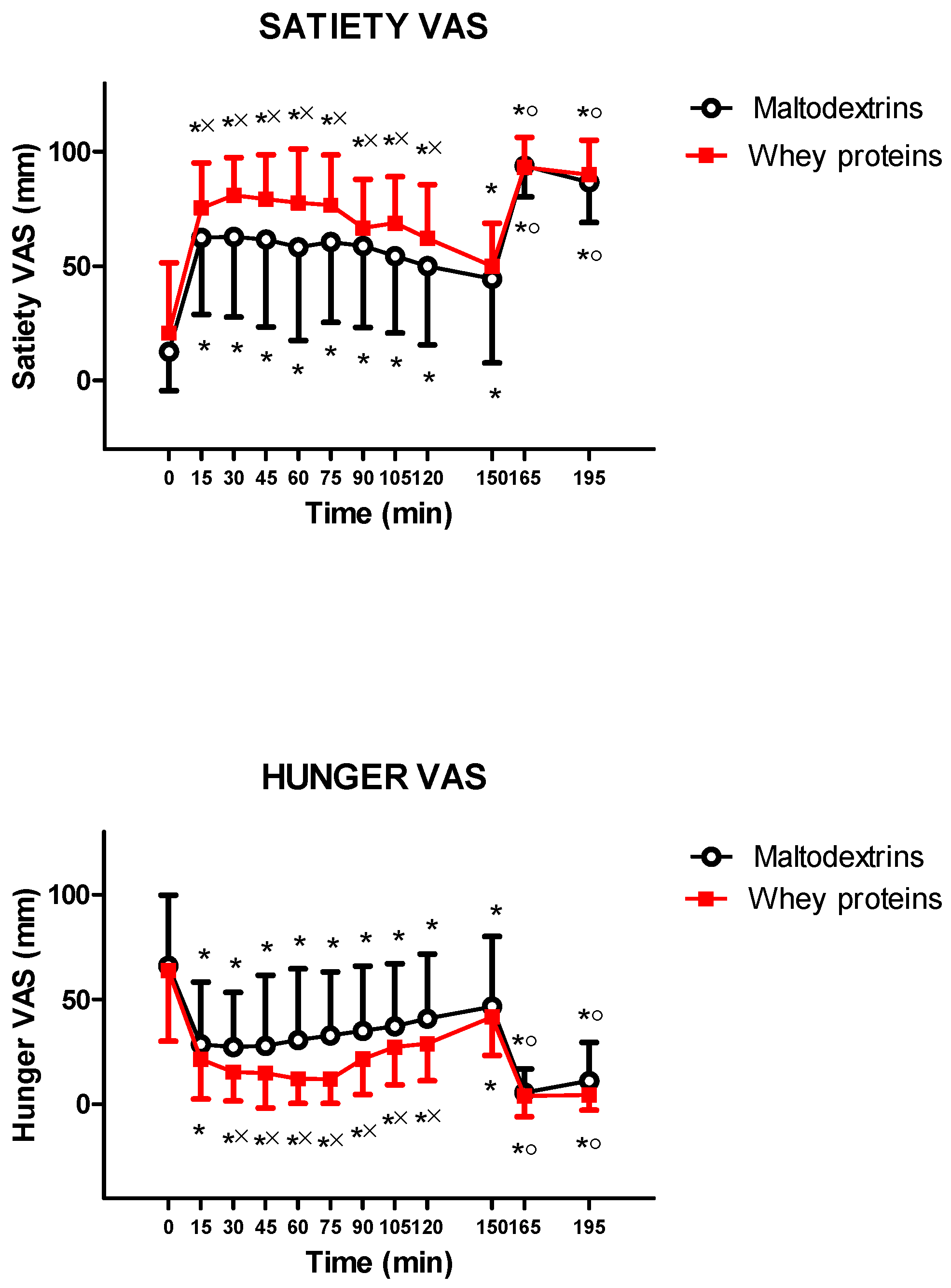
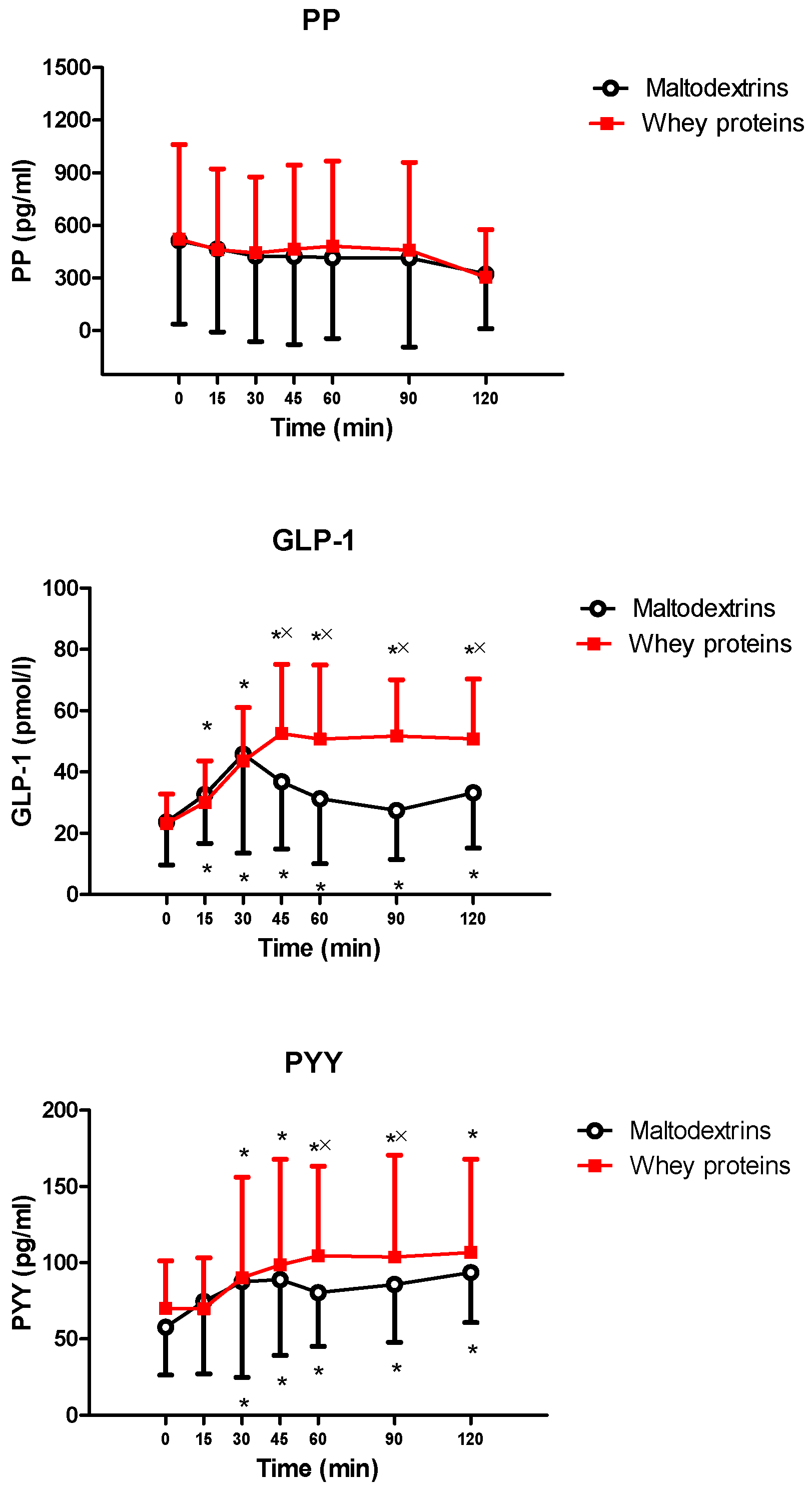
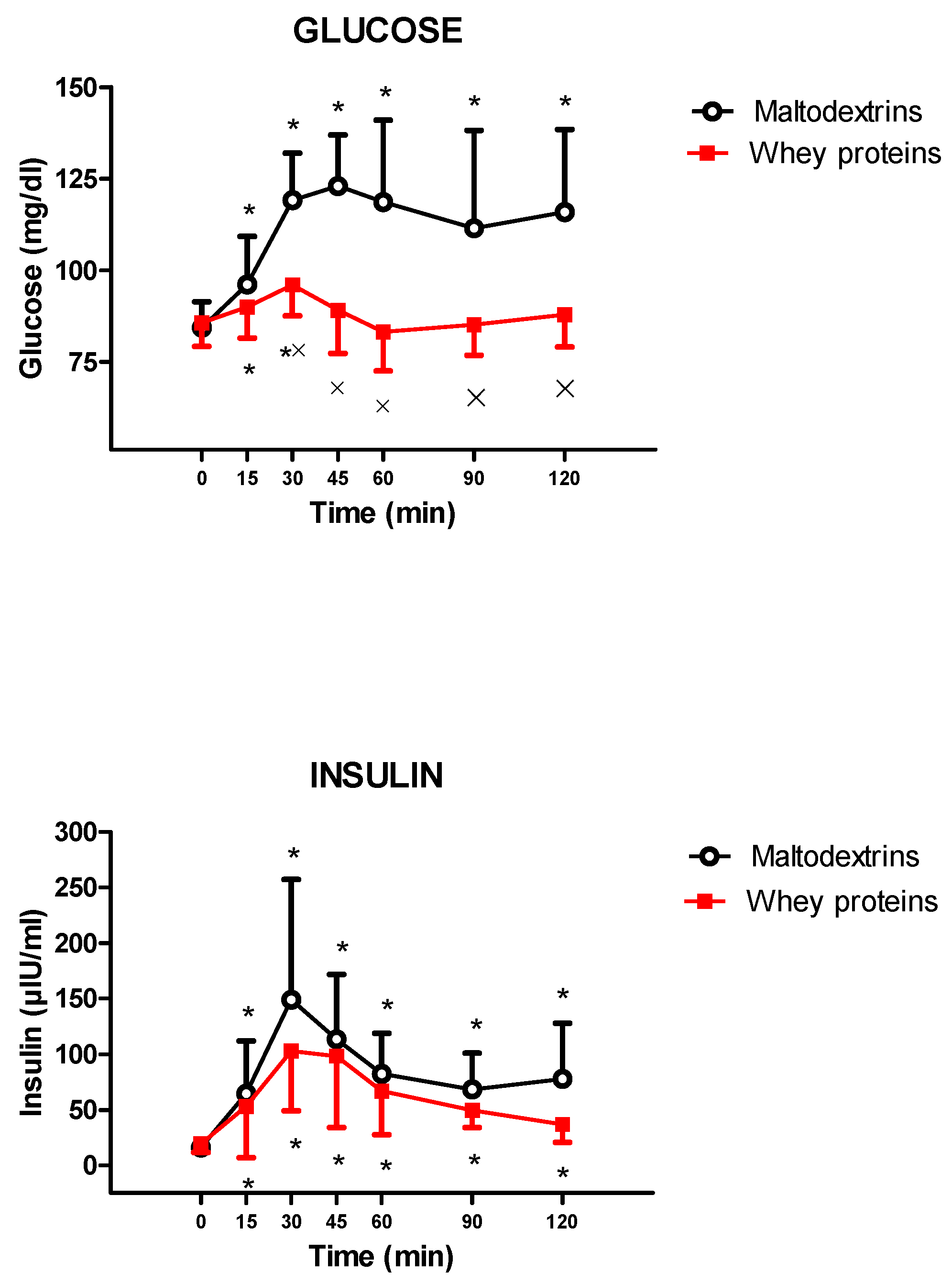
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | branched-chain amino acid |
| BMI | body mass index |
| CCK | cholecystokinin |
| CV | coefficient of variation |
| DPP-IV | dipeptidyl protease IV |
| FFM | fat-free mass |
| FM | fat mass |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide 1 |
| GMP | glycomacropeptide |
| PP | pancreatic polypeptide |
| PYY | peptide YY |
| sd | standard deviation |
| VAS | visual analogic scale |
References
- Brown, L.; Poudyal, H.; Panchal, S.K. Functional foods as potential therapeutic options for metabolic syndrome. Obes. Rev. 2015, 16, 914–941. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.Q.; Lorenzen, J.K.; Bendsen, N.T.; Rasmussen, C.; Astrup, A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: A review of the evidence from controlled clinical trials. Adv. Nutr. 2013, 4, 418–438. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.; Moughan, P.J.; Henare, S.J.; Ganesh, S. Effect of time of consumption of preloads on measures of satiety in healthy normal weight women. Appetite 2012, 59, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Effect of whey protein and glycomacropeptide on measures of satiety in normal-weight adult women. Appetite 2014, 78, 172–178. [Google Scholar] [CrossRef]
- Chungchunlam, S.M.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Dietary whey protein influences plasma satiety-related hormones and plasma amino acids in normal-weight adult women. Eur. J. Clin. Nutr. 2015, 69, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O. Biochemical and metabolic mechanisms by which dietary whey protein may combat obesity and Type 2 diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Akhavan, T.; Anderson, G.H. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr. 2007, 26, 704S–712S. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Read, R.S. Dietary supplements in sport. Sports Med. 1993, 15, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Kahlhöfer, J.; Karschin, J.; Silberhorn-Bühler, H.; Breusing, N.; Bosy-Westphal, A. Effect of low-glycemic-sugar-sweetened beverages on glucose metabolism and macronutrient oxidation in healthy men. Int. J. Obes. 2016, 40, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.L.; Broughton, K.S. Insulinotropic Effects of Whey: Mechanisms of Action, Recent Clinical Trials, and Clinical Applications. Ann. Nutr. Metab. 