Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion
Abstract
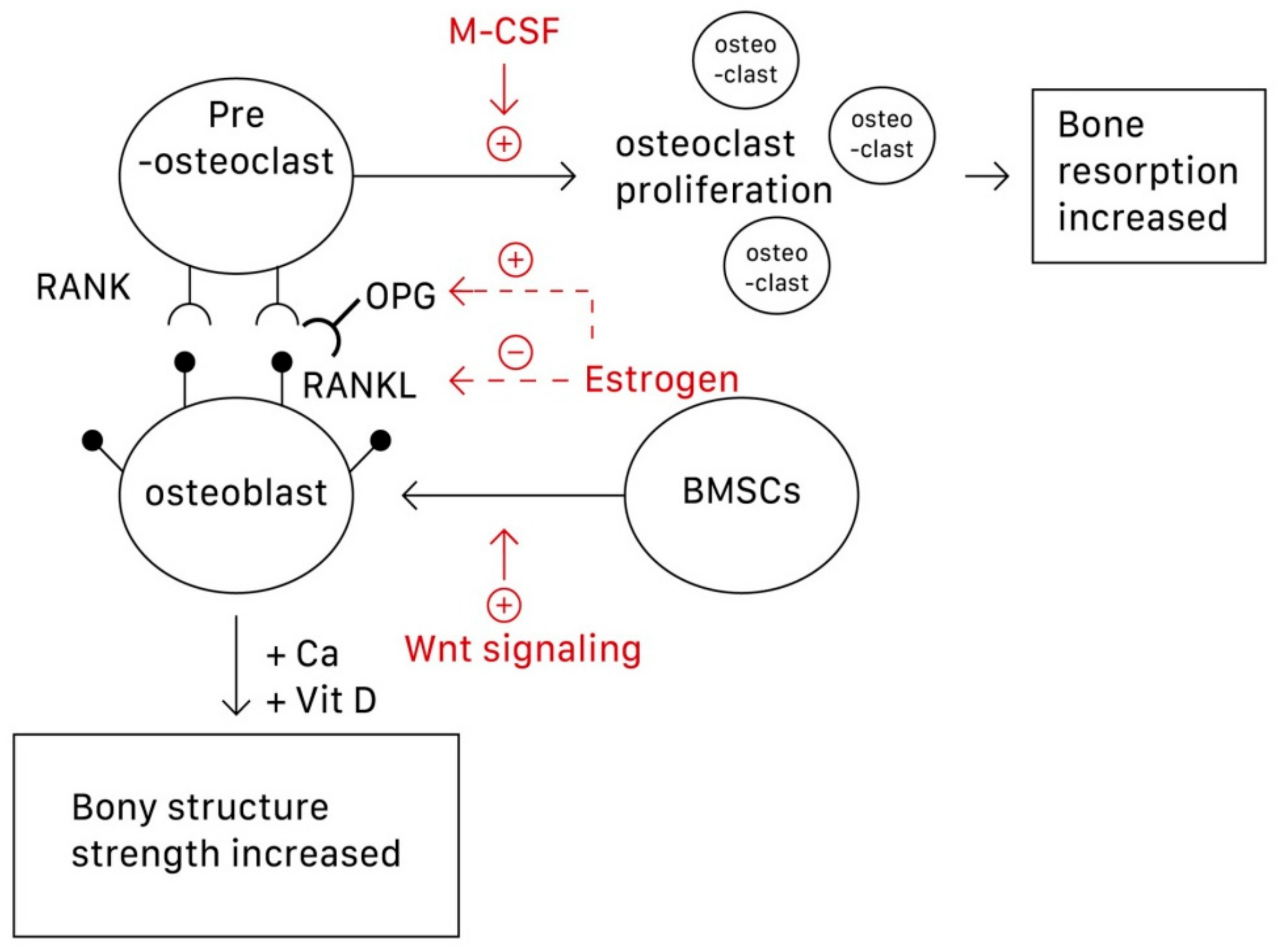
1. Introduction
2. Calcium and Vitamin D Supplementation
2.1. Calcium
2.2. Vitamin D
2.3. Combined Use of Calcium and Vitamin D
3. Lifestyle
4. Fall Prevention
5. Exercise
6. Physical Modalities
6.1. Low-Intensity Pulsed Ultrasound
6.2. Electrical Stimulation
6.3. Whole-Body Vibration
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.R.; Wen, Y.T.; Kuo, C.L.; Chen, K.H. Calcium and vitamin D supplementation on bone health: Current evidence and recommendations. Int. J. Gerontol. 2014, 8, 183–188. [Google Scholar] [CrossRef]
- Leboime, A.; Confavreux, C.B.; Mehsen, N.; Paccou, J.; David, C.; Roux, C. Osteoporosis and mortality. Jt. Bone Spine 2010, 77 (Suppl. 2), S107–S112. [Google Scholar] [CrossRef]
- Center, J.R.; Bliuc, D.; Nguyen, N.D.; Nguyen, T.V.; Eisman, J.A. Osteoporosis medication and reduced mortality risk in elderly women and men. J. Clin. Endocrinol. Metab. 2011, 96, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Black, D.M.; Rubin, S.M. Lifetime risks of hip, colles’, or vertebral fracture and coronary heart disease among white postmenopausal women. Arch. Intern. Med. 1989, 149, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Seeman, E.; Delmas, P.D. Bone Quality—The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.E.; Percin, G.I.; Muller, J.T.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.K.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019, 568, 541–545. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical treatment for osteoporosis: From molecular to clinical opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef]
- Amjadi-Moheb, F.; Akhavan-Niaki, H. Wnt signaling pathway in osteoporosis: Epigenetic regulation, interaction with other signaling pathways, and therapeutic promises. J. Cell Physiol. 2019, 28207, 1–10. [Google Scholar] [CrossRef]
- Gallagher, J.; Sai, A. Molecular biology of bone remodeling: Implications for new therapeutic targets for osteoporosis. Maturitas 2010, 65, 301–307. [Google Scholar] [CrossRef]
- Hou, Y.C.; Wu, C.C.; Liao, M.T.; Shyu, J.F.; Hung, C.F.; Yen, T.H.; Lu, C.L.; Lu, K.C. Role of nutritional vitamin D in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef]
- Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; Mishra, G.; Mueck, A.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; Stute, P.; et al. Calcium in the prevention of postmenopausal osteoporosis: EMAS clinical guide. Maturitas 2018, 107, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Adam, G.P.; Langberg, V.N. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J. Controversies in medicine: The role of calcium and vitamin D supplements in adults. Med. J. Aust. 2019, 211, 468–473. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zeng, X.T.; Wang, J.; Liu, L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: A systematic review and meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef]
- Palombaro, K.M.; Black, J.D.; Buchbinder, R.; Jette, D.U. Effectiveness of exercise for managing osteoporosis in women postmenopause. Phys. Ther. 2013, 93, 1021–1025. [Google Scholar] [CrossRef][Green Version]
