The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil
Abstract
:1. Introduction
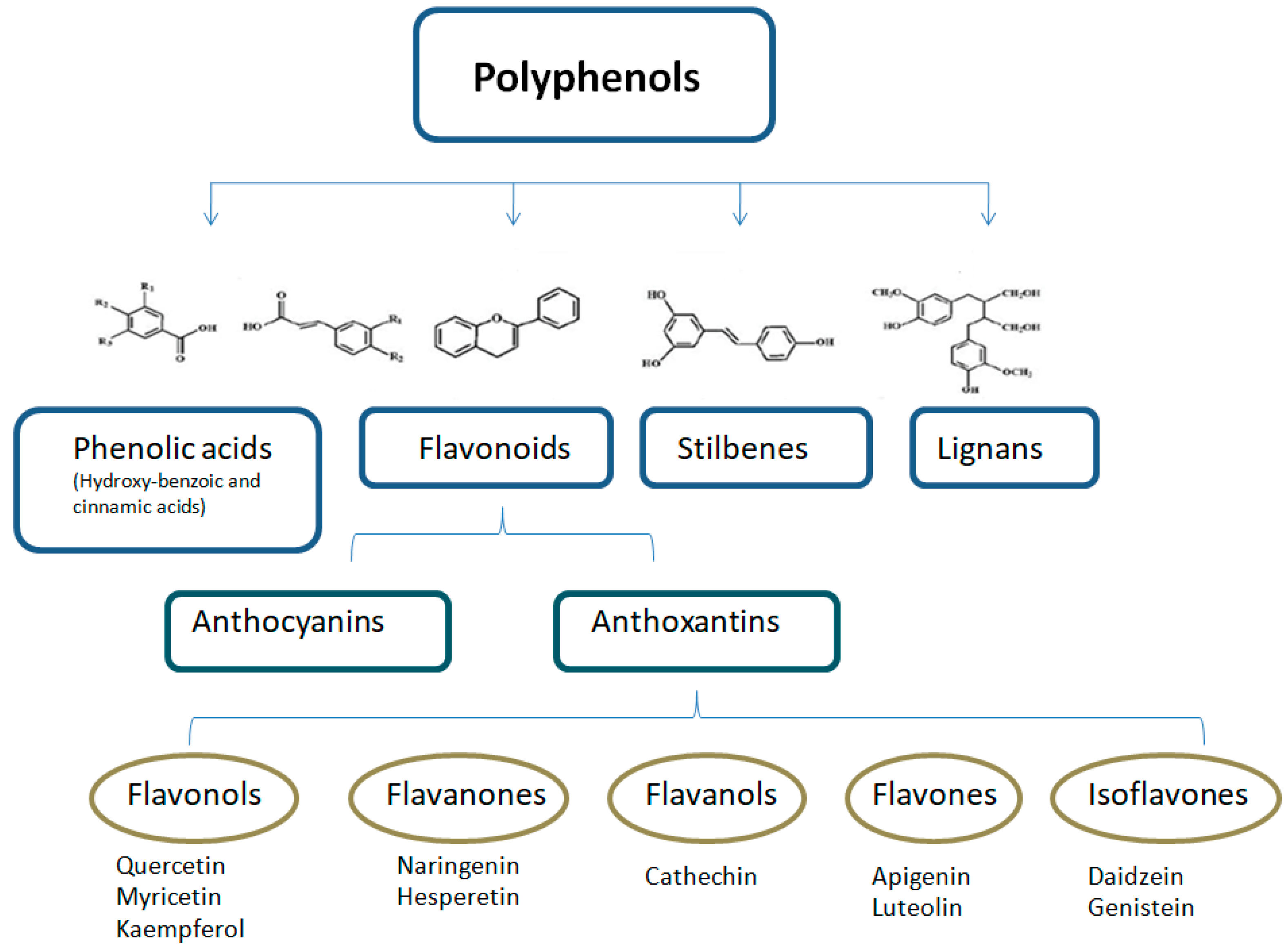
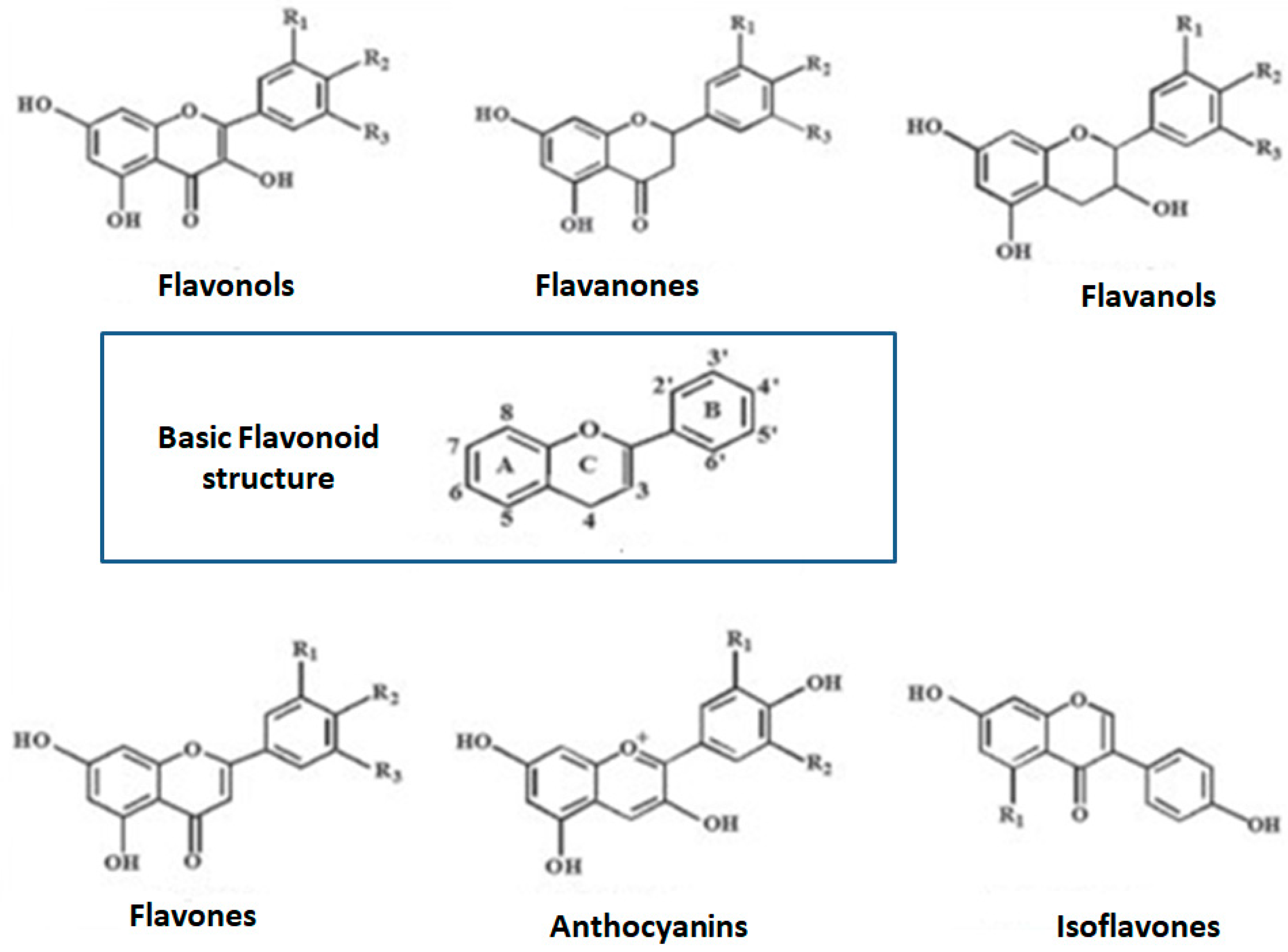
2. Polyphenols and Phenolic Compounds
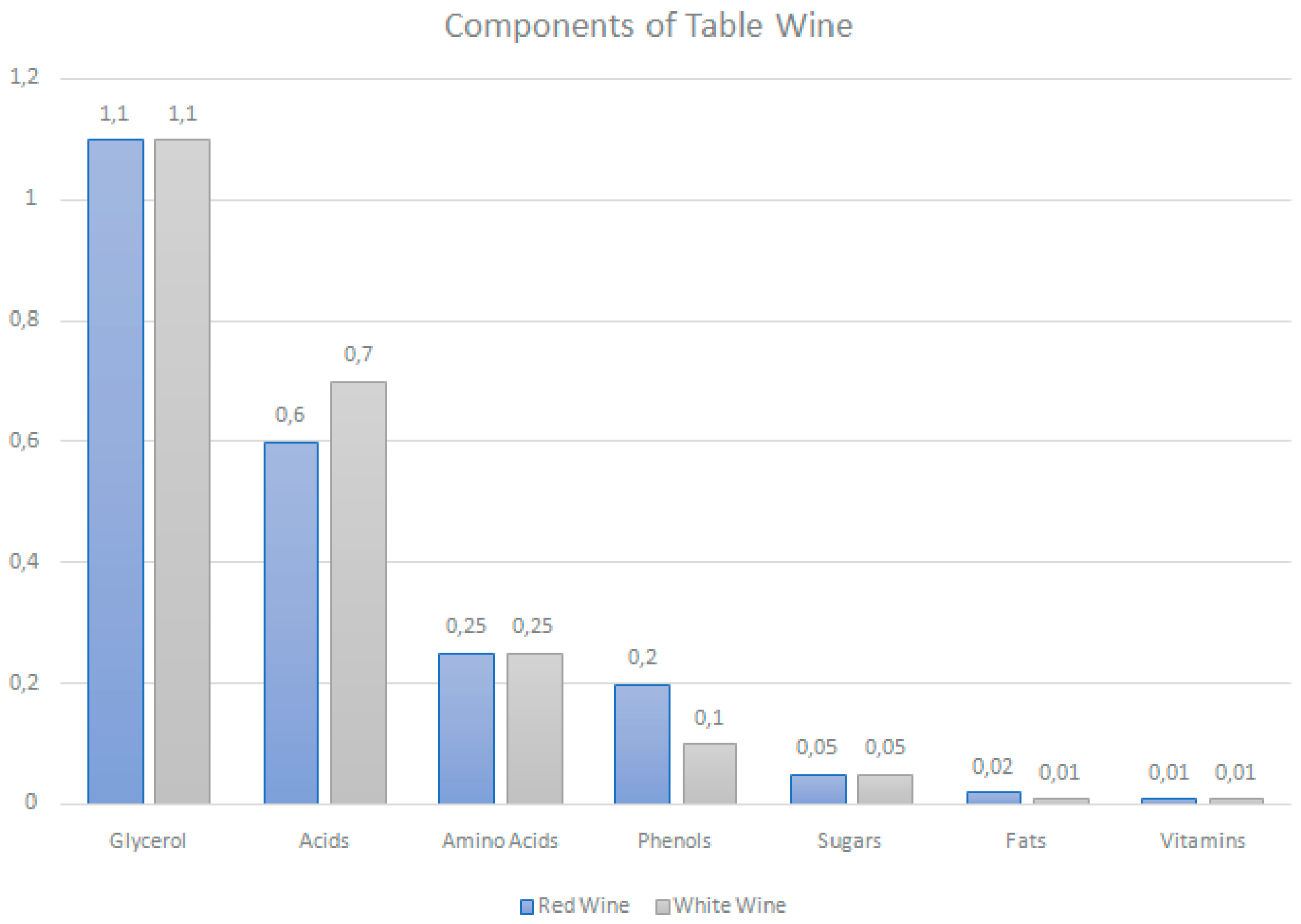
3. Polyphenols Content in Wine
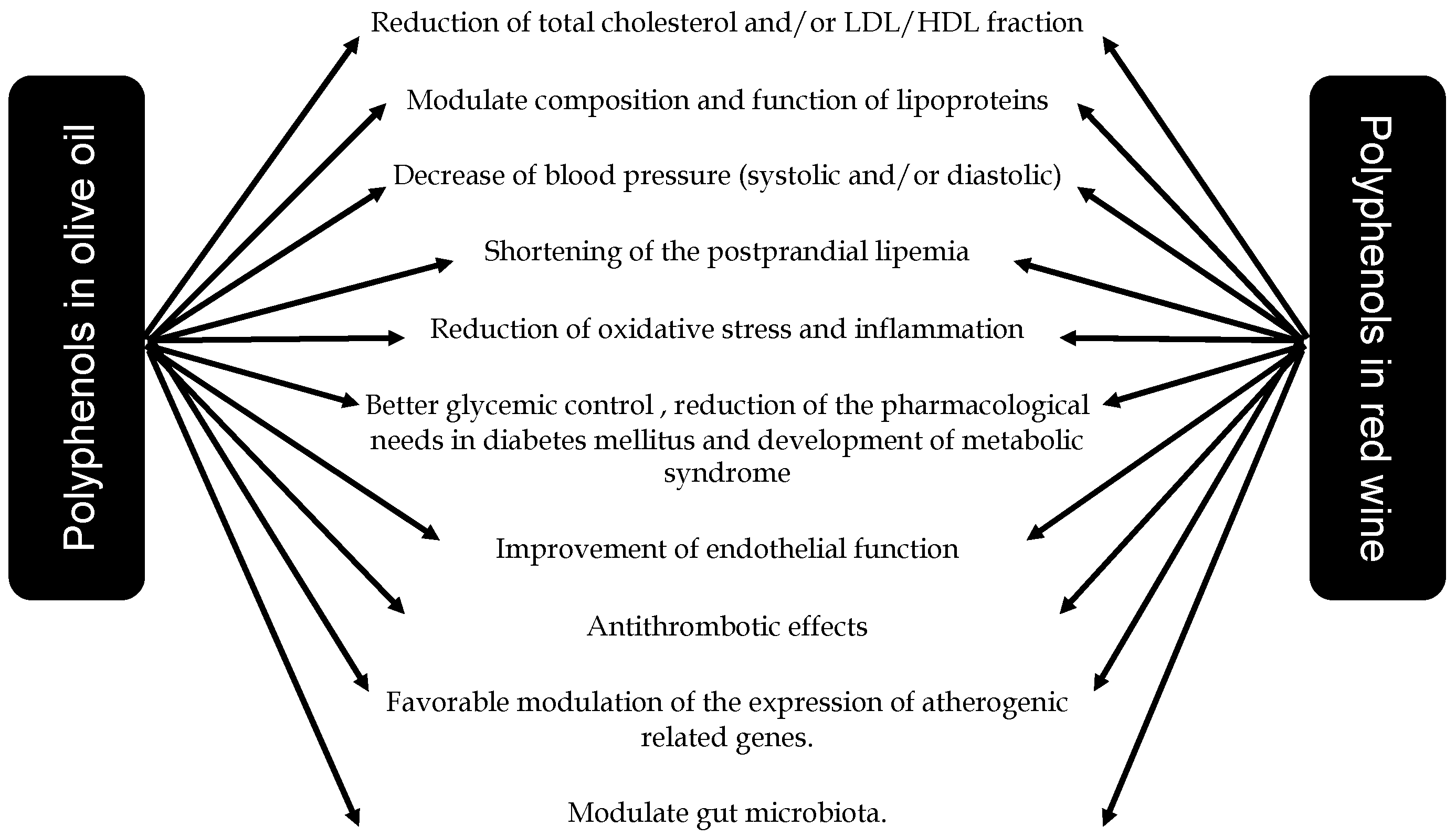
4. Red Wine Polyphenols and Cardiometabolic Diseases
4.1. Red Wine Polyphenols and Blood Lipids
4.2. Red Wine Polyphenols and Blood Pressure
4.3. Molecular Mechanisms of the Effects of Red Wine Polyphenols on the Atheromatous Plaque
4.4. Red Wine Polyphenols and Glucose Metabolism
5. Polyphenols Content in Olive Oil
6. Olive Oil Polyphenols and Cardiometabolic Diseases
6.1. Olive Oil Polyphenols and BP
6.2. Olive Oil Polyphenols and Lipids
6.3. Phenolic Compounds, Obesity, MetS and T2DM
6.4. Olive Oil Polyphenols and Endothelial Function
6.5. Olive Oil Polyphenols: Inflammation, Oxidative Stress and Hemostasis
6.6. Phenolic Compounds and Gut Microbiota
7. Polyphenols in Olive Oil and Red Wine and Nonalcoholic Fatty Liver Disease
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Apo | Apolipoproteins |
| BP | blood pressure |
| CVD | Cardiovascular disease |
| GLP-1 | glucagon-like peptide 1 |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | low-density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| MedDiet | Mediterranean style diet |
| NO | nitric oxide |
| ROS | Reactive Oxygen Species |
| RWPs | Red Wine Polyphenols |
| TC | Total cholesterol |
| TG | triglyceride |
| T2D | Type 2 diabetes |
| VLDL | Very Low-Density Lipoproteins |
References
- Guasch-Ferre, M.; Merino, J.; Sun, Q.; Fito, M.; Salas-Salvado, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovas, J.M.; Soriguer, F.; Abia, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martínez, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B1–B15. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Griswold, M.G.; Fullman, N.; Hawley, C.; Arian, N.; Zimsen, S.R.M.; Tymeson, H.D.; Venkateswaran, V.; Tapp, A.D.; Forouzanfar, M.H.; Salama, J.S.; et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef]
- Mukamal, K.; Lazo, M. Alcohol and cardiovascular disease. BMJ 2017, 356, j1340. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Bes-Rastrollo, M.; Toledo, E.; Garcia-Lopez, M.; Beunza, J.J.; Estruch, R.; Martinez-Gonzalez, M.A. Mediterranean alcohol-drinking pattern and mortality in the SUN (Seguimiento Universidad de Navarra) Project: A prospective cohort study. Br. J. Nutr. 2014, 111, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; Predimed, I. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Aguilera, C.M.; Gil, A. A Systematic Review of the Efficacy of Bioactive Compounds in Cardiovascular Disease: Phenolic Compounds. Nutrients 2015, 7, 5177–5216. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Ovaskainen, M.L.; Torronen, R.; Koponen, J.M.; Sinkko, H.; Hellstrom, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Schettino, C.; Sampaolo, S.; Galderisi, U.; Di Iorio, G.; Giordano, A.; Melone, M.A.B. Adult-onset brain tumors and neurodegeneration: Are polyphenols protective? J. Cell. Physiol. 2018, 233, 3955–3967. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, L.; Slowing, K.; Gomez-Serranillos, P.; Torres, F.; Valderrama, M.; Millan, J. Variability of polyphenol content in different types of wine and its potential application in the understanding of its biologic effects. Med. Clin. (Barc.) 2000, 114, 331–332. [Google Scholar]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Jackson, R. Wine Science: Principles, Practice, Perception, 2nd ed.; Elsevier Science and Technology Books: Cambridge, MA, USA, 2000. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: Chichester, UK, 2006; Volume 1. [Google Scholar]
- Artero, A.; Artero, A.; Tarin, J.J.; Cano, A. The impact of moderate wine consumption on health. Maturitas 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Cordova, A.C.; Sumpio, B.E. Polyphenols are medicine: Is it time to prescribe red wine for our patients? Int. J. Angiol. 2009, 18, 111–117. [Google Scholar] [CrossRef]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular Properties of Red Wine Compounds and Cardiometabolic Benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Bartolome, B.; Gomez-Cordoves, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Balga, I.; Leskó, A.; Ladányi, M.; Kallay, M. Influence of Ageing on Changes in Polyphenolic Compounds in Red Wines. Czech. J. Food Sci. 2014, 32, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Gronbaek, M.; Deis, A.; Sorensen, T.I.; Becker, U.; Schnohr, P.; Jensen, G. Mortality associated with moderate intakes of wine, beer, or spirits. BMJ 1995, 310, 1165–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- St Leger, A.S.; Cochrane, A.L.; Moore, F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet 1979, 1, 1017–1020. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Friedman, G.D.; Siegelaub, A.B. Alcohol consumption before myocardial infarction. Results from the Kaiser-Permanente epidemiologic study of myocardial infarction. Ann. Intern. Med. 1974, 81, 294–301. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Rosner, B.; Cole, D.S. Daily alcohol consumption and fatal coronary heart disease. Am. J. Epidemiol. 1978, 107, 196–200. [Google Scholar] [CrossRef]
