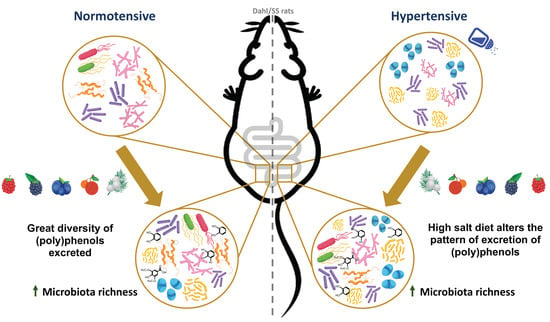
Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Preparation and Characterization of Diets
2.2.1. Fruit Puree (Berry Mixture)
2.2.2. Low-Salt and High-Salt diets
2.2.3. Determination of Proximate Composition
2.2.4. Enzymatic Hydrolysis of Glycosides
2.2.5. HPLC–MS Analysis of (Poly)Phenols from Diets
2.3. Animals
2.4. Plasma, Urine, Faeces and Tissues Treatment
2.4.1. Plasma Samples
2.4.2. Urine Samples
2.4.3. Faecal Samples
2.4.4. Tissues Samples (Liver and Heart)
2.5. Analysis of Berries Metabolites by UPLC–ESI-MS/MS
2.6. 16S rRNA Gene Sequencing Analysis of Gut Microbiota
2.7. Short Chain Fatty Acids (SCFAs) Analysis
2.8. Statistical Analysis
3. Results
3.1. Nutritional Composition of the Diets and Berry Mixture
3.2. (Poly)Phenols Content in Diets
3.3. Analysis of Aglycones after Enzyme Hydrolysis of Glycosides
3.4. Beneficial Effects of Berries against Chronic Salt Consumption
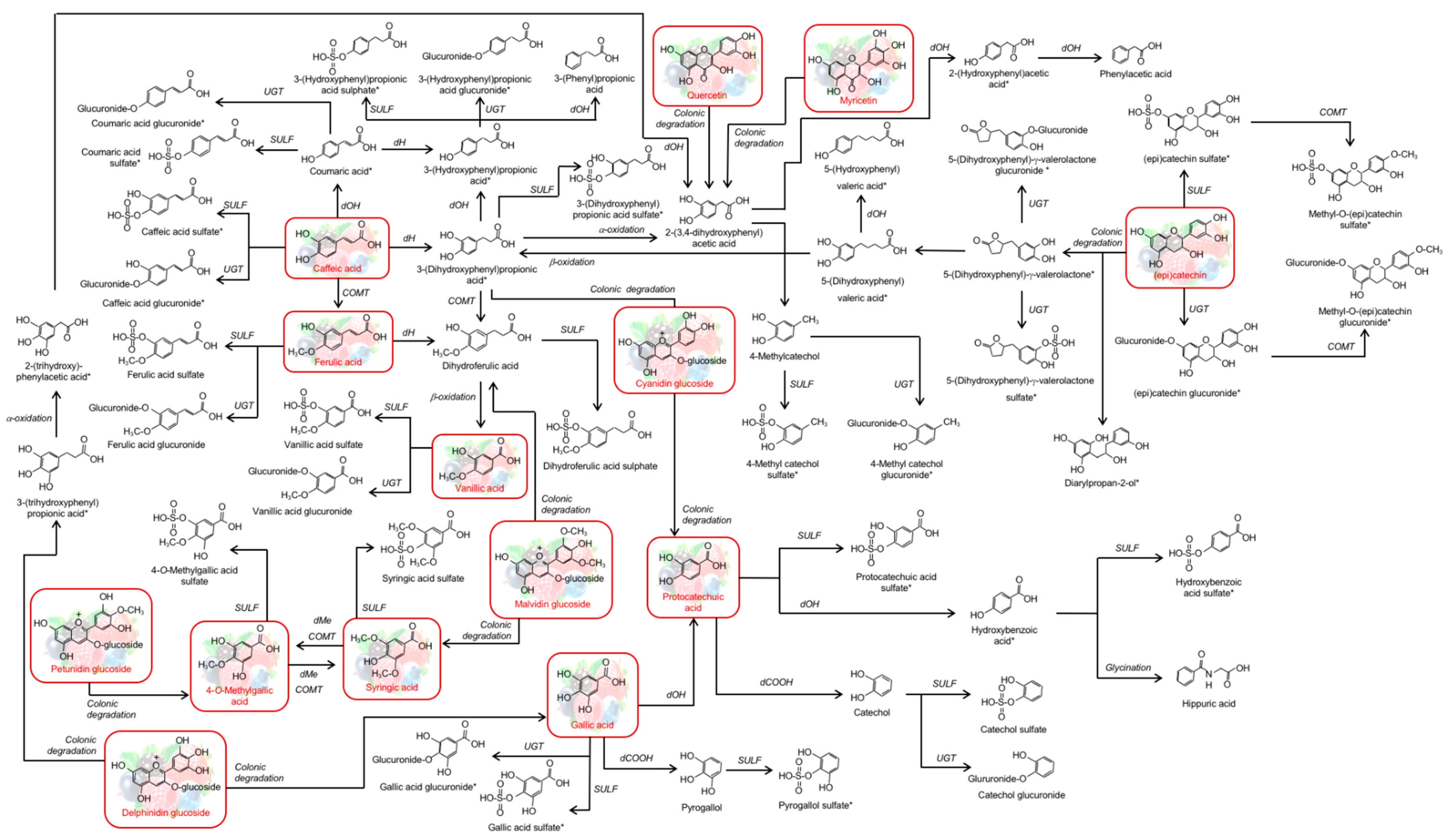
3.5. Metabolic Fate of (Poly)Phenols
3.5.1. Urinary (Poly)Phenols Excretion
3.5.2. Faecal (Poly)Phenol Excretion
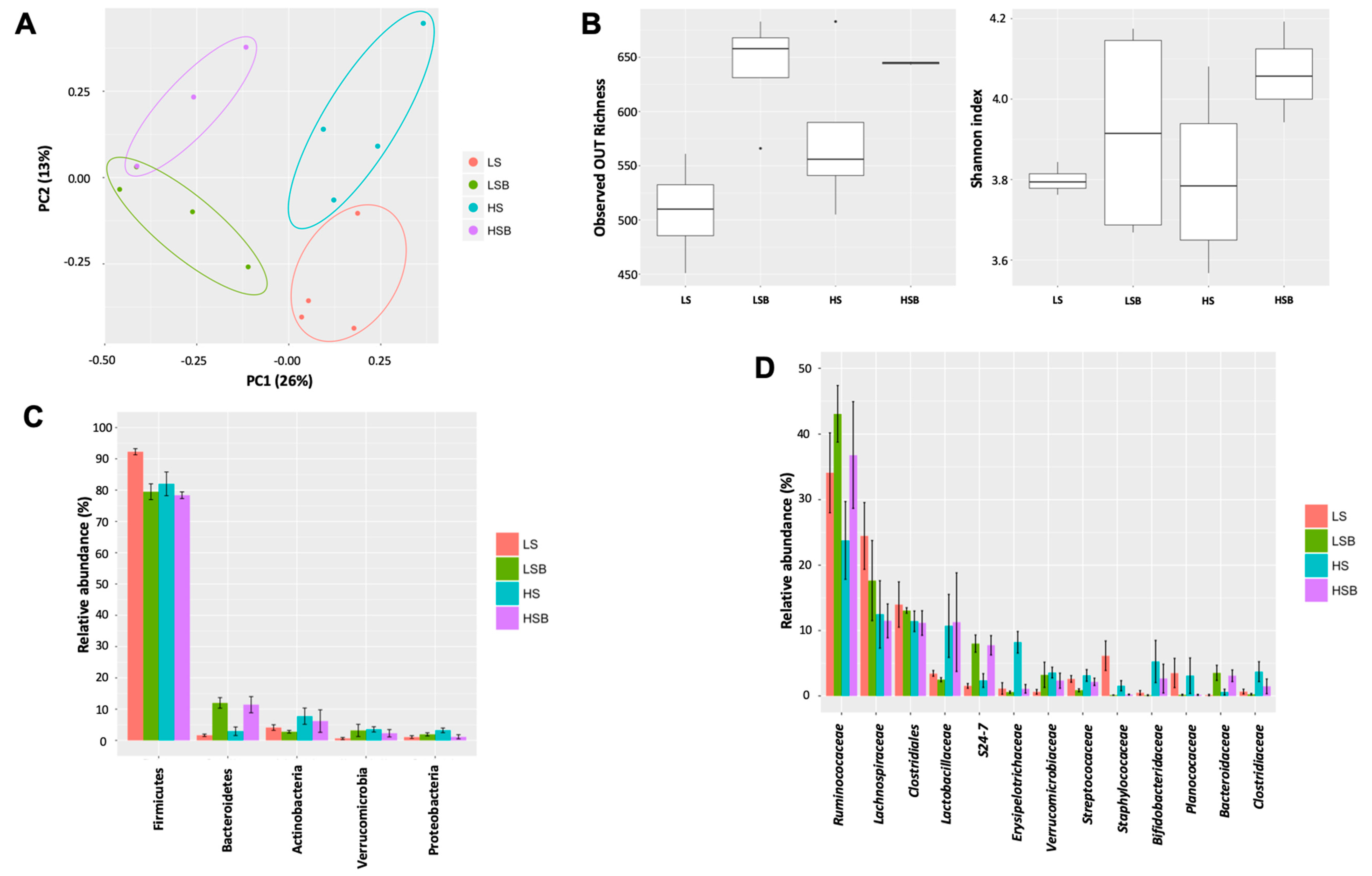
3.6. Salt and Berry Consumption Alter Gut Microbiota Composition
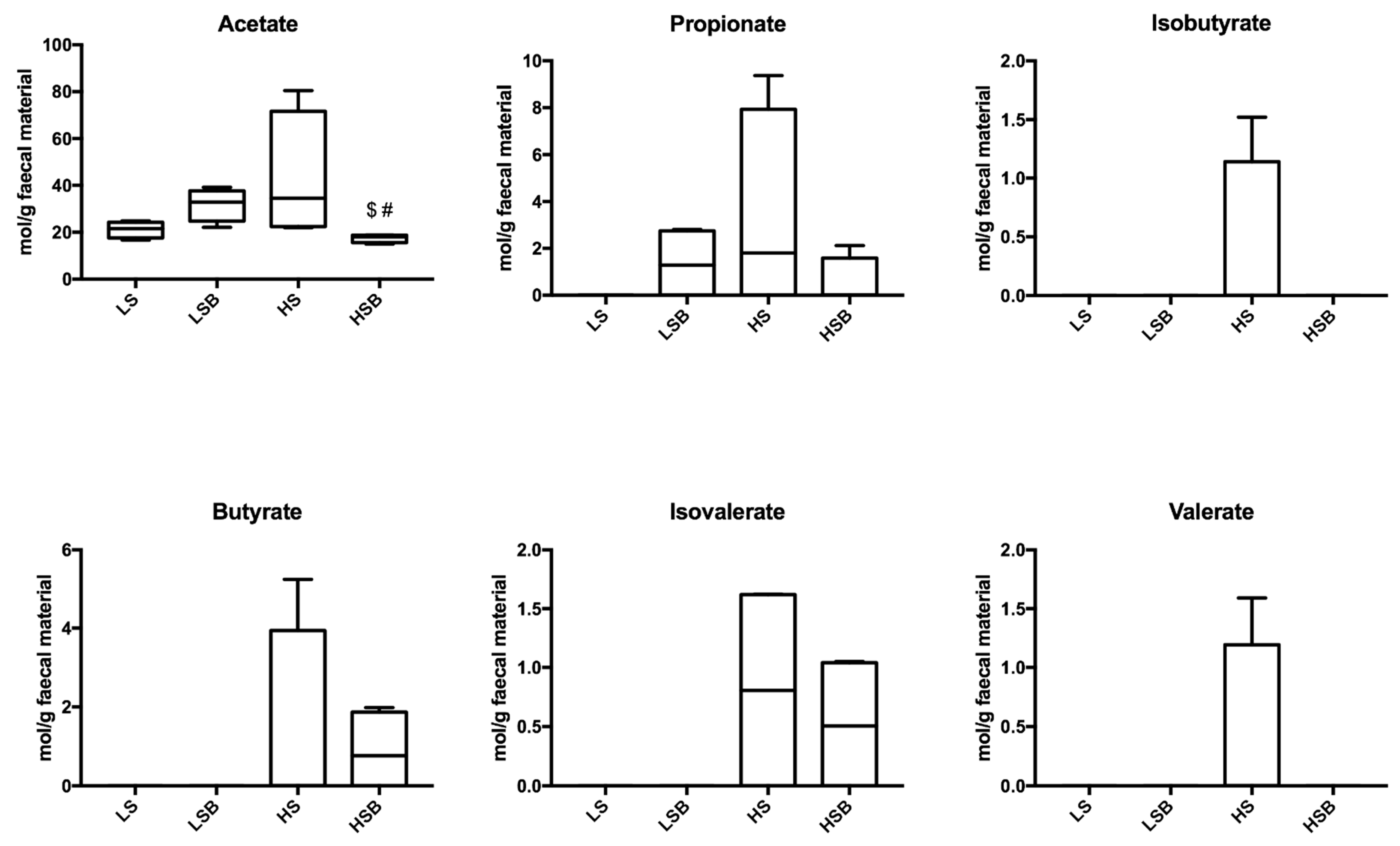
3.7. Salt and Berries Affect SCFA in Faeces
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- McAloon, C.J.; Boylan, L.M.; Hamborg, T.; Stallard, N.; Osman, F.; Lim, P.B.; Hayat, S.A. The changing face of cardiovascular disease 2000–2012: An analysis of the world health organisation global health estimates data. Int. J. Cardiol. 2016, 224, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected Effect of Dietary Salt Reductions on Future Cardiovascular Disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M.; Damasceno, A. Hypertension in developing countries. Lancet 2016, 380, 611–619. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global Sodium Consumption and Death from Cardiovascular Causes. N. Engl. J. Med. 2014, 371, 624–634. [Google Scholar] [CrossRef]
