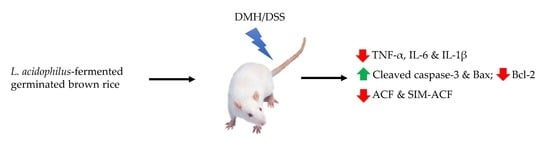
Lactobacillus acidophilus-Fermented Germinated Brown Rice Suppresses Preneoplastic Lesions of the Colon in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GBR and FGBR
2.2. Animals and Diets
2.3. Analysis of Aberrant Crypt Foci (ACF)
2.4. Analysis of Mucin-Producing ACF and Mucin-Depleted Foci (MDF)
2.5. Analysis of Cytokines
2.6. Analysis of Protein Expression
2.7. Statistical Analysis
3. Results
3.1. Effects of GBR and FGBR on Body Weight and Food Intake in Rats
3.2. Effects of GBR and FGBR on Colonic ACF in Rats
3.3. Effects of GBR and FGBR on Mucin Secretion and MDF in the Colon of Rats
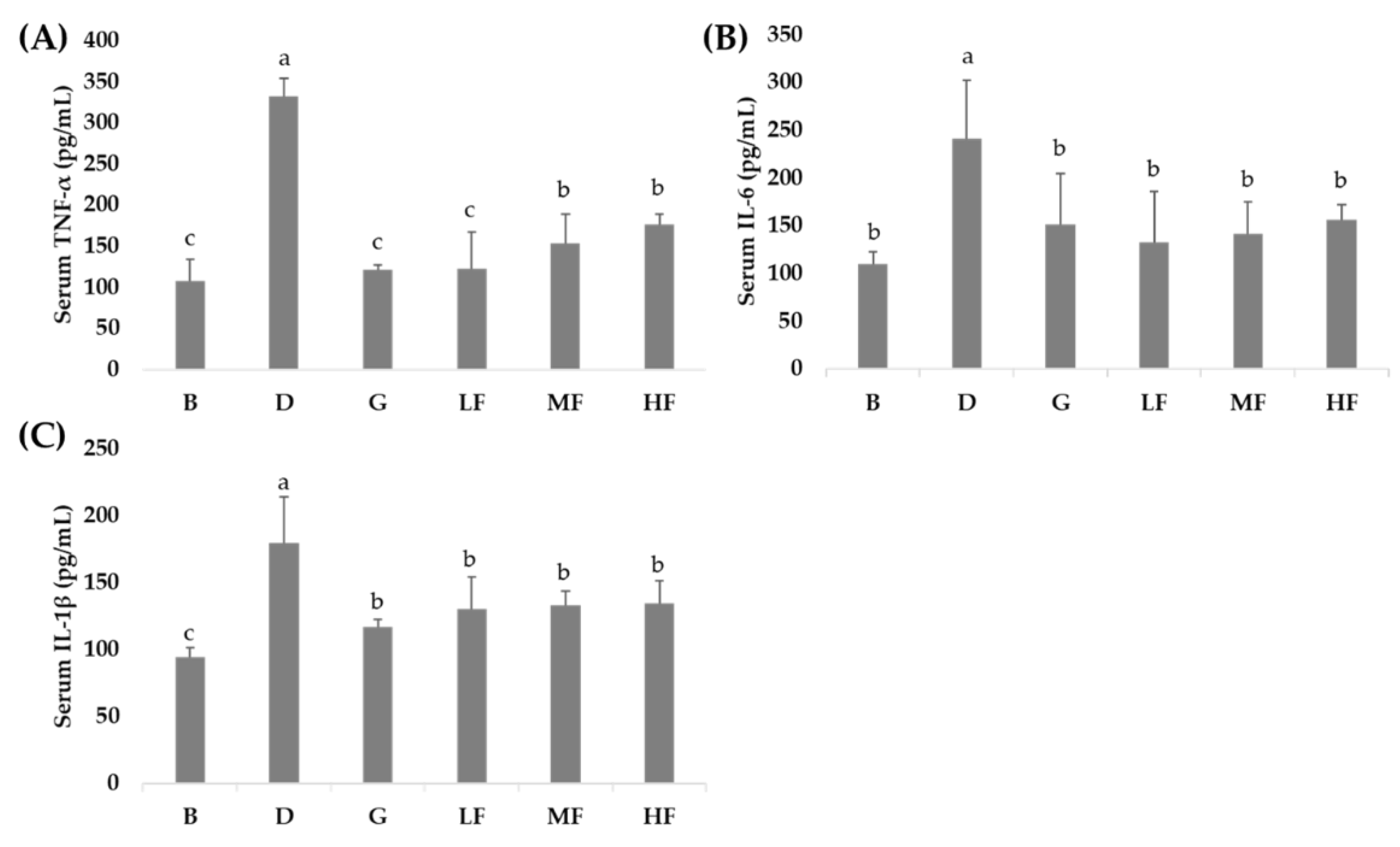
3.4. Effects of GBR and FGBR on Serum Pro-Inflammatory Cytokines in Rats
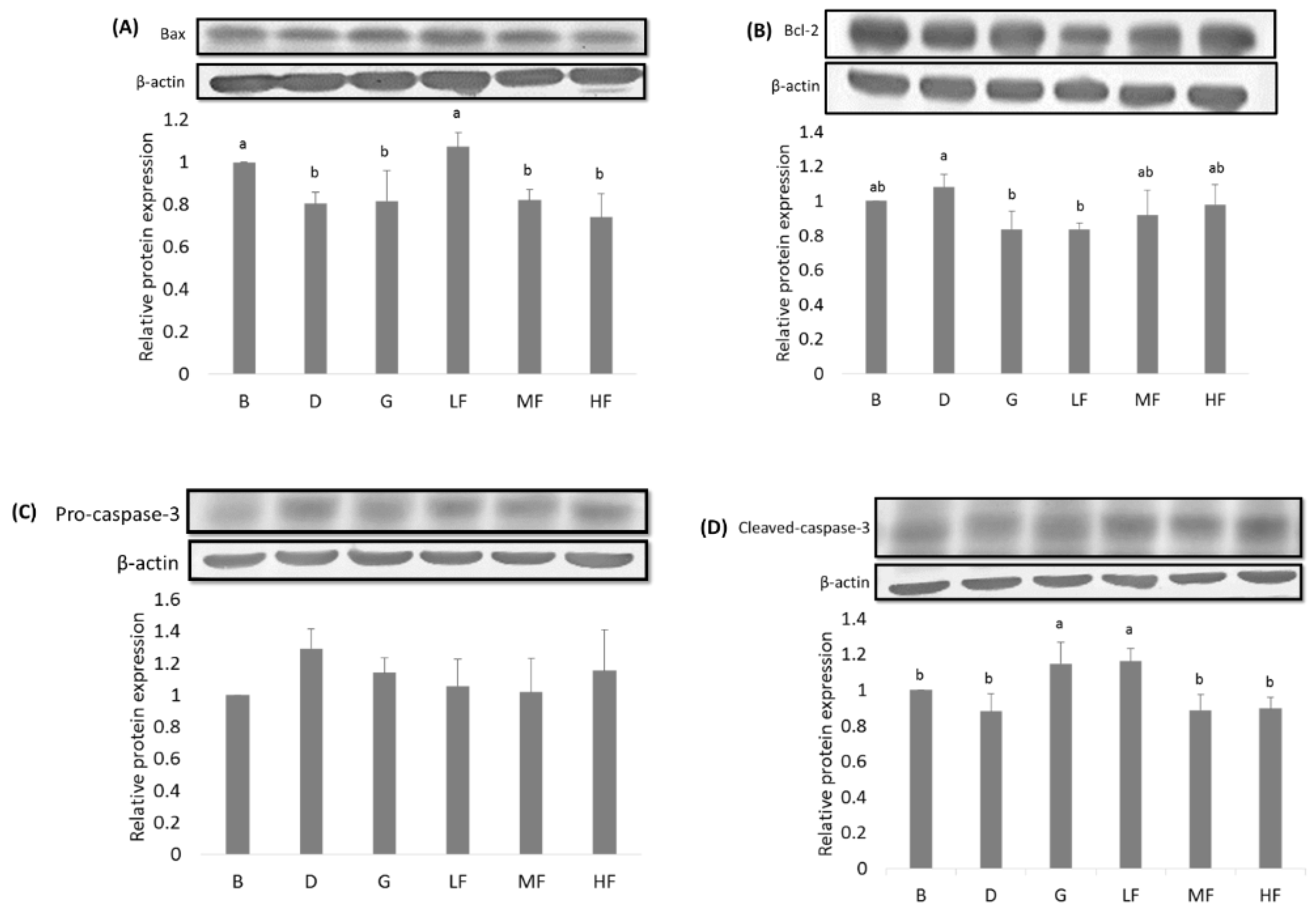
3.5. Effects of GBR and FGBR on the Expression of Apoptosis-Related Proteins in the Colon of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Saman, P.; Fuciños, P.; Vázquez, J.S.; Pandiella, S. Fermentability of Brown Rice and Rice Bran for Growth of Human Lactobacillus plantarum NCIMB. Food Technol. Biotechnol. 2011, 49, 128–132. [Google Scholar]
- Mattila-Sandholm, T.; Myllärinen, P.; Crittenden, R.; Mogensen, G.; Fondén, R.; Saarela, M. Technological challenges for future probiotic foods. Int. Dairy J. 2002, 12, 173–182. [Google Scholar] [CrossRef]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the Species and Application in Food Production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef]
- Hübner, F.; Arendt, E.K. Germination of Cereal Grains as a Way to Improve the Nutritional Value: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Probiotic Fermentation of Plant Based Products: Possibilities and Opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Nout, M. Rich nutrition from the poorest—Cereal fermentations in Africa and Asia. Food Microbiol. 2009, 26, 685–692. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive Properties of Dietary Rice Bran: Current Status and Future Prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Subhasree, R.S.; Ramaiyan, B.; Dinesh Babu, P. Evaluation of brown rice and germinated brown rice as an alternative substrate for probiotic food formulation using Lactobacillus spp. isolated from goat milk. Int. Food Res. J. 2013, 20, 2967–2971. [Google Scholar]