- Kong, S.H.; Kim, J.H.; Hong, A.R.; Cho, N.H.; Shin, C.S. Dietary calcium intake and risk of cardiovascular disease, stroke, and fracture in a population with low calcium intake. Am. J. Clin. Nutr. 2017, 106, 27–34. [Google Scholar] [CrossRef]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium intake and risk of fracture: Systematic review. BMJ 2015, 351, 4580. [Google Scholar] [CrossRef]
- Chel, V.G.; Ooms, M.E.; Popp-Snijders, C.; Pavel, S.; Schothorst, A.A.; Meulemans, C.C.; Lips, P. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J. Bone Miner. Res. 1998, 13, 1238–1242. [Google Scholar] [CrossRef]
- Hanley, D.A.; Cranney, A.; Jones, G.; Whiting, S.J.; Leslie, W.D.; Cole, D.E.; Atkinson, S.A.; Josse, R.G.; Feldman, S.; Kline, G.A. Vitamin D in adult health and disease: A review and guideline statement from Osteoporosis Canada. CMAJ 2010, 182, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, G.; Holick, M.F. Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord. 2010, 11, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, B.; Moghimi, J.; Foroutan, M.; Mirmohammadkhani, M.; Ghorbani, A. Comparison of the effect of vitamin D on osteoporosis and osteoporotic patients with healthy individuals referred to the bone density measurement center. Biomol. Concepts 2019, 10, 44–50. [Google Scholar] [CrossRef]
- Dhaliwal, R.; Mikhail, M.; Usera, G.; Stolberg, A.; Islam, S.; Ragolia, L.; Aloia, J.F. The relationship of Physical performance and Osteoporosis prevention with vitamin D in older African Americans (PODA) Contemporary. Contemp. Clin. Trials 2018, 65, 39–45. [Google Scholar] [CrossRef]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength A Randomized Clinical Trial. JAMA 2019, 27, 736–745. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; et al. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Abrahamsen, B.; Masud, T.; Avenell, A.; Anderson, F.; Meyer, H.E.; Cooper, C.; Smith, H.; LaCroix, A.Z.; Torgerson, D.; Johansen, A.; et al. Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 2010, 12, 5463. [Google Scholar] [CrossRef]
- Crandall, C.J.; Aragaki, A.K.; LeBoff, M.S.; Li, W.; Wactawski-Wende, J.; Cauley, J.A.; Margolis, K.L.; Manson, J.E. Calcium plus vitamin D supplementation and height loss: Findings from the Women’s Health Initiative Calcium and vitamin D clinical trial. Menopause 2016, 23, 1277–1286. [Google Scholar] [CrossRef]
- Kai, M.C.; Anderson, M.; Lau, E. Exercise interventions: Defusing the world’s osteoporosis time bomb. Bull. World Health Organ. 2003, 81, 827–830. [Google Scholar]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Bruyere, O.; Devogelaer, J.P.; Goemaere, S.; Hollevoet, N.; Kaufman, J.M.; Milisen, K.; et al. Non-pharmacological management of osteoporosis: A consensus of the belgian bone club. Osteoporos. Int. 2011, 22, 2769–2788. [Google Scholar] [CrossRef] [PubMed]
- Hoidrup, S.; Gronbaek, M.; Gottschau, A.; Lauritzen, J.B.; Schroll, M. Alcohol intake, beverage preference, and risk of hip fracture in men and women. Copenhagen centre for prospective population studies. Am. J. Epidemiol. 1999, 149, 993–1001. [Google Scholar] [CrossRef]