- Mohan, S.; Campbell, N.R.C. Salt and high blood pressure. Clin. Sci. 2009, 117, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.K.; Love, R.A. Etiological role of sodium chloride intake in essential hypertension in humans. J. Am. Med. Assoc. 1957, 164, 397–400. [Google Scholar] [CrossRef]
- Baldo, M.P.; Rodrigues, S.L.; Mill, J.G. High salt intake as a multifaceted cardiovascular disease: New support from cellular and molecular evidence. Heart Fail. Rev. 2015, 20, 461–474. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Medica 2002, 44, 20–23. [Google Scholar] [PubMed]
- Lee, I.T.; Chan, Y.C.; Lin, C.W.; Lee, W.J.; Sheu, W.H.-H. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet. Med. 2008, 25, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Low-calorie cranberry juice supplementation reduces plasma oxidized LDL and cell adhesion molecule concentrations in men. Br. J. Nutr. 2008, 99, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008, 87, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A.; Figueroa, A.; Navaei, N.; Wong, A.; Kalfon, R.; Ormsbee, L.T.; Feresin, R.G.; Elam, M.L.; Hooshmand, S.; Payton, M.E.; et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015, 115, 369–377. [Google Scholar] [CrossRef]
- Feresin, R.G.; Johnson, S.A.; Pourafshar, S.; Campbell, J.C.; Jaime, S.J.; Navaei, N.; Elam, M.L.; Akhavan, N.S.; Alvarez-Alvarado, S.; Tenenbaum, G.; et al. Impact of daily strawberry consumption on blood pressure and arterial stiffness in pre- and stage 1-hypertensive postmenopausal women: A randomized controlled trial. Food Funct. 2017, 8, 4139–4149. [Google Scholar] [CrossRef]