- Katyama, M.; Yoshimi, N.; Yamada, Y.; Sakata, K.; Kuno, T.; Yoshida, K.; Qiao, Z.; Vihn, P.Q.; Iwasaki, T.; Kobayashi, H.; et al. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol. Rep. 2002, 9, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Phutthaphadoong, S.; Yamada, Y.; Hirata, A.; Tomita, H.; Hara, A.; Limtrakul, P.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncol. Rep. 2010, 23, 53–59. [Google Scholar] [PubMed]
- Onuma, K.; Kanda, Y.; Suzuki Ikeda, S.; Sakaki, R.; Nonomura, T.; Kobayashi, M.; Osaki, M.; Shikanai, M.; Kobayashi, H.; Okada, F. Fermented Brown Rice and Rice Bran with Aspergillus oryzae (FBRA) Prevents Inflammation-Related Carcinogenesis in Mice, through Inhibition of Inflammatory Cell Infiltration. Nutrients 2015, 7, 10237–10250. [Google Scholar] [CrossRef]
- Itoh, M.; Nishibori, N.; Sagara, T.; Horie, Y.; Motojima, A.; Morita, K. Extract of Fermented Brown Rice Induces Apoptosis of Human Colorectal Tumor Cells by Activating Mitochondrial Pathway. Phytother. Res. 2012, 26, 1661–1666. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, S.Y.; Hong, S.M.; Hwang, S.G.; Park, D.K. The ethyl acetate extract of Phellinus linteus grown on germinated brown rice induces G0/G1 cell cycle arrest and apoptosis in human colon carcinoma HT29 cells. Phytother. Res. 2010, 24, 1019–1026. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.B.; Lee, S.J.; Song, M. Phellinus linteus Grown on Germinated Brown Rice Increases Cetuximab Sensitivity of KRAS-Mutated Colon Cancer. Int. J. Mol. Sci. 2017, 18, 1746. [Google Scholar] [CrossRef]
- Park, D.K.; Lim, Y.H.; Park, H.J. Antrodia camphorata grown on germinated brown rice inhibits HT-29 human colon carcinoma proliferation through inducing G0/G1 phase arrest and apoptosis by targeting the beta-catenin signaling. J. Med. Food 2013, 16, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Li, S.C.; Lin, H.P.; Shih, C.K. Germinated brown rice combined with Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis inhibits colorectal carcinogenesis in rats. Food Sci. Nutr. 2019, 7, 216–224. [Google Scholar] [PubMed]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef]
