Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty
Abstract
1. Introduction
2. Chronic Obstructive Pulmonary Disease
2.1. Assessment of CRF in COPD
2.2. Creatine Supplementation in COPD
2.3. Polyunsaturated Fatty Acid Supplementation in COPD
2.4. Micronutrient Supplementation in COPD
2.5. Dietary Nitrate Supplementation in COPD
2.6. Nutrition Support Supplementation in COPD
2.7. Summary of Nutrition Interventions in COPD
3. Heart Failure
3.1. Micronutrient Supplementation in HFrEF
3.2. Amino Acid Supplementation in HFrEF
3.3. Polyunsaturated Fatty Acid Supplementation in HFrEF
3.4. Nutrition Support Supplementation in HFrEF
3.5. Summary of Nutrition Interventions in HFrEF
3.6. Hypocaloric Diets in HFpEF
3.7. Unsaturated Fatty Acid Supplementation in HFpEF
3.8. Dietary Approaches to Stop Hypertension in HFpEF
3.9. Dietary Nitrate Supplementation in HFpEF
3.10. Summary of Nutrition Interventions in HFpEF
4. Obesity
4.1. Hypocaloric Diets in Obesity
4.2. Macronutrient Composition of Hypocaloric Diets in Obesity
4.3. Summary of Nutrition Interventions in Obesity
5. Sarcopenia and Frailty
5.1. Nutrition Interventions in Sarcopenia and Frailty
5.2. Dietary Patterns in Sarcopenia and Frailty
5.3. Protein Supplementation in Sarcopenia and Frailty
5.4. Summary of Nutrition Interventions in Sarcopenia and Frailty
6. Future Directions
7. Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Arena, R.; Borlaug, B.A.; Carbone, S.; Canada, J.M.; Kirkman, D.L.; Garten, R.; Rodriguez-Miguelez, P.; Guazzi, M.; Lavie, C.J.; et al. Exercise Intolerance in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2209–2225. [Google Scholar] [CrossRef]
- Mandsager, K.; Harb, S.; Cremer, P.; Phelan, D.; Nissen, S.E.; Jaber, W. Association of Cardiorespiratory Fitness with Long-term Mortality Among Adults Undergoing Exercise Treadmill Testing. JAMA Netw. Open 2018, 1, e183605. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Kaminsky, L.A.; Arena, R.; Blair, S.N.; Franklin, B.A.; Myers, J.; Ross, R. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog. Cardiovasc. Dis. 2017, 60, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.S.; Lee, I.-M.; Naito, H.; Kakigi, R.; Goto, S.; Kanazawa, M.; Okamoto, T.; Tsukamoto, K.; Muto, T.; Tanaka, H.; et al. Cardiorespiratory fitness, body mass index, and cancer mortality: A cohort study of Japanese men. BMC Public Health 2014, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Falk, R.S.; Heir, T.; Sandvik, L.; Vos, L.; Erikssen, J.E.; Tretli, S. Measured cardiorespiratory fitness and self-reported physical activity: Associations with cancer risk and death in a long-term prospective cohort study. Cancer Med. 2016, 5, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Myers, J.; Faselis, C.; Panagiotakos, D.B.; Doumas, M.; Pittaras, A.; Manolis, A.; Kokkinos, J.P.; Karasik, P.; Greenberg, M.; et al. Exercise capacity and mortality in older men: A 20-year follow-up study. Circulation 2010, 122, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; Imboden, M.T.; Arena, R.; Myers, J. Reference Standards for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing Using Cycle Ergometry: Data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) Registry. Mayo Clin. Proc. 2017, 92, 228–233. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Barlow, C.E.; DeFina, L.F.; Radford, N.B.; Berry, J.D.; Cooper, K.H.; Haskell, W.L.; Jones, L.W.; Lakoski, S.G. Cardiorespiratory Fitness and Long-Term Survival in “Low-Risk” Adults. J. Am. Heart Assoc. 2012, 1, e001354. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. HF-ACTION Investigators, for the Efficacy and Safety of Exercise Training in Patients with Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Carbone, S.; Kachur, S.; O’Keefe, E.L.; Elagizi, A. Effects of Physical Activity, Exercise, and Fitness on Obesity-Related Morbidity and Mortality. Curr. Sports Med. Rep. 2019, 18, 292–298. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Abbate, A.; Van Tassell, B.W.; Canada, J.M.; Dixon, D.L.; Arena, R.A.; Biondi-Zoccai, G. Pharmacologic and surgical interventions to improve functional capacity in heart failure. Heart Fail. Clin. 2015, 11, 117–124. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J.; Wildman, R.; Kleiner, S.; VanDusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International society of sports nutrition position stand: Diets and body composition. J. Int. Soc. Sports Nutr. 2017, 14, 16. [Google Scholar] [CrossRef]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Manson, J.E.; Prentice, R.L.; Aragaki, A.K.; Snetselaar, L.; Beresford, S.A.; Kuller, L.H.; Johnson, K.; Lane, D.; et al. Low-Fat Dietary Pattern and Cancer Mortality in the Women’s Health Initiative (WHI) Randomized Controlled Trial. JNCI Cancer Spectr. 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017, 92, 266–279. [Google Scholar] [CrossRef]