- Oudot, C.; Gomes, A.; Nicolas, V.; Le Gall, M.; Chaffey, P.; Broussard, C.; Calamita, G.; Mastrodonato, M.; Gena, P.; Perfettini, J.-L.; et al. CSRP3 mediates polyphenols-induced cardioprotection in hypertension. J. Nutr. Biochem. 2019, 66, 29–42. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Maier, C.S. The chemistry of gut microbial metabolism of polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.K.P.; Knight, R.; Spector, T.D. Can Your Microbiome Tell You What to Eat? Cell Metab. 2015, 22, 960–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Dew, T.; Oliveira, P.B.; Williamson, G.; Ferreira, R.B.; Santos, C.N. Analysis of phenolic compounds in Portuguese wild and commercial berries after multienzyme hydrolysis. J. Agric. Food Chem. 2013, 61, 4053–4062. [Google Scholar] [CrossRef]
- Latimer, D.G.W., Jr. (Ed.) Official Methods of Analysis of Association of Official Analytical Chemists, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Kjeldahl, J. New method for the determination of nitrogen in organic substances. Z. Anal. Chem. 1883, 22, 366–383. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Macià, A.; Valls, R.M.; Fernández, S.; Solà, R.; Motilva, M.-J. Improved method for identifying and quantifying olive oil phenolic compounds and their metabolites in human plasma by microelution solid-phase extraction plate and liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.-P.; Pantaleón, A.; Rozek, A.; Soler, A.; Valls, J.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Rapid analysis of procyanidins and anthocyanins in plasma by microelution SPE and ultra-HPLC. J. Sep. Sci. 2010, 33, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Serra, A.; Macià, A.; Borràs, X.; Romero, M.P.; Motilva, M.J. Validation of determination of plasma metabolites derived from thyme bioactive compounds by improved liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Rubió, L.; Borràs, X.