2016, 69, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child Obes. 2015, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Frantsve-Hawley, J.; Bader, J.D.; Welsh, J.A.; Wright, J.T. A systematic review of the association between consumption of sugar-containing beverages and excess weight gain among children under age 12. J. Public Health Dent. 2017, 77 (Suppl. 1), S43–S66. [Google Scholar] [CrossRef]
- Serdula, M.K.; Ivery, D.; Coates, R.J.; Freedman, D.S.; Williamson, D.F.; Byers, T. Do obese children become obese adults? A review of the literature. Prev. Med. 1993, 22, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.P.; Chan, R.S.; Nelson, E.A.; Chan, J.C. Role of low-glycemic index diet in management of childhood obesity. Obes. Rev. 2011, 12, 492–498. [Google Scholar] [CrossRef]
- Bellissimo, N.; Desantadina, M.V.; Pencharz, P.B.; Berall, G.B.; Thomas, S.G.; Anderson, G.H. A comparison of short-term appetite and energy intakes in normal weight and obese boys following glucose and whey-protein drinks. Int. J. Obes. 2008, 32, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Larnkjaer, A.; Arnberg, K.; Michaelsen, K.F.; Jensen, S.M.; Mølgaard, C. Effect of increased intake of skimmed milk, casein, whey or water on body composition and leptin in overweight adolescents: A randomized trial. Pediatr. Obes. 2015, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Anderson, G.H.; Vien, S.; Bellissimo, N.; McCrindle, B.W.; Hamilton, J.K. Obesity, sex and pubertal status affect appetite hormone responses to a mixed glucose and whey protein drink in adolescents. Clin. Endocrinol. 2014, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Resnik, M.; Compri, E.; Agosti, F.; De Col, A.; Monteleone, P.; Marazzi, N.; Bonomo, S.M.; Müller, E.E.; Sartorio, A. The cholestyramine-induced decrease of PYY postprandial response is negatively correlated with fat mass in obese women. Horm. Metab. Res. 2011, 43, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Agosti, F.; Compri, E.; Giunta, M.; Marazzi, N.; Muller, E.E.; Cella, S.G.; Sartorio, A. Anorexigenic postprandial responses of PYY and GLP1 to slow ice cream consumption: Preservation in obese adolescents, but not in obese adults. Eur. J. Endocrinol. 