- Carbone, S.; Billingsley, H.E.; Rodriguez-Miguelez, P.; Kirkman, D.L.; Garten, R.; Franco, R.L.; Lee, D.; Lavie, C.J. Lean Mass Abnormalities in Heart Failure: The Role of Sarcopenia, Sarcopenic Obesity, and Cachexia. Curr. Probl. Cardiol. 2019, S0146–S2806. [Google Scholar] [CrossRef]
- Cote, C.G.; Pinto-Plata, V.; Kasprzyk, K.; Dordelly, L.J.; Celli, B.R. The 6-Min Walk Distance, Peak Oxygen Uptake, and Mortality in COPD. Chest 2007, 132, 1778–1785. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Polkey, M.I.; Spruit, M.A.; Edwards, L.D.; Watkins, M.L.; Pinto-Plata, V.; Vestbo, J.; Calverley, P.M.A.; Tal-Singer, R.; Agustí, A.; Bakke, P.S.; et al. Six-Minute-Walk Test in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 382–386. [Google Scholar] [CrossRef]
- Hernandes, N.A.; Wouters, E.F.M.; Meijer, K.; Annegarn, J.; Pitta, F.; Spruit, M.A. Reproducibility of 6-minute walking test in patients with COPD. Eur. Respir. J. 2011, 38, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.J.; Vincent, E.E.; Greenhaff, P.L.; Fox, J.; Steiner, M.C.; Singh, S.J.; Morgan, M.D. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 233–239. [Google Scholar] [CrossRef]
- Fuld, J.P.; Kilduff, L.P.; Neder, J.A.; Pitsiladis, Y.; Lean ME, J.; Ward, S.A.; Cotton, M.M. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2005, 60, 531–537. [Google Scholar] [CrossRef]
- Faager, G.; Soderlund, K.; Skold, C.M.; Rundgren, S.; Tollback, A.; Jakobsson, P. Creatine supplementation and physical training in patients with COPD: A double blind, placebo-controlled study. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 445–453. [Google Scholar] [CrossRef]
- Broekhuizen, R.; Wouters, E.F.M.; Creutzberg, E.C.; Weling-Scheepers, C.A.P.M.; Schols, A.M.W.J. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 2005, 60, 376–382. [Google Scholar] [CrossRef]
- Kerley, C.P.; Cahill, K.; Bolger, K.; McGowan, A.; Burke, C.; Faul, J.; Cormican, L. Dietary nitrate supplementation in COPD: An acute, double-blind, randomized, placebo-controlled, crossover trial☆. Nitric Oxide 2015, 44, 105–111. [Google Scholar] [CrossRef]
- Friis, A.L.; Steenholt, C.B.; Løkke, A.; Hansen, M. Dietary beetroot juice-effects on physical performance in COPD patients: A randomized controlled crossover trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1765–1773. [Google Scholar] [CrossRef]
- Behnia, M.; Wheatley, C.M.; Avolio, A.; Johnson, B.D. Influence of dietary nitrate supplementation on lung function and exercise gas exchange in COPD patients. Nitric Oxide 2018, 76, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A secondary analysis of a randomized trial. Respir. Res. 2012, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Muller, P.T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.C.; Barton, R.L.; Singh, S.J.; Morgan MD, L. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2003, 58, 745–751. [Google Scholar] [CrossRef] [PubMed]
- van de Bool, C.; Rutten EP, A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A.M.W.J. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia. Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Yamada, K.; Yanagida, S.; Homma, M.; Dairiki, K.; Sasaki, H.; Kawagoshi, A.; Satake, M.; et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012, 106, 1526–1534. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef]
- Gurgun, A.; Deniz, S.; Argin, M.; Karapolat, H. Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: A prospective, randomized and controlled study. Respirology 2013, 18, 495–500. [Google Scholar] [CrossRef]
- van Wetering, C.R.; Hoogendoorn, M.; Broekhuizen, R.; Geraerts-Keeris, G.J.W.; De Munck, D.R.A.J.; Rutten-van Molken, M.P.M.H.; Schols, A.M.W.J. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: A prespecified subgroup analysis of the INTERCOM trial. J. Am. Med. Dir. Assoc. 2010, 11, 179–187. [Google Scholar] [CrossRef]