- Kannus, P.; Sievänen, H.; Palvanen, M.; Järvinen, T.; Parkkari, J. Prevention of falls and consequent injuries in elderly people. Lancet 2005, 366, 1885–1893. [Google Scholar] [CrossRef]
- Morrison, A.; Fan, T.; Sen, S.S.; Weisenfluh, L. Epidemiology of falls and osteoporotic fractures: A systematic review. Clinicoecon. Outcomes Res. 2013, 5, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Woltman, K.; den Hoed, P.T. Osteoporosis in patients with a low-energy fracture: 3 years of screening in an osteoporosis outpatient clinic. J. Trauma 2010, 69, 169–173. [Google Scholar] [CrossRef]
- Carter, N.D.; Kannus, P. Exercise in the prevention of falls in older people. Sports Med. 2001, 31, 427–438. [Google Scholar] [CrossRef]
- Kannus, P. Preventing osteoporosis, falls, and fractures among elderly people: Promotion of lifelong physical activity is essential. BMJ 1999, 318, 205–206. [Google Scholar] [CrossRef]
- Lee, M.; Pittler, M.; Shin, B.C.; Ernst, E. Tai chi for osteoporosis: A systematic review. Osteoporos. Int. 2008, 19, 139–146. [Google Scholar] [CrossRef]
- Maciaszek, J.; Osiński, W.; Szeklicki, R.; Stemplewski, R. Effect of tai chi on body balance: Randomized controlled trial in men with osteopenia or osteoporosis. Am. J. Chin. Med. 2007, 35, 1–9. [Google Scholar] [CrossRef]
- Qin, L.; Choy, W.; Leung, K.; Leung, P.C.; Au, S.; Hung, W.; Dambacher, M.; Chan, K. Beneficial effects of regular tai chi exercise on musculoskeletal system. J. Bone Miner. Metab. 2005, 23, 186–190. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, P.D.; Cran, G.W.; Beringer, T.R.; Kernohan, G.; Orr, J.; Dunlop, L.; Murray, L.J. Factors affecting adherence to use of hip protectors amongst residents of nursing homes—A correlation study. Int. J. Nurs. Stud. 2007, 44, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Gass, M.; Dawson-Hughes, B. Preventing osteoporosis-related fractures: An overview. Am. J. Med. 2006, 119, 3–11. [Google Scholar] [CrossRef]
- Järvinen, T.L.; Sievänen, H.; Khan, K.M.; Heinonen, A.; Kannus, P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ 2008, 336, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Chen, Y.C.; Yang, R.S.; Tsauo, J.Y. Effects of exercise programmes on quality of life in osteoporotic and osteopenic postmenopausal women: A systematic review and meta-analysis. Clin. Rehabil 2009, 23, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Borer, K.T. Physical activity in the prevention and amelioration of osteoporosis in women: Interaction of mechanical, hormonal and dietary factors. Sports Med. 2005, 35, 779–830. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Nsir, H.; Imbesi, R.; Musumeci, G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol. Histopathol. 2016, 31, 1183–1194. [Google Scholar]
- ACSM Information on Resistance Training for Health and Fitness. Available online: https://www.prescriptiontogetactive.com/app/uploads/resistance-training-ACSM.pdf (accessed on 26 October 2019).
- Frontera, W.R.; DeLisa, J.A.; Basford, J.R.; Bockenek, W.L.; Chae, J.; Robinson, L.R. DeLisa’s Physical Medicine and Rehabilitation: Principles and Practice, 6th ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2019; p. 1115. [Google Scholar]