- Moore, R.D.; Pearson, T.A. Moderate alcohol consumption and coronary artery disease. A review. Medicine (Baltimore) 1986, 65, 242–267. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Giovannucci, E.L.; Willett, W.C.; Colditz, G.A.; Ascherio, A.; Rosner, B.; Stampfer, M.J. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991, 338, 464–468. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Speizer, F.E.; Hennekens, C.H. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N. Engl. J. Med. 1988, 319, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Rhoads, G.G.; Kagan, A. Coffee, alcohol and risk of coronary heart disease among Japanese men living in Hawaii. N. Engl. J. Med. 1977, 297, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Niki, K.; Konstantinos, T.; Dimitri, P.M. Alcohol and the Cardiovascular System: A Double-Edged Sword. Curr. Pharm. Des. 2014, 20, 6276–6288. [Google Scholar]
- Gronbaek, M.; Becker, U.; Johansen, D.; Gottschau, A.; Schnohr, P.; Hein, H.O.; Jensen, G.; Sorensen, T.I. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann. Intern. Med. 2000, 133, 411–419. [Google Scholar] [CrossRef]
- Rimm, E.B.; Klatsky, A.; Grobbee, D.; Stampfer, M.J. Review of moderate alcohol consumption and reduced risk of coronary heart disease: Is the effect due to beer, wine, or spirits. BMJ 1996, 312, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Mostofsky, E.; Mukamal, K.J.; Giovannucci, E.L.; Stampfer, M.J.; Rimm, E.B. Key Findings on Alcohol Consumption and a Variety of Health Outcomes From the Nurses’ Health Study. Am. J. Public Health 2016, 106, 1586–1591. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.R.F. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillen, M.; Lamuela-Raventos, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Members, N.L.C.; Myers, G.L.; Christenson, R.H.; Cushman, M.; Ballantyne, C.M.; Cooper, G.R.; Pfeiffer, C.M.; Grundy, S.M.; Labarthe, D.R.; Levy, D.; et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice guidelines: Emerging biomarkers for primary prevention of cardiovascular disease. Clin. Chem. 2009, 55, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avellone, G.; Di Garbo, V.; Campisi, D.; De Simone, R.; Raneli, G.; Scaglione, R.; Licata, G. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. Eur. J. Clin. Nutr. 2006, 60, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Sacanella, E.; Mota, F.; Chiva-Blanch, G.; Antunez, E.; Casals, E.; Deulofeu, R.; Rotilio, D.; Andres-Lacueva, C.; Lamuela-Raventos, R.M.; et al. Moderate consumption of red wine, but not gin, decreases erythrocyte superoxide dismutase activity: A randomised cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 46–53. [Google Scholar] [CrossRef]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. Espen. 2015, 10, e224–e233. [Google Scholar] [CrossRef]
- Naissides, M.; Mamo, J.C.; James, A.P.; Pal, S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis 2006, 185, 438–445. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults With Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Droste, D.W.; Iliescu, C.; Vaillant, M.; Gantenbein, M.; De Bremaeker, N.; Lieunard, C.; Velez, T.; Meyer, M.; Guth, T.; Kuemmerle, A.; et al. A daily glass of red wine associated with lifestyle changes independently improves blood lipids in patients with carotid arteriosclerosis: Results from a randomized controlled trial. Nutr. J. 2013, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, D.; Miura, Y.; Yagasaki, K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci. 2003, 73, 1393–1400. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell. Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.; Cho, I.; Kim, S.; Kwon, D.; Ha, T. Dietary resveratrol alters lipid metabolism-related gene expression of mice on an atherogenic diet. J. Hepatol. 2008, 49, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, X.; Jin, Z. Effect of Resveratrol on Serum and Liver Lipid Profile and Antioxidant Activity in Hyperlipidemia Rats. Asian-Australas. J. Anim. Sci. 2008, 21, 890–895. [Google Scholar] [CrossRef]
- Cho, I.J.; Ahn, J.Y.; Kim, S.; Choi, M.S.; Ha, T.Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 2008, 367, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Nihei, T.; Miura, Y.; Yagasaki, K. Inhibitory effect of resveratrol on proteinuria, hypoalbuminemia and hyperlipidemia in nephritic rats. Life Sci. 2001, 68, 2845–2852. [Google Scholar] [CrossRef]
- Rivera, L.; Moron, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharm. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Rocha, K.K.; Souza, G.A.; Ebaid, G.X.; Seiva, F.R.; Cataneo, A.C.; Novelli, E.L. Resveratrol toxicity: Effects on risk factors for atherosclerosis and hepatic oxidative stress in standard and high-fat diets. Food Chem. Toxicol. 2009, 47, 1362–1367. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Zou, J.; Cao, K.; Xu, Y.; Wu, J.M. Effects of red wine and wine polyphenol resveratrol on platelet aggregation in vivo and in vitro. Int. J. Mol. Med. 2002, 9, 77–79. [Google Scholar] [CrossRef]
- Turrens, J.F.; Lariccia, J.; Nair, M.G. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic. Res. 1997, 27, 557–562. [Google Scholar] [CrossRef]
- Castro, M.; Veiga, A.P.M.; Pacheco, M.R. Plasma lipid profile of experimentally induced hyperlipidemic New Zealand white rabbits is not affected by resveratrol. J. Appl. Res. 2009, 9, 18–22. [Google Scholar]