- Greenhaff, P.L. Creatine and its application as an ergogenic aid. Int. J. Sport Nutr. 1995, 5, S100–S110. [Google Scholar] [CrossRef] [PubMed]
- Fiaccadori, E.; Del Canale, S.; Vitali, P.; Coffrini, E.; Ronda, N.; Guariglia, A. Skeletal muscle energetics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypoxemia. Chest 1987, 92, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Popovic, D.; Lavie, C.J.; Arena, R. Obesity, body composition and cardiorespiratory fitness in heart failure with preserved ejection fraction. Future Cardiol. 2017, 13, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Brose, A.; Parise, G.; Tarnopolsky, M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 11–19. [Google Scholar] [CrossRef] [PubMed]
- de Batlle, J.; Sauleda, J.; Balcells, E.; Gomez, F.P.; Mendez, M.; Rodriguez, E.; Barreiro, E.; Ferrer, J.J.; Romieu, I.; Gea, J.; et al. Association between Omega3 and Omega6 fatty acid intakes and serum inflammatory markers in COPD. J. Nutr. Biochem. 2012, 23, 817–821. [Google Scholar] [CrossRef]
- Fernández-Lahera, J.; Romera, D.; Gómez Mendieta, A.; Martínez Verdasco, A.; Fernández-Bujarrabal, J.; Santiago, A.; Alcolea, S.; Martínez-Abad, Y.; Prados, C.; Villasante, C.; et al. Prevalence of vitamin D deficiency in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2015, 46, PA3977. [Google Scholar]
- Jung, J.Y.; Kim, Y.S.; Kim, S.K.; Kim, H.Y.; Oh, Y.M.; Lee, S.M.; Seo, J.B.; Lee, S.-D. Relationship of vitamin D status with lung function and exercise capacity in COPD. Respirology 2015, 20, 782–789. [Google Scholar] [CrossRef]
- Veeranki, S.; Winchester, L.J.; Tyagi, S.C. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim. Biophys. Acta 2015, 1852, 732–741. [Google Scholar] [CrossRef]
- Kuo, H.-K.; Liao, K.-C.; Leveille, S.G.; Bean, J.F.; Yen, C.-J.; Chen, J.-H.; Yu, Y.-H.; Tai, T.-Y. Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 434–439. [Google Scholar] [CrossRef]
- Breese, B.C.; McNarry, M.A.; Marwood, S.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1441–R1450. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Fulford, J.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of walking and running: A placebo-controlled study. J. Appl. Physiol. 2011, 110, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Justus, N.W.; Hauser, J.I.; Case, A.H.; Helms, C.C.; Basu, S.; Rogers, Z.; Lewis, M.T.; Miller, G.D. Dietary nitrate supplementation improves exercise performance and decreases blood pressure in COPD patients. Nitric Oxide 2015, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.; Basham, J.E.; Yong, T.; Chazan, A.; Finlay, P.; Barnes, S.; Bardin, P.G.; Campbell, D. A double blind randomized placebo control crossover trial on the effect of dietary nitrate supplementation on exercise tolerance in stable moderate chronic obstructive pulmonary disease. BMC Pulm. Med. 2015, 15, 52. [Google Scholar] [CrossRef]
- Wang, Y.X.; Poon, K.S.; Randall, D.J.; Pang, C.C. Endothelium-derived nitric oxide partially mediates salbutamol-induced vasodilatations. Eur. J. Pharmacol. 1993, 250, 335–340. [Google Scholar] [CrossRef]
- Ceconi, C.; Fox, K.M.; Remme, W.J.; Simoons, M.L.; Bertrand, M.; Parrinello, G.; Kluft, C.; Blann, A.; Cokkinos, D.; Ferrari, R. ACE inhibition with perindopril and endothelial function. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc. Res. 2007, 73, 237–246. [Google Scholar] [CrossRef]
- Cacciatore, F.; Bruzzese, G.; Vitale, D.F.; Liguori, A.; de Nigris, F.; Fiorito, C.; Infante, T.; Donatelli, F.; Minucci, P.B.; Ignarro, L.J.; et al. Effects of ACE inhibition on circulating endothelial progenitor cells, vascular damage, and oxidative stress in hypertensive patients. Eur. J. Clin. Pharmacol. 2011, 67, 877–883. [Google Scholar] [CrossRef]
- van de Bool, C.; Steiner, M.C.; Schols, A.M.W.J. Nutritional targets to enhance exercise performance in chronic obstructive pulmonary disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 553–560. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Brooks, D.; White, J.; Goldstein, R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, CD000998. [Google Scholar] [CrossRef]
- Collins, P.F.; Elia, M.; Stratton, R.J. Nutritional support and functional capacity in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respirology 2013, 18, 616–629. [Google Scholar] [CrossRef]