- Jenab, M.; Chen, J.-M.; Thompson, L.U. Sialomucin production in aberrant crypt foci relates to degree of dysplasia and rate of cell proliferation. Cancer Lett. 2001, 165, 19–25. [Google Scholar] [CrossRef]
- Caderni, G.; Femia, A.P.; Giannini, A.; Favuzza, A.; Luceri, C.; Salvadori, M.; Dolara, P. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: Correlation with carcinogenesis. Cancer Res. 2003, 63, 2388–2392. [Google Scholar] [PubMed]
- Islam, A.; Gallaher, D.D. Wheat Type (Class) Influences Development and Regression of Colon Cancer Risk Markers in Rats. Nutr. Cancer 2015, 67, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Kozaki, K.; Nishikawa, Y.; Yamamoto, M.; Fukami, H.; Inoue, M.; Wakabayashi, K.; Tatematsu, M. Development and distribution of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced aberrant crypt foci in the rat large intestine. Jpn. J. Cancer Res. 1999, 90, 720–725. [Google Scholar] [CrossRef]
- Uchida, K.; Kado, S.; Ando, M.; Nagata, Y.; Takagi, A.; Onoue, M. A mucinous histochemical study on malignancy of aberrant crypt foci (ACF) in rat colon. J. Vet. Med. Sci. 2001, 63, 145–149. [Google Scholar] [CrossRef]
- Milosevic, V.; Vukmirovic, F.; Zindovic, M.; Krstic, M.; Milenkovic, S.; Jancic, S. Interplay between expression of leptin receptors and mucin histochemical aberrations in colorectal adenocarcinoma. Rom. J. Morphol. 2015, 56, 709–716. [Google Scholar]
- Kim, S.; Guo, J.; O’Sullivan, M.G.; Gallaher, D.D.; Turesky, R.J. Comparative DNA adduct formation and induction of colonic aberrant crypt foci in mice exposed to 2-amino-9H-pyrido[2-b]indole, 2-amino-3,4-dimethylimidazo[4–f]quinoline, and azoxymethane. Environ. Mol. Mutagen. 2016, 57, 125–136. [Google Scholar] [CrossRef]
- Guina, T.; Biasi, F.; Calfapietra, S.; Nano, M.; Poli, G. Inflammatory and redox reactions in colorectal carcinogenesis. Ann. N. Y. Acad. Sci. 2015, 1340, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latifah, S.Y.; Armania, N.; Tze, T.H.; Azhar, Y.; Nordiana, A.H.; Norazalina, S.; Hairuszah, I.; Saidi, M.; Maznah, I. Germinated brown rice (GBR) reduces the incidence of aberrant crypt foci with the involvement of beta-catenin and COX-2 in azoxymethane-induced colon cancer in