- Bau, P.F.; Bau, C.H.; Rosito, G.A.; Manfroi, W.C.; Fuchs, F.D. Alcohol consumption, cardiovascular health, and endothelial function markers. Alcohol 2007, 41, 479–488. [Google Scholar] [CrossRef]
- Xin, X.; He, J.; Frontini, M.G.; Ogden, L.G.; Motsamai, O.I.; Whelton, P.K. Effects of alcohol reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2001, 38, 1112–1117. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Coffey, R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993, 265, H774–H778. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Ricci, T.; Jantzen, P.; Coffey, R.G. Endothelium-dependent vasorelaxation caused by various plant extracts. J. Cardiovasc. Pharm. 1995, 26, 90–95. [Google Scholar] [CrossRef]
- Fitzpatrick, D.F.; Fleming, R.C.; Bing, B.; Maggi, D.A.; O’Malley, R.M. Isolation and characterization of endothelium-dependent vasorelaxing compounds from grape seeds. J. Agric. Food Chem. 2000, 48, 6384–6390. [Google Scholar] [CrossRef]
- Zenebe, W.; Pechanova, O.; Andriantsitohaina, R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol. Res. 2003, 52, 425–432. [Google Scholar]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharm. 2011, 667, 258–264. [Google Scholar] [CrossRef]
- Bernatova, I.; Pechanova, O.; Babal, P.; Kysela, S.; Stvrtina, S.; Andriantsitohaina, R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H942–H948. [Google Scholar] [CrossRef] [Green Version]
- Diebolt, M.; Bucher, B.; Andriantsitohaina, R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension 2001, 38, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Extract of wine phenolics improves aortic biomechanical properties in stroke-prone spontaneously hypertensive rats (SHRSP). J. Nutr. Sci. Vitam. (Tokyo) 1999, 45, 95–106. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr. Case-control and cohort studies of moderate alcohol consumption and stroke. Clin. Chim. Acta 1996, 246, 107–119. [Google Scholar] [CrossRef]
- Platisa, M.M.; Gal, V.; Nestorovic, Z.; Gojkovic-Bukarica, L. Quantification of the acute effect of a low dose of red wine by nonlinear measures of RR and QT interval series in healthy subjects. Comput. Biol. Med. 2014, 53, 291–296. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, K.; Wheatcroft, S.; Kearney, M. Heart and blood vessels. In Clinical Nutrition, 2nd ed.; Elia, M., Ljunqvist, O., Sratton, R.J., Lanhan New, R.J., Eds.; Wiley Blackwell: Oxford, UK, 2013. [Google Scholar]
- Da Luz, P.L.; Coimbra, S.R. Wine, alcohol and atherosclerosis: Clinical evidences and mechanisms. Braz. J. Med. Biol. Res. 2004, 37, 1275–1295. [Google Scholar] [CrossRef] [Green Version]
- Ndiaye, M.; Chataigneau, M.; Lobysheva, I.; Chataigneau, T.; Schini-Kerth, V.B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. 2005, 19, 455–457. [Google Scholar] [CrossRef]
- Wallerath, T.; Poleo, D.; Li, H.; Forstermann, U. Red wine increases the expression of human endothelial nitric oxide synthase: A mechanism that may contribute to its beneficial cardiovascular effects. J. Am. Coll. Cardiol. 2003, 41, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Leiro, J.; Alvarez, E.; Arranz, J.A.; Laguna, R.; Uriarte, E.; Orallo, F. Effects of cis-resveratrol on inflammatory murine macrophages: Antioxidant activity and down-regulation of inflammatory genes. J. Leukoc. Biol. 2004, 75, 1156–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCarrone, M.; Lorenzon, T.; Guerrieri, P.; Agro, A.F. Resveratrol prevents apoptosis in K562 cells by inhibiting lipoxygenase and cyclooxygenase activity. Eur. J. Biochem. 1999, 265, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L.; Garcia-Barcina, M.; Gutierrez-de Juan, V.; Bidaurrazaga, J.; de Luco, M.F.; Gutierrez-Stampa, M.; Larzabal, M.; Hijona, E.; Sarasqueta, C.; Echenique-Elizondo, M.; et al. Effect of resveratrol on alcohol-induced mortality and liver lesions in mice. BMC Gastroenterol. 2006, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Boscolo, P.; del Signore, A.; Sabbioni, E.; Di Gioacchino, M.; Di Giampaolo, L.; Reale, M.; Conti, P.; Paganelli, R.; Giaccio, M. Effects of resveratrol on lymphocyte proliferation and cytokine release. Ann. Clin. Lab. Sci. 2003, 33, 226–231. [Google Scholar]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharm. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Media, J.; Divine, G.; Jiang, H.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Discrepant in vitro and in vivo immunological effects. Biochem. Pharm. 2003, 66, 2427–2435. [Google Scholar] [CrossRef]
- Wang, M.J.; Huang, H.M.; Hsieh, S.J.; Jeng, K.C.; Kuo, J.S. Resveratrol inhibits interleukin-6 production in cortical mixed glial cells under hypoxia/hypoglycemia followed by reoxygenation. J. Neuroimmunol. 2001, 112, 28–34. [Google Scholar] [CrossRef]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Shen, F.; Chen, S.J.; Dong, X.J.; Zhong, H.; Li, Y.T.; Cheng, G.F. Suppression of IL-8 gene transcription by resveratrol in phorbol ester treated human monocytic cells. J. Asian Nat. Prod. Res. 2003, 5, 151–157. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Colio, L.M.; Valderrama, M.; Alvarez-Sala, L.A.; Bustos, C.; Ortego, M.; Hernandez-Presa, M.A.; Cancelas, P.; Gomez-Gerique, J.; Millan, J.; Egido, J. Red wine intake prevents nuclear factor-kappaB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia. Circulation 2000, 102, 1020–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oak, M.H.; Chataigneau, M.; Keravis, T.; Chataigneau, T.; Beretz, A.; Andriantsitohaina, R.; Stoclet, J.C.; Chang, S.J.; Schini-Kerth, V.B. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arter. Thromb. Vasc. Biol. 2003, 23, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Colio, L.M.; Munoz-Garcia, B.; Martin-Ventura, J.L.; Alvarez-Sala, L.A.; Castilla, M.; Bustamante, A.; Lamuela-Raventos, R.M.; Gomez-Gerique, J.; Fernandez-Cruz, A.; Millan, J.; et al. Ethanol beverages containing polyphenols decrease nuclear factor kappa-B activation in mononuclear cells and circulating MCP-1 concentrations in healthy volunteers during a fat-enriched diet. Atherosclerosis 2007, 192, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.R.; Varghese, S.; Vitseva, O.; Vita, J.A.; Freedman, J.E. The antiinflammatory effects of purple grape juice consumption in subjects with stable coronary artery disease. Arter. Thromb. Vasc. Biol. 2004, 24, e179–e180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef]
- Frankel, E.; Waterhouse, A.; Teissedre, P.-L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- Miyagi, Y.; Miwa, K.; Inoue, H. Inhibition of human low-density lipoprotein oxidation by flavonoids in red wine and grape juice. Am. J. Cardiol. 1997, 80, 1627–1631. [Google Scholar] [CrossRef]
- Floreani, M.; Napoli, E.; Quintieri, L.; Palatini, P. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 2003, 72, 2741–2750. [Google Scholar] [CrossRef]
- Jang, J.H.; Surh, Y.J. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat. Res. 2001, 496, 181–190. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Araim, O.; Ballantyne, J.; Waterhouse, A.L.; Sumpio, B.E. Inhibition of vascular smooth muscle cell proliferation with red wine and red wine polyphenols. J. Vasc. Surg. 2002, 35, 1226–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mnjoyan, Z.H.; Fujise, K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: A role of p53-p21(WAF1/CIP1) pathway. Biochem. Biophys. Res. Commun. 2003, 311, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Ghosh-Choudhury, N.; Das, F.; Mahimainathan, L.; Kamat, A.; Kasinath, B.S.; Abboud, H.E.; Choudhury, G.G. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: Role of PTP1B. FASEB J. 2008, 22, 3469–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Lim, S.C.; Lee, M.Y.; Lee, J.W.; Oh, W.K.; Kim, S.K.; Kang, K.W. Inhibition of neointimal formation by trans-resveratrol: Role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food Res. 2010, 54, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M.; Bertelli, A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 1995, 17, 1–3. [Google Scholar] [PubMed]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Pasquel, F.J. Management of Inpatient Hyperglycemia and Diabetes in Older Adults. Diabetes Care 2017, 40, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, X.; Brown, J.; Vistisen, D.; Sicree, R.; Shaw, J.; Nichols, G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010, 87, 293–301. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasnyo, P.; Molnar, G.A.; Mohas, M.; Marko, L.; Laczy, B.; Cseh, J.; Mikolas, E.; Szijarto, I.A.; Merei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [Green Version]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Crandall, J.P.; Oram, V.; Trandafirescu, G.; Reid, M.; Kishore, P.; Hawkins, M.; Cohen, H.W.; Barzilai, N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Mendez-del Villar, M.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Perez-Rubio, K.G.; Lizarraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou Seif, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014, 2014, 816307. [Google Scholar] [CrossRef]
- Wong, R.H.; Raederstorff, D.; Howe, P.R. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharm. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2007, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.; Mesa, M.; Sanchez-Rodriguez, E.; María, C.; García, D. Compuestos bioactivos del aceite de oliva virgen. Nutr. Clin. Med. 2018, 12, 80–94. [Google Scholar]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Perez-Caballero, A.I.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean Diet and Cardiovascular Risk: Beyond Traditional Risk Factors. Crit. Rev. Food Sci. Nutr. 2016, 56, 788–801. [Google Scholar] [CrossRef]
- Doménech, M.; Roman, P.; Lapetra, J.; Corte, F.J.G.D.L.; Sala-Vila, A.; Torre, R.D.L.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Lamuela-Raventós, R.-M.; et al. Mediterranean Diet Reduces 24-Hour Ambulatory Blood Pressure, Blood Glucose, and Lipids. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive Oil Polyphenols Decrease Blood Pressure and Improve Endothelial Function in Young Women with Mild Hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharm. 2017, 83, 114–128. [Google Scholar] [CrossRef] [Green Version]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with Hydroxytyrosol and Punicalagin Improves Early Atherosclerosis Markers Involved in the Asymptomatic Phase of Atherosclerosis in the Adult Population: A Randomized, Placebo-Controlled, Crossover Trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mata, P.; Garrido, J.A.; Ordovas, J.M.; Blazquez, E.; Alvarez-Sala, L.A.; Rubio, M.J.; Alonso, R.; de Oya, M. Effect of dietary monounsaturated fatty acids on plasma lipoproteins and apolipoproteins in women. Am. J. Clin. Nutr. 1992, 56, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Miranda, J.; Perez-Martinez, P.; Marin, C.; Moreno, J.A.; Gomez, P.; Perez-Jimenez, F. Postprandial lipoprotein metabolism, genes and risk of cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Schnohr, P.; Jensen, G.B.; Benn, M. Nonfasting triglycerides, cholesterol, and ischemic stroke in the general population. Ann. Neurol. 2011, 69, 628–634. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Perez-Martinez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopoulos, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharm. 2011, 9, 258–270. [Google Scholar] [CrossRef]