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Mosele, J.I.; Martín-Peláez, S.; Macià, A.; Farràs, M.; Valls, R.M.; Catalán, Ú.; Motilva, M.J. Faecal microbial metabolism of olive oil phenolic compounds: In Vitro and In Vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.-P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.-R.; Motilva, M.-J. Comparative study of UPLC–MS/MS and HPLC–MS/MS to determine procyanidins and alkaloids in cocoa samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Andersen, S.J.; Hennebel, T.; Gildemyn, S.; Coma, M.; Desloover, J.; Berton, J.; Tsukamoto, J.; Stevens, C.; Rabaey, K. Electrolytic membrane extraction enables production of fine chemicals from biorefinery sidestreams. Environ. Sci. Technol. 2014, 48, 7135–7142. [Google Scholar] [CrossRef]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Natarajan, N.; Pluznick, J.L. From microbe to man: The role of microbial short chain fatty acid metabolites in host cell biology. Am. J. Physiol.-Cell Physiol. 2014, 307, C979–C985. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 2014, 5, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Renal and cardiovascular sensory receptors and blood pressure regulation. Am. J. Physiol.-Ren. Physiol. 2013, 305, F439–F444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.-X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, M.; Mente, A.; Rangarajan, S.; McQueen, M.J.; Wang, X.; Liu, L.; Yan, H.; Lee, S.F.; Mony, P.; Devanath, A.; et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N. Engl. J. Med. 2014, 371, 612–623. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Aspects Med. 2010, 31, 495–502. [Google Scholar] [CrossRef]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- McCain, M.L.; Parker, K.K. Mechanotransduction: The role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflug. Arch. Eur. J. Physiol. 