rats. Nutr. J. 2010, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Saki, E.; Saiful, Y.L.; Mohd Ali, R.; Ahmad, Z. Chemopreventive Effects of Germinated Rough Rice Crude Extract in Inhibiting Azoxymethane-Induced Aberrant Crypt Foci Formation in Sprague-Dawley Rats. Biomed. Res. Int. 2017, 2017, 9517287. [Google Scholar] [CrossRef] [PubMed]
- Tuntipopipat, S.; Muangnoi, C.; Thiyajai, P.; Srichamnong, W.; Charoenkiatkul, S.; Praengam, K. A bioaccessible fraction of parboiled germinated brown rice exhibits a higher anti-inflammatory activity than that of brown rice. Food Funct. 2015, 6, 1480–1488. [Google Scholar] [CrossRef]
- Peran, L.; Camuesco, D.; Comalada, M.; Bailon, E.; Henriksson, A.; Xaus, J.; Zarzuelo, A.; Galvez, J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J. Appl. Microbiol. 2007, 103, 836–844. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Lee, J.H.; Kim, J.H.; Che, X.; Ma, H.W.; Seo, D.H.; Kim, T.I.; Kim, W.H.; Kim, S.W.; et al. Lactobacillus acidophilus suppresses intestinal inflammation by inhibiting endoplasmic reticulum stress. J. Gastroenterol. Hepatol. 2019, 34, 178–185. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Martoni, C.J.; Gundamaraju, R.; Ahuja, K.D.K.; Ball, M.; Eri, R. A human origin strain Lactobacillus acidophilus DDS-1 exhibits superior in vitro probiotic efficacy in comparison to plant or dairy origin probiotics. Int. J. Med Sci. 2018, 15, 840–848. [Google Scholar] [CrossRef]
- Li, S.C.; Hsu, W.F.; Chang, J.S.; Shih, C.K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar]
- Sivamaruthi, B.; Kesika, P.; Chaiyasut, C. A comprehensive review on functional properties of fermented rice bran. Pharmacogn. Rev. 2018, 12, 218–224. [Google Scholar] [CrossRef]
- Kondo, S.; Kuda, T.; Nemoto, M.; Usami, Y.; Takahashi, H.; Kimura, B. Protective effects of rice bran fermented by Saccharomyces cerevisiae Misaki-1 and Lactobacillus plantarum Sanriki-SU8 in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2016, 16, 44–49. [Google Scholar] [CrossRef]