- Langsted, A.; Freiberg, J.J.; Tybjaerg-Hansen, A.; Schnohr, P.; Jensen, G.B.; Nordestgaard, B.G. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: The Copenhagen City Heart Study with 31 years of follow-up. J. Intern. Med. 2011, 270, 65–75. [Google Scholar] [CrossRef]
- Van Wijk, D.F.; Stroes, E.S.; Kastelein, J.J. Lipid measures and cardiovascular disease prediction. Dis. Markers 2009, 26, 209–216. [Google Scholar] [CrossRef]
- Bayturan, O.; Tuzcu, E.M.; Lavoie, A.; Hu, T.; Wolski, K.; Schoenhagen, P.; Kapadia, S.; Nissen, S.E.; Nicholls, S.J. The metabolic syndrome, its component risk factors, and progression of coronary atherosclerosis. Arch. Intern. Med. 2010, 170, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Barzi, F.; Jamrozik, K.; Lam, T.H.; Ueshima, H.; Whitlock, G.; Woodward, M. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation 2004, 110, 2678–2686. [Google Scholar]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.T.; Gudnason, V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Watts, G.; Mikhailidis, D.; Pérez-Martínez, P.; Mora, S.; Bilianou, H.; Panotopoulos, G.; Katsiki, N.; Ooi, T.; Lopez-Miranda, J.; et al. Postprandial Hypertriglyceridaemia Revisited In The Era Of Non-Fasting Lipid Profile Testing: A 2019 Expert Panel Statement. Curr. Vasc. Pharm. 2019, 17, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Watts, G.; Mikhailidis, D.; Pérez-Martínez, P.; Mora, S.; Bilianou, H.; Panotopoulos, G.; Katsiki, N.; Ooi, T.; Lopez-Miranda, J.; et al. Postprandial Hypertriglyceridaemia Revisited in the Era of Non-Fasting Lipid Profile Testing: A 2019 Expert Panel Statement, Main Text. Curr. Vasc. Pharm. 2019, 17, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.; Watts, G.; Mikhailidis, D.; Pérez-Martínez, P.; Mora, S.; Bilianou, H.; Panotopoulos, G.; Katsiki, N.; Ooi, T.; Lopez-Miranda, J.; et al. Postprandial Hypertriglyceridaemia Revisited in the Era of Non-Fasting Lipid Profile Testing: A 2019 Expert Panel Statement, Narrative Review. Curr. Vasc. Pharm. 2019, 17, 515–537. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State of the Art on Functional Virgin Olive Oils Enriched with Bioactive Compounds and Their Properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar] [CrossRef] [Green Version]
- Marrugat, J.; Covas, M.-I.; Fitó, M.; Schroder, H.; Miró-Casas, E.; Gimeno, E.; Carmen López-Sabater, M.; de la Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oil on lipids and LDL oxidation: A randomized controlled trial. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.; Catapano, A. Treating High Density Lipoprotein Cholesterol (HDL-C): Quantity versus Quality. Curr. Pharm. Des. 2012, 19, 3841–3857. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Birner-Gruenberger, R.; Schittmayer, M.; Holzer, M.; Marsche, G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog. Lipid Res. 2014, 56, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Otocka-Kmiecik, A.; Mikhailidis, D.P.; Nicholls, S.J.; Davidson, M.; Rysz, J.; Banach, M. Dysfunctional HDL: A novel important diagnostic and therapeutic target in cardiovascular disease? Prog. Lipid Res. 2012, 51, 314–324. [Google Scholar] [CrossRef]
- Pedret, A.; Catalán, Ú.; Fernández-Castillejo, S.; Farràs, M.; Valls, R.-M.; Rubió, L.; Canela, N.; Aragonés, G.; Romeu, M.; Castañer, O.; et al. Impact of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on the HDL Proteome in Hypercholesterolemic Subjects: A Double Blind, Randomized, Controlled, Cross-Over Clinical Trial (VOHF Study). PLoS ONE 2015, 10, e0129160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized TrialOlive Oil Polyphenols and Heart Disease Risk. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Fitó, M.; López-Sabater, M.C.; Poulsen, H.E.; Nyyssönen, K.; Schröder, H.; Salonen, J.T.; De la Torre-Carbot, K.; Zunft, H.-F.; De la Torre, R.; et al. The effect of olive oil polyphenols on antibodies against oxidized LDL. A randomized clinical trial. Clin. Nutr. 2011, 30, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, J.; Tousoulis, D.; Toutouza, M.; Toutouzas, P.; Stefanadis, C. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am. Heart J. 2004, 147, 106–112. [Google Scholar] [CrossRef]
- Tortosa, A.; Bes-Rastrollo, M.; Sanchez-Villegas, A.; Basterra-Gortari, F.J.; Nunez-Cordoba, J.M.; Martinez-Gonzalez, M.A. Mediterranean diet inversely associated with the incidence of metabolic syndrome: The SUN prospective cohort. Diabetes Care 2007, 30, 2957–2959. [Google Scholar] [CrossRef] [Green Version]