- Gusi, N.; Raimundo, A.; Leal, A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC Musculoskelet. Disord. 2006, 7, 92. [Google Scholar] [CrossRef]
- Forwood, M.R.; Burr, D.B. Physical activity and bone mass: Exercises in futility? Bone Miner. 1993, 21, 89–112. [Google Scholar] [CrossRef]
- Shigeta, H.; Goto, S.; Hyakutake, S. Effect of regular exercise on bone mineral density in pre, peri, and postmenopausal women. Osteoporos. Int. 1996, 6, 238. [Google Scholar] [CrossRef]
- Nikander, R.; Sievanen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Sinaki, M.; Pfeifer, M.; Preisinger, E.; Itoi, E.; Rizzoli, R.; Boonen, S.; Geusens, P.; Minne, H.W. The role of exercise in the treatment of osteoporosis. Curr. Osteoporos. Rep. 2010, 8, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J.; Chrischilles, E.A.; Cooper, C.; Lane, A.W.; Riggs, B.L. How many women have osteoporosis? J. Bone Miner. Res. 2005, 20, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, W.M.; Bloomfield, S.A.; Little, K.D.; Nelson, M.E.; Yingling, V.R. American College of Sports Medicine Position Stand: Physical activity and bone health. Med. Sci. Sports Exerc. 2004, 36, 1985–1996. [Google Scholar] [CrossRef]
- Lirani-Galvao, A.P.; Lazaretti-Castro, M. Physical approach for prevention and treatment of osteoporosis. Arq. Bras. Endocrinol. Metabol. 2010, 54, 171–178. [Google Scholar] [CrossRef]
- Monici, M.; Bernabei, P.A.; Basile, V.B.; Giovanni, R.; Antonio, C.; Luca, B.; Leonardo, M.; Augusto, C. Can ultrasound counteract bone loss? Effect of low-intensity ultrasound stimulation on a model of osteoclastic precursor. Acta Astronaut. 2007, 60, 383–390. [Google Scholar] [CrossRef]
- Unsworth, J.; Kaneez, S.; Harris, S.; Ridgway, J.; Fenwick, S.; Chenery, D.; Harrison, A. Pulsed low intensity ultrasound enhances mineralisation in preosteoblast cells. Ultrasound Med. Biol. 2007, 33, 1468–1474. [Google Scholar] [CrossRef]
- Yang, R.S.; Lin, W.L.; Chen, Y.Z.; Tang, C.H.; Huang, T.H.; Lu, B.Y.; Fu, W.M. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone 2005, 36, 276–283. [Google Scholar] [CrossRef]
- Azuma, Y.; Ito, M.; Harada, Y.; Takagi, H.; Ohta, T.; Jingushi, S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J. Bone Miner. Res. 2001, 16, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Hantes, M.E.; Mavrodontidis, A.N.; Zalavras, C.G.; Karantanas, A.H.; Karachalios, T.; Malizos, K.N. Low-intensity transosseous ultrasound accelerates osteotomy healing in a sheep fracture model. J. Bone Jt. Surg. Am. 2004, 86, 2275–2282. [Google Scholar] [CrossRef] [PubMed]
- Pilla, A.A.; Mont, M.A.; Nasser, P.R. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J. Orthop. Trauma 1990, 4, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, A.; Inui, K.; Azuma, Y.; Khan, S.A.; Figueiredo, M.; Kaufman, J.J.; Siffert, R.S. Low-intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbits. J. Bone Jt. Surg. Br. 2000, 82, 1077–1082. [Google Scholar] [CrossRef]