- Islam, J.; Koseki, T.; Watanabe, K.; Ardiansyah, M.; Budijanto, S.; Oikawa, A.; Alauddin, M.; Goto, T.; Aso, H.; Komai, M.; et al. Dietary Supplementation of Fermented Rice Bran Effectively Alleviates Dextran Sodium Sulfate-Induced Colitis in Mice. Nutrients 2017, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Ogasa, S.; Kuwahara, T.; Bando, Y.; Hagiwara, M.; Arimochi, H.; Nakanishi, S.; Iwasaki, T.; Ohnishi, Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig. Dis. Sci. 2008, 53, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, H.-J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharm. 2014, 154, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Park, H.J. Ethanol Extract of Antrodia camphorata Grown on Germinated Brown Rice Suppresses Inflammatory Responses in Mice with Acute DSS-Induced Colitis. Evid. Based Complement. Altern. Med. 2013, 2013, 914524. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Walia, S.; Kamal, R.; Dhawan, D.K.; Kanwar, S.S. Chemoprevention by Probiotics During 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Dig. Dis. Sci. 2018, 63, 900–909. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-Y.; Hwang, I.-G.; Joung, E.-M.; Kim, T.-M.; Kim, D.-J.; Park, D.-S.; Lee, J.-S.; Jeong, H.-S. Antiproliferation Effects of Germinated-Korean Rough Rice Extract on Human Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2010, 39, 325–330. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, S.-H.; Hwang, I.-G.; Kim, T.-M.; Park, D.-S.; Kim, J.-H.; Kim, D.-J.; Lee, J.-S.; Jeong, H.-S. Antioxidant Activity and Anticancer Effects of Rough Rice (Oryza sativa L.) by Germination Periods. J. Korean Soc. Food Sci. Nutr. 2012, 41, 14–19. [Google Scholar] [CrossRef]
- Kim, D.-J.; Oh, S.-K.; Yoon, M.-R.; Chun, A.-R.; Choi, I.-S.; Lee, D.-H.; Lee, J.-S.; Yu, K.-W.; Kim, Y.-K. The Change in Biological Activities of Brown Rice and Germinated Brown Rice. J. Korean Soc. Food Sci. Nutr. 2011, 40, 781–789. [Google Scholar] [CrossRef]
- Chung, S.I.; Lee, S.C.; Yi, S.J.; Kang, M.Y. Antioxidative and antiproliferative activities of ethanol extracts from pigmented giant embryo rice (Oryza sativa L. cv. Keunnunjami) before and after germination. Nutr. Res. Pract. 2018, 12, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Huang, C.T.; Lin, Y.C.; Jung, S.M.; Lin, T.Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Soltan Dallal, M.M.; Mojarrad, M.; Baghbani, F.; Raoofian, R.; Mardaneh, J.; Salehipour, Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Arch. Iran. Med. 2015, 18, 167–172. [Google Scholar] [PubMed]