- Perez-Martinez, P.; Garcia-Rios, A.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean diet rich in olive oil and obesity, metabolic syndrome and diabetes mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 5. Lifestyle management: Standards of Medical Care in Diabetesd2019. Diabetes Care 2019, 42 (Suppl. 1), S46–S60. [Google Scholar] [CrossRef] [Green Version]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61, 1700300. [Google Scholar] [CrossRef]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health Benefits of the Mediterranean Diet:An Update of Research Over the Last 5 Years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Olive oil and CVD: Accruing evidence of a protective effect. Br. J. Nutr. 2012, 108, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of Diabetes With Mediterranean Diets: A Subgroup Analysis of a Randomized TrialPrevention of Diabetes With Mediterranean Diets. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresserra-Rimbau, A.; Guasch-Ferré, M.; Salas-Salvadó, J.; Toledo, E.; Corella, D.; Castañer, O.; Guo, X.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Intake of total polyphenols and some classes of polyphenols is inversely associated with diabetes in elderly people at high cardiovascular disease risk. J. Nutr. 2016, 146, 767–777. [Google Scholar]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Portillo, M.P.; Moreno, J.J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef]
- Xiao, J.B.; Hogger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Gomez-Marin, B.; Gomez-Delgado, F.; Lopez-Moreno, J.; Alcala-Diaz, J.F.; Jimenez-Lucena, R.; Torres-Peña, J.D.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Yubero-Serrano, E.M.; del Mar Malagon, M.; et al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: The Cordioprev randomized trial. Am. J. Clin. Nutr. 2018, 108, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Basterra-Gortari, F.J.; Ruiz-Canela, M.; Martínez-González, M.A.; Babio, N.; Sorlí, J.V.; Fito, M.; Ros, E.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. Effects of a Mediterranean Eating Plan on the Need for Glucose-Lowering Medications in Participants With Type 2 Diabetes: A Subgroup Analysis of the PREDIMED Trial. Diabetes Care 2019, 42, 1390–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Cirs. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive Value of Brachial Flow-Mediated Dilation for Incident Cardiovascular Events in a Population-Based Study: The Multi-Ethnic Study of Atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, D.; Paolillo, S.; Della Ratta, G.; Mariniello, A.; Formisano, T.; Maria Pellegrino, A.; Perrone-Filardi, P. Endothelial function as a marker of pre-clinical atherosclerosis: Assessment techniques and clinical implications. Monaldi Arch. Chest Dis. 2013, 80, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancheti, S.; Shah, P.; Phalgune, D.S. Correlation of endothelial dysfunction measured by flow-mediated vasodilatation to severity of coronary artery disease. Indian Heart J. 2018, 70, 622–626. [Google Scholar] [CrossRef]
- Anderson, T.; Charbonneau, F.; M Title, L.; Buithieu, J.; Rose, M.; Conradson, H.; Hildebrand, K.; Fung, M.; Verma, S.; Lonn, E. Microvascular Function Predicts Cardiovascular Events in Primary Prevention Long-Term Results from the Firefighters and Their Endothelium (FATE) Study. Circulation 2011, 123, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; Caterina, R.D. Olive Oil and Red Wine Antioxidant Polyphenols Inhibit Endothelial Activation. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.A.; López-Miranda, J.; Gómez, P.; Benkhalti, F.; El Boustani, E.-S.; Pérez-Jiménez, F. Effect of phenolic compounds of virgin olive oil on LDL oxidation resistance. Med. Clin. (Barc.) 2003, 120, 128–131. [Google Scholar]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic Content of Virgin Olive Oil Improves Ischemic Reactive Hyperemia in Hypercholesterolemic Patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Valls, R.-M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.-I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatzi, K.; Papamichael, C.; Karatzis, E.; Papaioannou, T.G.; Voidonikola, P.T.; Vamvakou, G.D.; Lekakis, J.; Zampelas, A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: A synergistic effect of components of the Mediterranean diet. J. Am. Coll. Nutr. 2008, 27, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pena, J.D.; Garcia-Rios, A.; Delgado-Casado, N.; Gomez-Luna, P.; Alcala-Diaz, J.F.; Yubero-Serrano, E.M.; Gomez-Delgado, F.; Leon-Acuna, A.; Lopez-Moreno, J.; Camargo, A.; et al. Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis 2018, 269, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Garcia-Rios, A.; Perez-Martinez, P.; Fuentes, F.; Jiménez-Gomez, Y.; Gomez-Luna, M.J.; Parnell, L.D.; Marin, C.; Lai, C.Q.; Perez-Jimenez, F.; et al. Gene variations of nitric oxide synthase regulate the effects of a saturated fat rich meal on endothelial function. Clin. Nutr. 2011, 30, 234–238. [Google Scholar] [CrossRef]
- Jimenez-Morales, A.I.; Ruano, J.; Delgado-Lista, J.; Fernandez, J.M.; Camargo, A.; Lopez-Segura, F.; Villarraso, J.C.; Fuentes-Jimenez, F.; Lopez-Miranda, J.; Perez-Jimenez, F. NOS3 Glu298Asp polymorphism interacts with virgin olive oil phenols to determine the postprandial endothelial function in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1694–E1702. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef]
- Perez-Jimenez, F.; Alvarez de Cienfuegos, G.; Badimon, L.; Barja, G.; Battino, M.; Blanco, A.; Bonanome, A.; Colomer, R.; Corella-Piquer, D.; Covas, I.; et al. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Investig. 2005, 35, 421–424. [Google Scholar]