- Shepherd, A.I.; Wilkerson, D.P.; Dobson, L.; Kelly, J.; Winyard, P.G.; Jones, A.M.; Benjamin, N.; Shore, A.C.; Gilchrist, M. The effect of dietary nitrate supplementation on the oxygen cost of cycling, walking performance and resting blood pressure in individuals with chronic obstructive pulmonary disease: A double blind placebo controlled, randomised control trial. Nitric Oxide Biol. Chem. 2015, 48, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhu, Y.; Ma, Y.; Xu, Z.; Zao, Y.; Wang, J.; Lin, Y.; Comer, G.M. Effect of supplementing a high-fat, low-carbohydrate enteral formula in COPD patients. Nutrition 2003, 19, 229–232. [Google Scholar] [CrossRef]
- al-Saady, N.M.; Blackmore, C.M.; Bennett, E.D. High fat, low carbohydrate, enteral feeding lowers PaCO2 and reduces the period of ventilation in artificially ventilated patients. Intensive Care Med. 1989, 15, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Angelillo, V.A.; Bedi, S.; Durfee, D.; Dahl, J.; Patterson, A.J.; O’Donohue, W.J.J. Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann. Intern. Med. 1985, 103, 883–885. [Google Scholar] [CrossRef]
- Frankfort, J.D.; Fischer, C.E.; Stansbury, D.W.; McArthur, D.L.; Brown, S.E.; Light, R.W. Effects of high- and low-carbohydrate meals on maximum exercise performance in chronic airflow obstruction. Chest 1991, 100, 792–795. [Google Scholar] [CrossRef]
- Witte, K.K.A.; Nikitin, N.P.; Parker, A.C.; von Haehling, S.; Volk, H.-D.; Anker, S.D.; Clark, A.L.; Cleland, J.G.F. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur. Heart J. 2005, 26, 2238–2244. [Google Scholar] [CrossRef]
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Quinzani, F.; Guidetti, F.; Rovetta, R.; Nodari, S.; Gheorghiade, M.; Metra, M. Effects of oral amino Acid supplements on functional capacity in patients with chronic heart failure. Clin. Med. Insights. Cardiol. 2014, 8, 39–44. [Google Scholar] [CrossRef]
- Aquilani, R.; Viglio, S.; Iadarola, P.; Opasich, C.; Testa, A.; Dioguardi, F.S.; Pasini, E. Oral Amino Acid Supplements Improve Exercise Capacities in Elderly Patients with Chronic Heart Failure. Am. J. Cardiol. 2008, 101, S104–S110. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Gualco, A.; Verri, M.; Testa, A.; Pasini, E.; Viglio, S.; Iadarola, P.; Pastoris, O.; Dossena, M.; et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 1127–1135. [Google Scholar] [CrossRef]
- Pineda-Juárez, J.A.; Sánchez-Ortiz, N.A.; Castillo-Martínez, L.; Orea-Tejeda, A.; Cervantes-Gaytán, R.; Keirns-Davis, C.; Pérez-Ocampo, C.; Quiroz-Bautista, K.; Tenorio-Dupont, M.; Ronquillo-Martínez, A. Changes in body composition in heart failure patients after a resistance exercise program and branched chain amino acid supplementation. Clin. Nutr. 2016, 35, 41–47. [Google Scholar] [CrossRef]
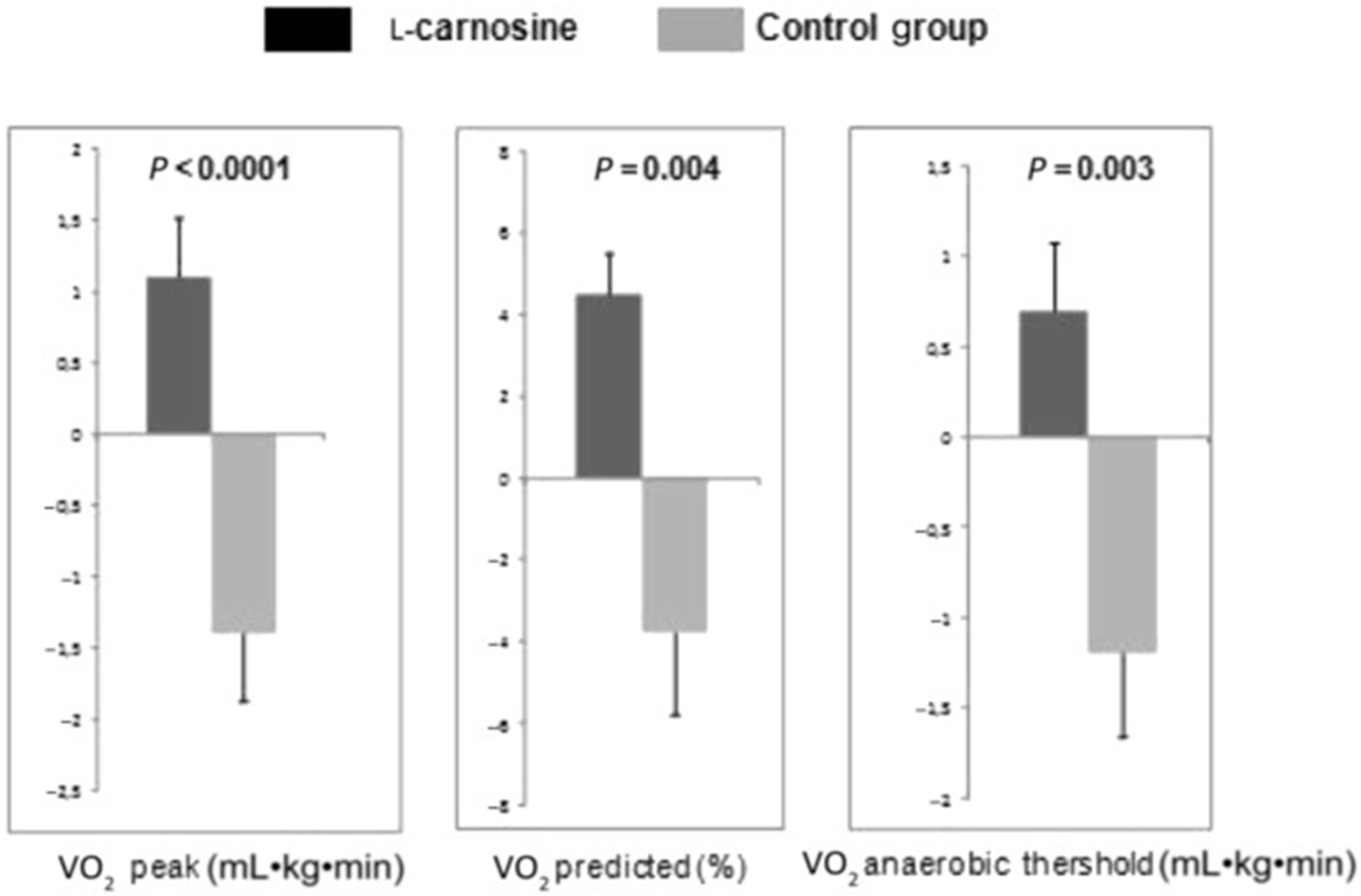
- Lombardi, C.; Carubelli, V.; Lazzarini, V.; Vizzardi, E.; Bordonali, T.; Ciccarese, C.; Castrini, A.I.; Dei Cas, A.; Nodari, S.; Metra, M. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition 2015, 31, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Kato, T.S.; Ji, R.; Zizola, C.; Brunjes, D.L.; Deng, Y.; Akashi, H.; Armstrong, H.F.; Kennel, P.J.; Thomas, T.; et al. Supplementation of l-Alanyl-l-Glutamine and Fish Oil Improves Body Composition and Quality of Life in Patients With Chronic Heart Failure. Circ. Hear. Fail. 2015, 8, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Nodari, S.; Triggiani, M.; Campia, U.; Manerba, A.; Milesi, G.; Cesana, B.M.; Gheorghiade, M.; Dei Cas, L. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 870–879. [Google Scholar] [CrossRef]
- Rozentryt, P.; von Haehling, S.; Lainscak, M.; Nowak, J.U.; Kalantar-Zadeh, K.; Polonski, L.; Anker, S.D. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: A randomized, double-blind pilot study. J. Cachexia. Sarcopenia Muscle 2010, 1, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hirai, D.M.; Zelt, J.T.; Jones, J.H.; Castanhas, L.G.; Bentley, R.F.; Earle, W.; Staples, P.; Tschakovsky, M.E.; McCans, J.; O’Donnell, D.E.; et al. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am. J. Physiol. Integr. Comp. Physiol. 2016, 312, R13–R22. [Google Scholar] [CrossRef]