2013, 168, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Piscitelli, F.; Aveta, T.; Agosti, F.; De Col, A.; Bini, S.; Cella, S.G.; Di Marzo, V.; Sartorio, A. Anticipatory and consummatory effects of (hedonic) chocolate intake are associated with increased circulating levels of the orexigenic peptide ghrelin and endocannabinoids in obese adults. Food Nutr. Res. 2015, 59, 29678. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.M.; Tan, T.M.; Bloom, S.R. Gastrointestinal hormones and their role in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Tringali, G.; De Micheli, R.; Abbruzzese, L.; Bortolotti, M.; et al. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr. Res. 2018, 52, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Locatelli, L.; Cella, S.G.; Bonomo, S.M.; Giunta, M.; Molinari, F.; Sartorio, A.; Müller, E.E. Muscle expressions of MGF, IGF-IEa, and myostatin in intact and hypophysectomized rats: Effects of rhGH and testosterone alone or combined. Horm. Metab. Res. 2009, 41, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Giunta, M.; Rigamonti, A.E.; Agosti, F.; Patrizi, A.; Compri, E.; Cardinale, M.; Sartorio, A. Combination of external load and whole body vibration potentiates the GH-releasing effect of squatting in healthy females. Horm. Metab. Res. 2013, 45, 611–616. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging health properties of whey proteins and their clinical implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef]
- Zafar, T.A.; Waslien, C.; Al Raefaei, A.; Alrashidi, N.; Al Mahmoud, E. Whey protein sweetened beverages reduce glycemic and appetite responses and food intake in young females. Nutr. Res. 2013, 33, 303–310. [Google Scholar] [CrossRef]
- Akhavan, T.; Luhovyy, B.L.; Anderson, G.H. Effect of drinking compared with eating sugars or whey protein on short-term appetite and food intake. Int. J. Obes. 2011, 35, 562–569. [Google Scholar] [CrossRef]