- Giugliano, D.; Esposito, K. Mediterranean diet and metabolic diseases. Curr. Opin. Lipidol. 2008, 19, 63–68. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: An in-depth review of the PREDIMED. Nutr. Diabetes 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Salvado, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gomez-Gracia, E.; Vinoles, E.; Aros, F.; Herrera, C.; et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruano, J.; López-Miranda, J.; de la Torre, R.; Delgado-Lista, J.; Fernández, J.; Caballero, J.; Covas, M.I.; Jiménez, Y.; Pérez-Martínez, P.; Marín, C.; et al. Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. Am. J. Clin. Nutr. 2007, 86, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza-Miranda, R.E.; Rangel, O.; Marin, C.; Pérez-Martínez, P.; Delgado-Lista, J.; Mariscal, C.; Peña-Orihuela, P.J.; Morales, I.; Malagón, M.M.; Tinahones, F.; et al. Virgin olive oil rich in phenolic compounds modulates the expression of atherosclerosis-related genes in vascular endothelium. Eur. J. Nutr. 2015, 55, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Rangel, O.; Mariscal, C.; Tormos, C.; Perez-Martinez, P.; Delgado-Lista, J.; Marin, C.; Quintana-Navarro, G.; Cerdá, C.; Sáez, G.; Lopez-Segura, F.; et al. Frying oils with high natural or added antioxidants content, which protect against postprandial oxidative stress, also protect against DNA oxidation damage. Eur. J. Nutr. 2016, 56. [Google Scholar]
- Capurso, C.; Massaro, M.; Scoditti, E.; Vendemiale, G.; Capurso, A. Vascular effects of the Mediterranean diet Part I: Anti-hypertensive and anti-thrombotic effects. Vasc. Pharmacol. 2014, 63, 118–126. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; Lista, J.D.; Pérez-Martínez, P.; López-Segura, F.; Fuentes, F.; Cortés, B.; Lozano, A.; López-Miranda, J. Olive oil and haemostasis: A review on its healthy effects. Public Health Nutr. 2006, 9, 1083–1088. [Google Scholar] [CrossRef]
- Smith, R.D.; Kelly, C.N.M.; Fielding, B.A.; Hauton, D.; Silva, K.D.R.R.; Nydahl, M.C.; Miller, G.J.; Williams, C.M. Long-term monounsaturated fatty acid diets reduce platelet aggregation in healthy young subjects. Br. J. Nutr. 2007, 90, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Pastori, D.; Farcomeni, A.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Bucci, T.; Carnevale, R.; Violi, F. Mediterranean diet reduces thromboxane A2 production in atrial fibrillation patients. Clin. Nutr. 2015, 34, 899–903. [Google Scholar] [CrossRef]
- Misikangas, M.; Freese, R.; Turpeinen, A.M.; Mutanen, M. High Linoleic Acid, Low Vegetable, and High Oleic Acid, High Vegetable Diets Affect Platelet Activation Similarly in Healthy Women and Men. J. Nutr. 2001, 131, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Karantonis, H.C.; Fragopoulou, E.; Antonopoulou, S.; Rementzis, J.; Phenekos, C.; Demopoulos, C.A. Effect of fast-food Mediterranean-type diet on type 2 diabetics and healthy human subjects’ platelet aggregation. Diabetes Res. Clin. Pract. 2006, 72, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic Lipid Minor Constituents from Vegetable Oils. Comparison between Olive Oils and Others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Lopez, S.; Bermúdez, B.; Abia, R.; Muriana, F.J.G. Extra-virgin vs. refined olive oil on postprandial hemostatic markers in healthy subjects. J. Thromb. Haemost. JTH 2006, 4, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.F.; Jespersen, J.; Marckmann, P. Are olive oil diets antithrombotic? Diets enriched with olive, rapeseed, or sunflower oil affect postprandial factor VII differently. Am. J. Clin. Nutr. 1999, 70, 976–982. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Lopez-Miranda, J.; Cortés, B.; Perez-Martinez, P.; Lozano, A.; Gomez-Luna, R.; Gomez, P.; Gomez, M.J.; Criado, J.; Fuentes, F.; et al. Chronic dietary fat intake modifies the postprandial response of hemostatic markers to a single fatty test meal. Am. J. Clin. Nutr. 2008, 87, 317–322. [Google Scholar] [CrossRef]
- Bravo-Herrera, M.D.; López-Miranda, J.; Marín, C.; Gómez, P.; Gómez, M.J.; Moreno, J.A.; Pérez-Martínez, P.; Blanco, A.; Jiménez-Gómez, Y.; Pérez-Jiménez, F. Tissue factor expression is decreased in monocytes obtained from blood during Mediterranean or high carbohydrate diets. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 128–132. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; David Torres-Peña, J.; Perez-Jimenez, F.; Perez-Martinez, P. Gut Microbiota: A New Marker of Cardiovascular Disease. Curr. Pharm. Des. 2017, 23, 3233–3238. [Google Scholar]
- Dumas, M.-E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef] [Green Version]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Montes-Borrego, M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B.; et al. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J. Clin. Endocrinol. Metab. 2016, 101, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotronen, A.; Yki-Järvinen, H. Fatty Liver A Novel Component of the Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2008, 28, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Arcopinto, M.; Schiavo, A.; Salzano, A.; Bossone, E.; D’Assante, R.; Marsico, F.; Demelo-Rodriguez, P.; Baliga, R.R.; Cittadini, A.; Marra, A.M. Metabolic Syndrome in Heart Failure: Friend or Foe? Heart Fail Clin. 2019, 15, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Perla, F.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Povsic, M.; Wong, O.Y.; Perry, R.; Bottomley, J. A Structured Literature Review of the Epidemiology and Disease Burden of Non-Alcoholic Steatohepatitis (NASH). Adv. Ther. 2019, 36, 1574–1594. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Abenavoli, L.; Pellicano, R.; Boccuto, L. Role of genetic and metabolism in non-alcoholic fatty liver disease. Panminerva Med. 2018, 60, 41–43. [Google Scholar]