- Coggan, A.R.; Broadstreet, S.R.; Mahmood, K.; Mikhalkova, D.; Madigan, M.; Bole, I.; Park, S.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; et al. Dietary Nitrate Increases VO2peak and Performance but Does Not Alter Ventilation or Efficiency in Patients With Heart Failure With Reduced Ejection Fraction. J. Card. Fail. 2018, 24, 65–73. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Brubaker, P.; Morgan, T.; Haykowsky, M.; Hundley, G.; Kraus, W.E.; Eggebeen, J.; Nicklas, B.J. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients with Heart Failure with Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 315, 36–46. [Google Scholar] [CrossRef]
- Carbone, S.; Canada, J.M.; Buckley, L.F.; Trankle, C.R.; Billingsley, H.E.; Dixon, D.L.; Mauro, A.G.; Dessie, S.; Kadariya, D.; Mezzaroma, E.; et al. Dietary Fat, Sugar Consumption, and Cardiorespiratory Fitness in Patients with Heart Failure with Preserved Ejection Fraction. JACC Basic Transl. Sci. 2017, 2, 513–525. [Google Scholar] [CrossRef]
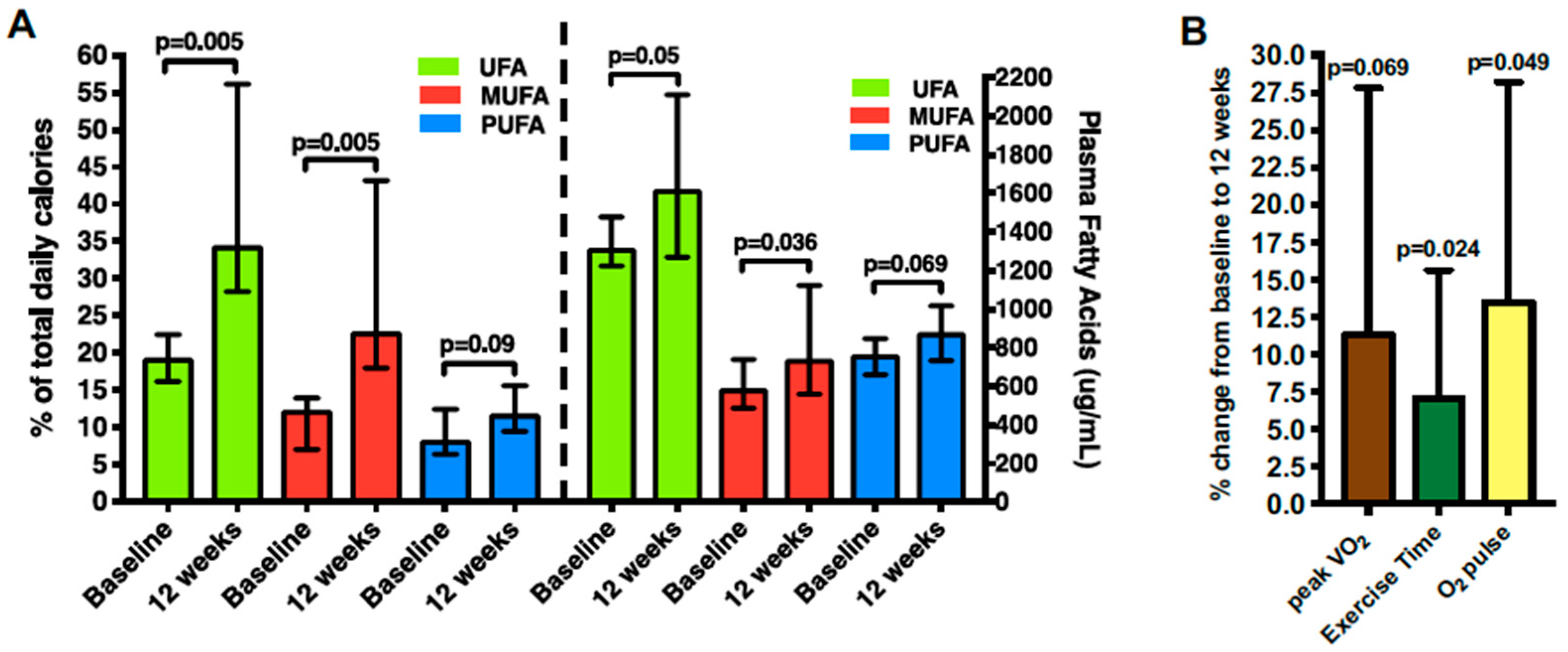
- Carbone, S.; Billingsley, H.E.; Canada, J.M.; Kadariya, D.; Medina De Chazal, H.; Rotelli, B.; Potere, N.; Paudel, B.; Markley, R.; Dixon, D.L.; et al. Unsaturated Fatty Acids to Improve Cardiorespiratory Fitness in Patients with Obesity and HFpEF- The UFA PRESERVED Pilot Study. JACC Basic Transl. Sci. 2019, 4, 563–565. [Google Scholar] [CrossRef]
- Hummel, S.L.; Seymour, E.M.; Brook, R.D.; Kolias, T.J.; Sheth, S.S.; Rosenblum, H.R.; Wells, J.M.; Weder, A.B. Low-Sodium Dietary Approaches to Stop Hypertension Diet Reduces Blood Pressure, Arterial Stiffness, and Oxidative Stress in Hypertensive Heart Failure with Preserved Ejection Fraction. Hypertension 2012, 60, 1200–1206. [Google Scholar] [CrossRef]
- Rifai, L.; Pisano, C.; Hayden, J.; Sulo, S.; Silver, M.A. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proc. (Bayl. Univ. Med. Cent). 2015, 28, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, H.A.; Eggebeen, J.; Marsh, A.P.; Brubaker, P.H.; Laurienti, P.J.; Burdette, J.H.; Basu, S.; Morgan, A.; Dos Santos, P.C.; Norris, J.L.; et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide 2017, 69, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Anstrom, K.J.; Lewis, G.D.; Shah, S.J.; Levine, J.A.; Koepp, G.A.; Givertz, M.M.; Felker, G.M.; LeWinter, M.M.; Mann, D.L.; et al. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients with Heart Failure with Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. JAMA 2018, 320, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Chalé, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of Whey Protein Supplementation on Resistance Exercise–Induced Changes in Lean Mass, Muscle Strength, and Physical Function in Mobility-Limited Older Adults. Journals Gerontol. Ser. A 2012, 68, 682–690. [Google Scholar] [CrossRef]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; von Berens, A.; Melin, M.; Cederholm, T.; Gustafsson, T.; et al. Nutritional Supplementation with Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 95–101. [Google Scholar] [CrossRef]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight Loss, Exercise, or Both and Physical Function in Obese Older Adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Wang, X.; You, T.; Lyles, M.F.; Demons, J.; Easter, L.; Berry, M.J.; Lenchik, L.; Carr, J.J. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: A randomized, controlled trial. Am. J. Clin. Nutr. 2009, 89, 1043–1052. [Google Scholar] [CrossRef]
- Amati, F.; Dube, J.J.; Shay, C.; Goodpaster, B.H. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J. Appl. Physiol. 2008, 105, 825–831. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.-Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012, 20, 1628–1638. [Google Scholar] [CrossRef]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.-C.; et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]