- Mayer, K. Childhood obesity prevention: Focusing on the community food environment. Fam. Community Health 2009, 32, 257–270. [Google Scholar] [CrossRef]
- Giezenaar, C.; Luscombe-Marsh, N.D.; Hutchison, A.T.; Standfield, S.; Feinle-Bisset, C.; Horowitz, M.; Chapman, I.; Soenen, S. Dose-Dependent Effects of Randomized Intraduodenal Whey-Protein Loads on Glucose, Gut Hormone, and Amino Acid Concentrations in Healthy Older and Younger Men. Nutrients 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.L. Sugars and health: A review of current evidence and future policy. Proc. Nutr. Soc. 2017, 76, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wellendorph, P.; Johansen, L.D.; Bräuner-Osborne, H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol. Pharmacol. 2009, 76, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, A.L.; Calderwood, D.; Hobson, L.; Green, B.D. Whey proteins have beneficial effects on intestinal enteroendocrine cells stimulating cell growth and increasing the production and secretion of incretin hormones. Food Chem. 2015, 189, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Caron, J.; Cudennec, B.; Domenger, D.; Belguesmia, Y.; Flahaut, C.; Kouach, M.; Lesage, J.; Goossens, J.F.; Dhulster, P.; Ravallec, R. Simulated GI digestion of dietary protein: Release of new bioactive peptides involved in gut hormone secretion. Food Res. Int. 2016, 89 Pt 1, 382–390. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Tomé, D.; Gaudichon, C.; Recio, I. Stimulation of CCK and GLP-1 secretion and expression in STC-1 cells by human jejunal contents and in vitro gastrointestinal digests from casein and whey proteins. Food Funct. 2018, 9, 4702–4713. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Burhol, P.G. Fasting and postprandial plasma pancreatic polypeptide (PP) levels in obesity. Int. J. Obes. 1984, 8, 393–397. [Google Scholar]
- Holst, J.J.; Schwartz, T.W.; Lovgreen, N.A.; Pedersen, O.; Beck-Nielsen, H. Diurnal profile of pancreatic polypeptide, pancreatic glucagon, gut glucagon and insulin in human morbid obesity. Int. J. Obes. 1983, 7, 529–538. [Google Scholar]
- Lassmann, V.; Vague, P.; Vialettes, B.; Simon, M.C. Low plasma levels of pancreatic polypeptide in obesity. Diabetes 1980, 29, 428–4230. [Google Scholar] [CrossRef]