- Boccuto, L.; Abenavoli, L. Genetic and Epigenetic Profile of Patients with Alcoholic Liver Disease. Ann. Hepatol. 2017, 16, 490–500. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Lama, A.; Pirozzi, C.; Mollica, M.P.; Trinchese, G.; Di Guida, F.; Cavaliere, G.; Calignano, A.; Mattace Raso, G.; Berni Canani, R.; Meli, R. Polyphenol-rich virgin olive oil reduces insulin resistance and liver inflammation and improves mitochondrial dysfunction in high-fat diet fed rats. Mol. Nutr. Food Res. 2017, 61, 1600418. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Lyon, C.J.; Hsueh, W.A. Nuclear Factor (Erythroid-Derived 2)-Like-2 Factor (Nrf2), a Key Regulator of the Antioxidant Response to Protect Against Atherosclerosis and Nonalcoholic Steatohepatitis. Curr. Diabetes Rep. 2013, 13, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, E.M.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Macarulla, M.T.; Aguirre, L.; Hijona, E.; Bujanda, L.; Milagro, F.; Martínez, J.A.; Portillo, M.P. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br. J. Nutr. 2011, 107, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Yang, Q.; Hao, J.; Guo, L.; Chen, X.; Hu, J.; Leng, L.; Jiang, Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int. Immunopharmacol. 2011, 11, 762–768. [Google Scholar] [CrossRef]
- Bujanda, L.; Hijona, E.; Larzabal, M.; Beraza, M.; Aldazabal, P.; García-Urkia, N.; Sarasqueta, C.; Cosme, A.; Irastorza, B.; González, A.; et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Marcolin, E.; San-Miguel, B.; Vallejo, D.; Tieppo, J.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Quercetin Treatment Ameliorates Inflammation and Fibrosis in Mice with Nonalcoholic Steatohepatitis. J. Nutr. 2012, 142, 1821–1828. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-J.; Jung, U.J.; Lee, M.-K.; Cho, S.-J.; Jung, H.-K.; Hong, J.H.; Park, Y.B.; Kim, S.R.; Shim, S.; Jung, J.; et al. Modulation of lipid metabolism by polyphenol-rich grape skin extract improves liver steatosis and adiposity in high fat fed mice. Mol. Nutr. Food Res. 2013, 57, 360–364. [Google Scholar] [CrossRef]
- Chang, H.C.; Peng, C.H.; Yeh, D.M.; Kao, E.S.; Wang, C.J. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014, 5, 734–739. [Google Scholar] [CrossRef]
- Guo, H.; Zhong, R.; Liu, Y.; Jiang, X.; Tang, X.; Li, Z.; Xia, M.; Ling, W. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition 2014, 30, 198–203. [Google Scholar] [CrossRef]
- Suda, I.; Ishikawa, F.; Hatakeyama, M.; Miyawaki, M.; Kudo, T.; Hirano, K.; Ito, A.; Yamakawa, O.; Horiuchi, S. Intake of purple sweet potato beverage effects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur. J. Clin. Nutr. 2008, 62, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Pepa, G.; Vetrani, C.; Lombardi, G.; Bozzetto, L.; Annuzzi, G.; Rivellese, A.A. Isocaloric dietary changes and non-alcoholic fatty liver disease in high cardiometabolic risk individuals. Nutrients 2017, 9, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Phenolic Compounds | Red Wine (mg/GAE/L) | White Wine (mg/GAE/L) |
|---|---|---|
| Catequin | 191 | 35 |
| Epigallocatechin | 82 | 21 |
| Gallic Acid | 95 | 7 |
| Cyanidin-3-glucoside | 3 | 0 |
| Malvidin-3-glucoside | 24 | 1 |
| Rutine | 9 | 0 |
| Quercetin | 8 | 0 |
| Myricetin | 9 | 0 |
| Caffeic acid | 7.1 | 2.8 |
| Resveratrol | 1.5 | 0 |
| Total content of phenolics | 2567 | 239 |
| Resource | Phenolic Compounds |
|---|---|
| Seed | gallic acid, (+)-catechin, epicatechin, dimeric procyanidin, proanthocyanidins |
| Skin | Proanthocyanidins, ellagic acid, myricetin, quercetin, kaempferol, trans-resveratrol |
| Leaf | myricetin, ellagic acid, kaempferol, quercetin, gallic acid |
| Stem | rutin, quercetin 3-O-glucuronide, trans-resveratrol, astilbin |
| Raisin | hydroxycinnamic acid, hydroxymethylfurfural |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. https://doi.org/10.3390/nu11112833
Ditano-Vázquez P, Torres-Peña JD, Galeano-Valle F, Pérez-Caballero AI, Demelo-Rodríguez P, Lopez-Miranda J, Katsiki N, Delgado-Lista J, Alvarez-Sala-Walther LA. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients. 2019; 11(11):2833. https://doi.org/10.3390/nu11112833
Chicago/Turabian StyleDitano-Vázquez, Paola, José David Torres-Peña, Francisco Galeano-Valle, Ana Isabel Pérez-Caballero, Pablo Demelo-Rodríguez, José Lopez-Miranda, Niki Katsiki, Javier Delgado-Lista, and Luis A. Alvarez-Sala-Walther. 2019. "The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil" Nutrients 11, no. 11: 2833. https://doi.org/10.3390/nu11112833
APA StyleDitano-Vázquez, P., Torres-Peña, J. D., Galeano-Valle, F., Pérez-Caballero, A. I., Demelo-Rodríguez, P., Lopez-Miranda, J., Katsiki, N., Delgado-Lista, J., & Alvarez-Sala-Walther, L. A. (2019). The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients, 11(11), 2833. https://doi.org/10.3390/nu11112833