- Edo Zulfafamy, K.; Ardiansyah, A.; Budijanto, S. Antioxidative Properties and Cytotoxic Activity Against Colon Cancer Cell WiDr of Rhizopus oryzae and Rhizopus oligosporus-Fermented Black Rice Bran Extract. Curr. Res. Nutr. Food Sci. J. 2018, 6, 23–34. [Google Scholar] [CrossRef]
- Jeon, T.-I.; Jung, C.-H.; Cho, J.-Y.; Park, D.K.; Moon, J.-H. Identification of an anticancer compound against HT-29 cells from Phellinus linteus grown on germinated brown rice. Asian Pac. J. Trop. Biomed. 2013, 3, 785–789. [Google Scholar] [CrossRef]
- Park, H.-J. Phellinus linteus grown on germinated brown rice Suppress metastasis and Induce Apoptosis of Colon Cancer Cells by suppressing NF-κB and Wnt/β-catenin Signaling Pathways. J. Funct. Foods 2015, 14, 289–298. [Google Scholar] [CrossRef]
- Tamaruay, K.; Intaket, R.; Kaewkumsan, P. The effect of fermentation of germinated brown rice and KKU URL0381 cultivar black rice on GABA content and chemical composition of by-product and fermented rice juice. Food Appl. Biosci. J. 2015, 3, 21–29. [Google Scholar]
- Mongkontanawat, N. Fermentation of Germinated Native Black Rice Milk Mixture by Probiotic Lactic Acid Bacteria. Int. J. Nutr. Food Eng. 2016, 10, 243–247. [Google Scholar]
- Song, L.; Du, A.; Xiong, Y.; Jiang, J.; Zhang, Y.; Tian, Z.; Yan, H. Gamma-Aminobutyric acid inhibits the proliferation and increases oxaliplatin sensitivity in human colon cancer cells. Tumour Biol. 2016, 37, 14885–14894. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of GABA (gamma—Aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Sung, J.; Lee, J.; Oh, S.-K.; Lee, J.-S.; Choi, W.-S. Changes in Phytochemical Content and Antiproliferative Activity of Germinated Geunnun and Ilpum Rice Varieties. J. Korean Soc. Food Sci. Nutr. 2013, 42, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Kook, M.C.; Seo, M.J.; Cheigh, C.I.; Pyun, Y.R.; Cho, S.C.; Park, H. Enhanced production of gamma-aminobutyric acid using rice bran extracts by Lactobacillus sakei B2. J. Microbiol. Biotechnol. 2010, 20, 763–766. [Google Scholar] [PubMed]