- Kondamudi, N.; Haykowsky, M.; Forman, D.E.; Berry, J.D.; Pandey, A. Exercise Training for Prevention and Treatment of Heart Failure. Prog. Cardiovasc. Dis. 2017, 60, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Tomczak, C.R.; Scott, J.M.; Paterson, D.I.; Kitzman, D.W. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J. Appl. Physiol. 2015, 119, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Buckley, L.F.; Trankle, C.R.; Dixon, D.L.; Buzzetti, R.; Arena, R.; Van Tassell, B.W.; Abbate, A. Obesity Contributes to Exercise Intolerance in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2016, 68, 2487–2488. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Niazi, A.K.; Lavie, C.J.; O’Keefe, J.H.; Ventura, H.O. Thiamine Supplementation for the Treatment of Heart Failure: A Review of the Literature. Congest. Hear. Fail. 2013, 19, 214–222. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis–Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Araújo, J.P.; Lourenço, P.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Nutritional markers and prognosis in cardiac cachexia. Int. J. Cardiol. 2011, 146, 359–363. [Google Scholar] [CrossRef]
- Bittner, V.; Weiner, D.H.; Yusuf, S.; Rogers, W.J.; McIntyre, K.M.; Bangdiwala, S.I.; Kronenberg, M.W.; Kostis, J.B.; Kohn, R.M.; Guillotte, M.; et al. Prediction of Mortality and Morbidity with a 6-Minute Walk Test in Patients with Left Ventricular Dysfunction. JAMA 1993, 270, 1702–1707. [Google Scholar] [CrossRef]
- Coats, A.J.S.; Pieske, B.; Linde, C.; Jankowska, E.A.; Ruschitzka, F.; Rutten, F.H.; Rosano, G.M.C.; Bueno, H.; Riley, J.P.; Cleland, J.G.F.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Abbate, A.; Arena, R.; Abouzaki, N.; Van Tassell, B.W.; Canada, J.; Shah, K.; Biondi-Zoccai, G.; Voelkel, N.F. Heart failure with preserved ejection fraction: Refocusing on diastole. Int. J. Cardiol. 2015, 179, 430–440. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Buckley, L.; Abbate, A. Primary and Secondary Diastolic Dysfunction in Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2018, 122, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits with Incident HFpEF and HFrEF. JACC. Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Shah, S.J. The HFpEF Obesity Phenotype. J. Am. Coll. Cardiol. 2016, 68, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Canada, J.M.; Antonio, A. Letter by Carbone et al Regarding Article, “Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction”. Circulation 2018, 137, 414–415. [Google Scholar] [CrossRef]
- Carbone, S.; Pandey, A.; Lavie, C.J. Editorial commentary: Obesity and heart failure with preserved ejection fraction: A single disease or two co-existing conditions? Trends Cardiovasc. Med. 2018, 28, 328–329. [Google Scholar] [CrossRef]
- Upadhya, B.; Haykowsky, M.J.; Eggebeen, J.; Kitzman, D.W. Exercise intolerance in heart failure with preserved ejection fraction: More than a heart problem. J. Geriatr. Cardiol. 2015, 12, 294–304. [Google Scholar]
- Oh, A.; Okazaki, R.; Sam, F.; Valero-Muñoz, M. Heart Failure with Preserved Ejection Fraction and Adipose Tissue: A Story of Two Tales. Front. Cardiovasc. Med. 2019, 6, 110. [Google Scholar] [CrossRef]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity paradox in cardiovascular disease: Where do we stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef]
- Billingsley, H.; Carbone, S.; Lavie, C. Dietary Fats and Chronic Noncommunicable Diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef]
- Hummel, S.L.; Karmally, W.; Gillespie, B.W.; Helmke, S.; Teruya, S.; Wells, J.; Trumble, E.; Jimenez, O.; Marolt, C.; Wessler, J.D.; et al. Home-Delivered Meals Postdischarge from Heart Failure Hospitalization. Circ. Hear. Fail. 2018, 11, e004886. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [PubMed]
- Ekkekakis, P.; Vazou, S.; Bixby, W.R.; Georgiadis, E. The mysterious case of the public health guideline that is (almost) entirely ignored: Call for a research agenda on the causes of the extreme avoidance of physical activity in obesity. Obes. Rev. 2016, 17, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P.; Lavie, C.J. Dangers and Long-Term Outcomes in Metabolically Healthy Obesity: The Impact of the Missing Fitness Component∗. J. Am. Coll. Cardiol. 2018, 71, 1866–1868. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Laddu, D.; Arena, R.; Ortega, F.B.; Alpert, M.A.; Kushner, R.F. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1506–1531. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Cahalin, L.P. Evaluation of cardiorespiratory fitness and respiratory muscle function in the obese population. Prog. Cardiovasc. Dis. 2014, 56, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Ruiz, J.R.; Labayen, I.; Lavie, C.J.; Blair, S.N. The Fat but Fit paradox: What we know and don’t know about it. Br. J. Sports Med. 2018, 52, 151–153. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2013, 129, S102–S138. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Nestel, P.J.; McGrane, M.T.; Dawood, T.; Schlaich, M.P.; Masuo, K.; Eikelis, N.; de Courten, B.; Mariani, J.A.; et al. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes 2010, 59, 71–79. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Buckley, J.D. Effects of a low carbohydrate weight loss diet on exercise capacity and tolerance in obese subjects. Obesity (Silver Spring) 2009, 17, 1916–1923. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Brinkworth, G.D. Long-term effects of a very low-carbohydrate weight loss diet on exercise capacity and tolerance in overweight and obese adults. J. Am. Coll. Nutr. 2014, 33, 267–273. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Brinkworth, G.D. Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. Eur. J. Nutr. 2013, 52, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A.; Schneider, S.M.; Sieber, C.C.; Topinkova, E.; Vandewoude, M.; Visser, M.; Zamboni, M.; Bahat, G.; Bauer, J.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.J.; Bottaro, M.; Motta, A.M.; Pitanga, F.; Guido, M.; Leite, T.K.M.; Bezerra, L.M.A.; Lima, R.M. Association between sarcopenia-related phenotypes and aerobic capacity indexes of older women. J. Sports Sci. Med. 2009, 8, 337–343. [Google Scholar] [PubMed]
- Jung, M.-H.; Ihm, S.-H.; Park, S.M.; Jung, H.O.; Hong, K.-S.; Baek, S.H.; Youn, H.-J. Effects of sarcopenia, body mass indices, and sarcopenic obesity on diastolic function and exercise capacity in Koreans. Metabolism 2019, 97, 18–24. [Google Scholar] [CrossRef]
- Emami, A.; Saitoh, M.; Valentova, M.; Sandek, A.; Evertz, R.; Ebner, N.; Loncar, G.; Springer, J.; Doehner, W.; Lainscak, M.; et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur. J. Heart Fail. 2018, 20, 1580–1587. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef]
- Morard, M.-D.; Besson, D.; Laroche, D.; Naaïm, A.; Gremeaux, V.; Casillas, J.-M. Fixed-distance walk tests at comfortable and fast speed: Potential tools for the functional assessment of coronary patients? Ann. Phys. Rehabil. Med. 2017, 60, 13–19. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zuniga, C.; Arai, H.; Boirie, Y.; Chen, L.-K.; Fielding, R.A.; Martin, F.C.; Michel, J.-P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Bibiloni, M.D.M.; Julibert, A.; Argelich, E.; Aparicio-Ugarriza, R.; Palacios, G.; Pons, A.; Gonzalez-Gross, M.; Tur, J.A. Western and Mediterranean Dietary Patterns and Physical Activity and Fitness among Spanish Older Adults. Nutrients 2017, 9, 704. [Google Scholar] [CrossRef]
- Tepper, S.; Alter Sivashensky, A.; Rivkah Shahar, D.; Geva, D.; Cukierman-Yaffe, T. The Association between Mediterranean Diet and the Risk of Falls and Physical Function Indices in Older Type 2 Diabetic People Varies by Age. Nutrients 2018, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Travison, T.G.; Kirn, D.R.; Koochek, A.; Reid, K.F.; von Berens, Å.; Zhu, H.; Folta, S.C.; Sacheck, J.M.; Nelson, M.E.; et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: Results from the VIVE2 randomized trial. J. Nutr. Health Aging 2017, 21, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, D.L.; Muth, B.J.; Stock, J.M.; Townsend, R.R.; Edwards, D.G. Cardiopulmonary exercise testing reveals subclinical abnormalities in chronic kidney disease. Eur. J. Prev. Cardiol. 2018, 25, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef]
- Jones, L.W.; Eves, N.D.; Haykowsky, M.; Freedland, S.J.; Mackey, J.R. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet. Oncol. 2009, 10, 598–605. [Google Scholar] [CrossRef]
- Vinke, P.; Jansen, S.M.; Witkamp, R.F.; van Norren, K. Increasing quality of life in pulmonary arterial hypertension: Is there a role for nutrition? Heart Fail. Rev. 2018, 23, 711–722. [Google Scholar] [CrossRef]
- Canada, J.M.; Abbate, A.; Collen, R.; Billingsley, H.; Buckley, L.F.; Carbone, S.; Trankle, C.R.; Idowu, M.O.; Kadariya, D.; Van Tassell, B.; et al. Relation of Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease to Left Ventricular Diastolic Function and Exercise Tolerance. Am. J. Cardiol. 2019, 123, 466–473. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Hobbelt, A.H.; Brügemann, J.; Rienstra, M. Time to implement fitness and reduction of fatness in atrial fibrillation therapy. EP Eur. 2016, 19, 513–514. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Robinson-Cohen, C.; Ellis, C.; Headley, S.A.E.; Tuttle, K.; Wood, R.J.; Evans, E.E.; Milch, C.M.; Moody, K.A.; Germain, M.; et al. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J. Am. Soc. Nephrol. 2018, 29, 250–259. [Google Scholar] [CrossRef]