- Inui, A.; Mizuno, N.; Ooya, M.; Suenaga, K.; Morioka, H.; Ogawa, T.; Ishida, M.; Baba, S. Cross-reactivities of neuropeptide Y and peptide YY with pancreatic polypeptide antisera: Evidence for the existence of pancreatic polypeptide in the brain. Brain Res. 1985, 330, 386–389. [Google Scholar] [CrossRef]
- Gao, Z.; Young, R.A.; Li, G.; Najafi, H.; Buettger, C.; Sukumvanich, S.S.; Wong, R.K.; Wolf, B.A.; Matschinsky, F.M. Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells. Endocrinology 2003, 144, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Dean, H.J.; Sellers, E.A. Children have type 2 diabetes too: An historical perspective. Biochem. Cell Biol. 2015, 93, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. Can milk proteins be a useful tool in the management of cardiometabolic health? An updated review of human intervention trials. Proc. Nutr. Soc. 2016, 75, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Leoncini, R.; Casnici, C.; Marelli, O.; De Col, A.; Tamini, S.; Lucchetti, E.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; et al. Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women. Nutrients 2019, 11, 247. https://doi.org/10.3390/nu11020247
Rigamonti AE, Leoncini R, Casnici C, Marelli O, De Col A, Tamini S, Lucchetti E, Cicolini S, Abbruzzese L, Cella SG, et al. Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women. Nutrients. 2019; 11(2):247. https://doi.org/10.3390/nu11020247
Chicago/Turabian StyleRigamonti, Antonello E., Roberto Leoncini, Claudia Casnici, Ornella Marelli, Alessandra De Col, Sofia Tamini, Elisa Lucchetti, Sabrina Cicolini, Laura Abbruzzese, Silvano G. Cella, and et al. 2019. "Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women" Nutrients 11, no. 2: 247. https://doi.org/10.3390/nu11020247
APA StyleRigamonti, A. E., Leoncini, R., Casnici, C., Marelli, O., De Col, A., Tamini, S., Lucchetti, E., Cicolini, S., Abbruzzese, L., Cella, S. G., & Sartorio, A. (2019). Whey Proteins Reduce Appetite, Stimulate Anorexigenic Gastrointestinal Peptides and Improve Glucometabolic Homeostasis in Young Obese Women. Nutrients, 11(2), 247. https://doi.org/10.3390/nu11020247