- Rutten, S.; Nolte, P.A.; Korstjens, C.M.; van Duin, M.A.; Klein-Nulend, J. Low-intensity pulsed ultrasound increases bone volume, osteoid thickness and mineral apposition rate in the area of fracture healing in patients with a delayed union of the osteotomized fibula. Bone 2008, 43, 348–354. [Google Scholar] [CrossRef]
- Kristiansen, T.K.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Roe, L.R. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study. J. Bone Jt. Surg. Am. 1997, 79, 961–973. [Google Scholar] [CrossRef]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J. Bone Jt. Surg. Am. 1994, 76, 26–34. [Google Scholar] [CrossRef]
- Carvalho, D.C.; Cliquet Junior, A. The action of low-intensity pulsed ultrasound in bones of osteopenic rats. Artif. Organs. 2004, 28, 114–118. [Google Scholar] [CrossRef]
- Perry, M.J.; Parry, L.K.; Burton, V.J.; Gheduzzi, S.; Beresford, J.N.; Humphrey, V.F.; Skerry, T.M. Ultrasound mimics the effect of mechanical loading on bone formation in vivo on rat ulnae. Med. Eng. Phys. 2009, 31, 42–47. [Google Scholar] [CrossRef]
- Warden, S.; Bennell, K.; Matthews, B.; Brown, D.J.; McMeeken, J.M.; Wark, J.D. Efficacy of low-intensity pulsed ultrasound in the prevention of osteoporosis following spinal cord injury. Bone 2001, 29, 431–436. [Google Scholar] [CrossRef]
- Marsh, D. Concepts of fracture union, delayed union, and uonunion. Clin. Orthop. Relat. Res. 1998, 355, 20–30. [Google Scholar]
- Brighton, C.T.; Wang, W.; Seldes, R.; Zhang, G.; Pollack, S.R. Signal transduction in electrically stimulated bone cells. J. Bone Jt. Surg. Am. 2001, 83, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Viapiana, O.; Gatti, D.; Zhang, G.; Pollack, S.R. Capacitively coupled electric field for pain relief in patients with vertebral fractures and chronic pain. Clin. Orthop. Relat. Res. 2010, 468, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Tabrah, F.; Hoffmeier, M.; Gilbert, F., Jr.; Batkin, S.; Bassett, C.A. Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (pemfs). J. Bone Miner. Res. 1990, 5, 437–442. [Google Scholar] [CrossRef]
- Giordano, N.; Battisti, E.; Geraci, S.; Marco, F.; Clorinda, S.; Mario, R.; Luigi, G.; Carlo, G. Effect of electromagnetic fields on bone mineral density and biochemical markers of bone turnover in osteoporosis: A single-blind, randomized pilot study. Curr. Ther. Res. 2001, 62, 187–193. [Google Scholar] [CrossRef]
- Lirani-Galvao, A.P.; Bergamaschi, C.T.; Silva, O.L.; Lazaretti-Castro, M. Electrical field stimulation improves bone mineral density in ovariectomized rats. Braz. J. Med. Biol. Res. 2006, 39, 1501–1505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trock, D.H. Electromagnetic fields and magnets: Investigational treatment for musculoskeletal disorders. Rheum. Dis. Clin. North Am. 2000, 26, 51–62. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, D.M.; Wang, C.T. Review of bone remodeling criteria in numerical simulation. J. Med. Biomech. 2007, 22, 417–422. [Google Scholar]
- Hsieh, Y.F.; Turner, C.H. Effects of loading frequency on mechanically induced bone formation. J. Bone Miner. Res. 2001, 16, 918–924. [Google Scholar] [CrossRef]
- Rubin, C.; Li, C.; Sun, Y.; Fritton, C.; McLeod, K. Non-invasive stimulation of trabecular bone formation via low magnitude, high frequency strain. Trans. ORS. 1995, 20, 548. [Google Scholar]