- Webber, D.M.; Hettiarachchy, N.S.; Li, R.; Horax, R.; Theivendran, S. Phenolic Profile and Antioxidant Activity of Extracts Prepared from Fermented Heat-Stabilized Defatted Rice Bran. J. Food Sci. 2014, 79, 2383. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.Y.; Abd Razak, D.; Jamaluddin, A.; Sharifudin, S.; Long, K. Bioactive compounds and antioxidant activity of rice bran fermented with lactic acid bacteria. Malays. J. Microbiol. 2015, 11, 156–162. [Google Scholar]
- Nisa, K.; Rosyida, V.T.; Nurhayati, S.; Indrianingsih, A.W.; Darsih, C.; Apriyana, W. Total phenolic contents and antioxidant activity of rice bran fermented with lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012020. [Google Scholar] [CrossRef]
- Jung, T.D.; Shin, G.H.; Kim, J.M.; Choi, S.I.; Lee, J.H.; Lee, S.J.; Park, S.J.; Woo, K.S.; Oh, S.K.; Lee, O.H. Comparative Analysis of gamma-Oryzanol, beta-Glucan, Total Phenolic Content and Antioxidant Activity in Fermented Rice Bran of Different Varieties. Nutrients 2017, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice Bran Fermented with Saccharomyces boulardii Generates Novel Metabolite Profiles with Bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef]
- Theilmann, M.C.; Goh, Y.J.; Nielsen, K.F.; Klaenhammer, T.R.; Barrangou, R.; Hachem, M.A. Lactobacillus acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals. mBio 2017, 8, e01421-17. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Rollán, G.C.; Gerez, C.L.; Leblanc, J.G. Lactic Fermentation as a Strategy to Improve the Nutritional and Functional Values of Pseudocereals. Front. Nutr. 2019, 6, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawai, K.; Prajapati, J. Safety aspects of fermented and probiotic foods. Int. J. Food Fermented 2017, 6, 45–55. [Google Scholar] [CrossRef]
- Linsalata, M.; Orlando, A.; Russo, F. Pharmacological and dietary agents for colorectal cancer chemoprevention: Effects on polyamine metabolism (Review). Int. J. Oncol. 2014, 45, 1802–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Zhang, Q.-X.; Lu, R.-R. Surface layer protein from Lactobacillus acidophilus NCFM inhibit intestinal pathogen-induced apoptosis in HT-29 cells. Int. J. Boil. Macromol. 2017, 96, 766–774. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Zhang, L.; Xu, S.; Zhang, Q.; Lu, R.-R. A surface-layer protein from Lactobacillus acidophilus NCFM induces autophagic death in HCT116 cells requiring ROS-mediated modulation of mTOR and JNK signaling pathways. Food Funct. 2019, 10, 4102–4112. [Google Scholar] [CrossRef]