- Snelson, M.; Clarke, E.R.; Coughlan, T.M. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients 2017, 9, 265. [Google Scholar] [CrossRef]
- Sheppard, V.B.; Hicks, J.; Makambi, K.; Hurtado-de-Mendoza, A.; Demark-Wahnefried, W.; Adams-Campbell, L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemp. Clin. Trials 2016, 46, 106–113. [Google Scholar] [CrossRef]
- O’Neill, L.M.; Guinan, E.; Doyle, S.L.; Bennett, A.E.; Murphy, C.; Elliott, J.A.; O’Sullivan, J.; Reynolds, J.V.; Hussey, J. The RESTORE Randomized Controlled Trial: Impact of a Multidisciplinary Rehabilitative Program on Cardiorespiratory Fitness in Esophagogastric cancer Survivorship. Ann. Surg. 2018, 268, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Travier, N.; Buckland, G.; Vendrell, J.J.; Fernandez-Veledo, S.; Peiro, I.; Del Barco, S.; Pernas, S.; Zamora, E.; Bellet, M.; Margeli, M.; et al. Changes in metabolic risk, insulin resistance, leptin and adiponectin following a lifestyle intervention in overweight and obese breast cancer survivors. Eur. J. Cancer Care (Engl.) 2018, 27, e12861. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B.; Heymsfield, S.B. Improving the Quality of Dietary ResearchImproving the Quality of Dietary ResearchImproving the Quality of Dietary Research. JAMA 2019, 322, 1549–1550. [Google Scholar] [CrossRef]
| Peak VO2 | 6 MWT | ISWT | 400 m Walk | |
|---|---|---|---|---|
| COPD | ||||
| Creatine [35,36,37] | ↔ | ?? | ↔ | |
| N-3 PUFA [38] | ↔ | ?? | ?? | |
| Vitamin D [43] | ?? | ?? | ?? | |
| Vitamin B12 [44] | ?? | ?? | ?? | |
| Dietary Nitrates [39,40,41,63,64] | ↔ | ↔ | ↑ | |
| Nutrition Support Supplements [45,46,47,49,50] | ?? | ↔ | ↔ | |
| HFrEF | ||||
| Multivitamin [76] | ?? | ↔ | ||
| Essential AA [77,78,79] | ↑ | ↑ | ||
| BCAA [80] | ↔ | ?? | ||
| L-Carnosine [81] | ↑ | ↑ | ||
| Glutamine + N-3 PUFA [82] | ↔ | ↔ | ||
| N-3 PUFA [83] | ↑ | ↔ | ||
| Nutrition Support Supplements [84] | ↔ | ↑ | ||
| Dietary Nitrates [85,86] | ↔ | ?? | ||
| HFpEF | ||||
| HCD [87] | ↑ | ?? | ||
| UFA Supplementation [88,89] | ?? | ?? | ||
| DASH Diet [90,91] | ?? | ↑ | ||
| Dietary Nitrates [92,93] | ↔ | ?? | ||
| Sarcopenia and Frailty | ||||
| Whey Protein [94] | ?? | ?? | ↔ | |
| Whey Protein + Vitamin D [95] | ?? | ?? | ↔ | |
| Peak VO2 | Units | |
|---|---|---|
| Diet | ||
| Villareal et al. (2011) | ↑ | ml/kg/min |
| Nicklas et al (2009) | ↑ | ml/kg/min |
| Straznicky et al (2010) | ↔ | ml/kgFFM/min |
| Foster-Schubert et al (2012) | ↔ | L/min |
| Exercise | ||
| Villareal et al. (2011) | ↑ | ml/kg/min |
| Nicklas et al. (2009) | N/A | ml/kg/min |
| Straznicky et al. (2010) | N/A | ml/kgFFM/min |
| Foster-Schubert et al. (2012) | ↑ | L/min |
| Diet and Exercise | ||
| Villareal et al. (2011) | ↑↑ | ml/kg/min |
| Nicklas et al. (2009) | ↑↑ | ml/kg/min |
| Straznicky et al. (2010) | ↑↑ | ml/kgFFM/min |
| Foster-Schubert et al. (2012) | ↑ | L/min |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billingsley, H.E.; Rodriguez-Miguelez, P.; Del Buono, M.G.; Abbate, A.; Lavie, C.J.; Carbone, S. Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty. Nutrients 2019, 11, 2849. https://doi.org/10.3390/nu11122849
Billingsley HE, Rodriguez-Miguelez P, Del Buono MG, Abbate A, Lavie CJ, Carbone S. Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty. Nutrients. 2019; 11(12):2849. https://doi.org/10.3390/nu11122849
Chicago/Turabian StyleBillingsley, Hayley E., Paula Rodriguez-Miguelez, Marco Giuseppe Del Buono, Antonio Abbate, Carl J. Lavie, and Salvatore Carbone. 2019. "Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty" Nutrients 11, no. 12: 2849. https://doi.org/10.3390/nu11122849
APA StyleBillingsley, H. E., Rodriguez-Miguelez, P., Del Buono, M. G., Abbate, A., Lavie, C. J., & Carbone, S. (2019). Lifestyle Interventions with a Focus on Nutritional Strategies to Increase Cardiorespiratory Fitness in Chronic Obstructive Pulmonary Disease, Heart Failure, Obesity, Sarcopenia, and Frailty. Nutrients, 11(12), 2849. https://doi.org/10.3390/nu11122849