- Flieger, J.; Karachalios, T.; Khaldi, L.; Raptou, P.; Lyritis, G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif. Tissue Int. 1998, 63, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Falempin, M.; In-Albon, S.F. Influence of brief daily tendon vibration on rat soleus muscle in non-weight-bearing situation. J. Appl. Physiol. 1999, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; Colli, R.; Introini, E.; Cardinale, M.; Tsarpela, O.; Madella, A.; Tihanyi, J.; Viru, A. Adaptive responses of human skeletal muscle to vibration exposure. Clin. Physiol. 1999, 19, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.; Rehfeld, G.; Resnicek, E. Balance training and exercise in geriatric patients. J. Musculoskelet. Neuronal Interact. 2000, 1, 61–65. [Google Scholar]
- Mester, J.; Kleinoder, H.; Yue, Z. Vibration training: Benefits and risks. J. Biomech. 2006, 39, 1056–1065. [Google Scholar] [CrossRef]
- Kiiski, J.; Heinonen, A.; Järvinen, T.L.; Kannus, P.; Sievänen, H. Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res. 2008, 23, 1318–1325. [Google Scholar] [CrossRef]
- Kiel, D.P.; Hannan, M.T.; Barton, B.A.; Bouxsein, M.L.; Sisson, E.; Lang, T.; Allaire, B.; Dewkett, D.; Carroll, D.; Magaziner, J.; et al. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: A randomized, placebo-controlled trial. J. Bone Miner. Res. 2015, 30, 1319–1328. [Google Scholar] [CrossRef]
- Burger, C.; Schade, V.; Lindner, C.; Radlinger, L.; Elfering, A. Stochastic resonance training reduces musculoskeletal symptoms in metal manufacturing workers: A controlled preventive intervention study. Work 2012, 42, 269–278. [Google Scholar]
- Van Nes, I.J.; Geurts, A.C.; Hendricks, H.T.; Duysens, J. Short-term effects of whole-body vibration on postural control in unilateral chronic stroke patients: Preliminary evidence. Am. J. Phys. Med. Rehabil. 2004, 83, 867–873. [Google Scholar] [CrossRef]
- Verschueren, S.M.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. 2004, 19, 352–359. [Google Scholar] [CrossRef]
- Griffin, M.J. Handbook of Human Vibration; Academic Press: London, UK, 1990. [Google Scholar]
- Kavounoudias, A.; Roll, R.; Roll, J.P. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci. Lett. 1999, 266, 181–184. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Gilhodes, J.; Roll, J. Vibration-induced postural posteffects. J. Neurophysiol. 1998, 79, 143–150. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-R.; Hou, P.-H.; Chen, K.-H. Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients 2019, 11, 2848. https://doi.org/10.3390/nu11122848
Chen L-R, Hou P-H, Chen K-H. Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients. 2019; 11(12):2848. https://doi.org/10.3390/nu11122848
Chicago/Turabian StyleChen, Li-Ru, Peng-Hsuan Hou, and Kuo-Hu Chen. 2019. "Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion" Nutrients 11, no. 12: 2848. https://doi.org/10.3390/nu11122848
APA StyleChen, L.-R., Hou, P.-H., & Chen, K.-H. (2019). Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients, 11(12), 2848. https://doi.org/10.3390/nu11122848