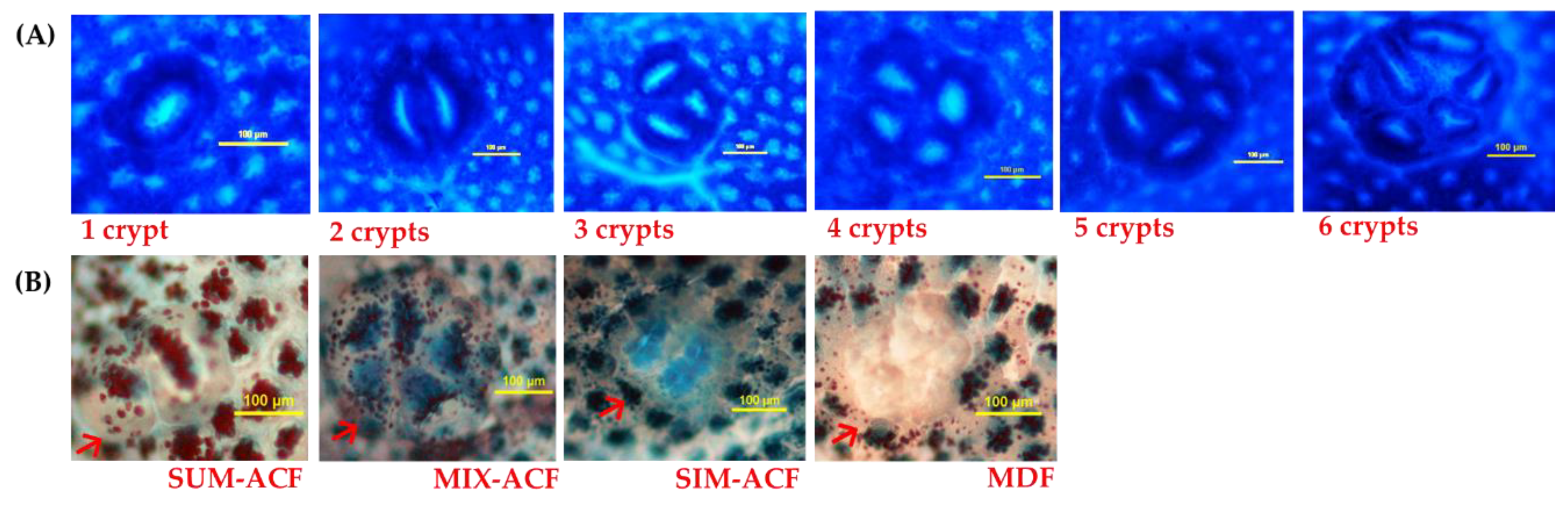
| Group 3 | ACF with | Number of ACF (number/cm2) | Number of AC (number/cm2) | ||
|---|---|---|---|---|---|
| 1 crypt | 2 crypts | 3 crypts | |||
| D | 2.2 ± 0.8 a | 3.3 ± 0.9 a | 1.5 ± 0.4 a | 7.8 ± 2.1 a | 16.6 ± 4.6 a |
| G | 1.6 ± 0.8 b | 2.8 ± 1.2 ab | 1.5 ± 0.8 a | 6.7 ± 2.7 ab | 14.8 ± 6.1 a |
| LF | 1.3 ± 0.4 b | 2.0 ± 0.7 b | 1.0 ± 0.3 b | 4.6 ± 0.9 b | 9.7 ± 2.0 b |
| MF | 1.5 ± 0.8 b | 2.7 ± 1.0 ab | 1.3 ± 0.7 ab | 6.1 ± 2.3 ab | 13.3 ± 5.2 ab |
| HF | 1.4 ± 0.6 b | 2.8 ± 1.5 ab | 1.4 ± 0.6 ab | 6.5 ± 2.8 ab | 14.7 ± 6.6 a |
| Group 3 | Number of ACF-Producing 4 (number/cm2) | MDF | ||
|---|---|---|---|---|
| SUM | MIX | SIM | ||
| D | 8.5 ± 3.8 a | 0.9 ± 0.5 b | 2.0 ± 1.8 a | 0.12 ± 0.25 a |
| G | 8.2 ± 5.2 a | 1.4 ± 0.8 ab | 1.9 ± 2.1 a | 0.12 ± 0.22 a |
| LF | 6.1 ± 1.7 a | 1.1 ± 0.7 b | 0.3 ± 0.3 b | 0 a |
| MF | 8.4 ± 4.3 a | 2.1 ± 1.2 a | 1.0 ± 1.3 ab | 0.08 ± 0.26 a |
| HF | 9.2 ± 4.5 a | 2.3 ± 1.7 a | 0.9 ± 0.8 ab | 0.14 ± 0.22 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-C.; Lin, H.-P.; Chang, J.-S.; Shih, C.-K. Lactobacillus acidophilus-Fermented Germinated Brown Rice Suppresses Preneoplastic Lesions of the Colon in Rats. Nutrients 2019, 11, 2718. https://doi.org/10.3390/nu11112718
Li S-C, Lin H-P, Chang J-S, Shih C-K. Lactobacillus acidophilus-Fermented Germinated Brown Rice Suppresses Preneoplastic Lesions of the Colon in Rats. Nutrients. 2019; 11(11):2718. https://doi.org/10.3390/nu11112718
Chicago/Turabian StyleLi, Sing-Chung, Han-Pei Lin, Jung-Su Chang, and Chun-Kuang Shih. 2019. "Lactobacillus acidophilus-Fermented Germinated Brown Rice Suppresses Preneoplastic Lesions of the Colon in Rats" Nutrients 11, no. 11: 2718. https://doi.org/10.3390/nu11112718
APA StyleLi, S.-C., Lin, H.-P., Chang, J.-S., & Shih, C.-K. (2019). Lactobacillus acidophilus-Fermented Germinated Brown Rice Suppresses Preneoplastic Lesions of the Colon in Rats. Nutrients, 11(11), 2718. https://doi.org/10.3390/nu11112718