2011, 462, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Kardum, N.; Glibetic, M. Polyphenols and their interactions with other dietary compounds: Implications for human health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar] [CrossRef]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Plasma kinetics and microbial biotransformation of grape seed flavanols in rats. J. Funct. Foods 2015, 12, 478–488. [Google Scholar] [CrossRef]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In Vivo and In Vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef] [Green Version]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Dew, T.; Figueira, M.E.; Mcdougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N.; Williamson, G. Urinary metabolite profiling identifies novel colonic metabolites and conjugates of phenolics in healthy volunteers. Mol. Nutr. Food Res. 2014, 58, 1414–1425. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Plumb, G.; Garcia Conesa, M.T.; Kroon, P.; Rhodes, M.; Ridley, S.; Williamson, G. Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora. J. Sci. Food Agric. 1999, 79, 390–392. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.J.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, P.R.; Istas, G.; Heiss, C.; Rodriguez-Mateos, A. Plasma and urinary phenolic profiles after acute and repetitive intake of wild blueberry. Molecules 2016, 21, 1120. [Google Scholar] [CrossRef]
- Hanhineva, K.; Lankinen, M.A.; Pedret, A.; Schwab, U.; Kolehmainen, M.; Paananen, J.; de Mello, V.; Sola, R.; Lehtonen, M.; Poutanen, K.; et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J. Nutr. 2015, 145, 7–17. [Google Scholar] [CrossRef]
- Vetrani, C.; Rivellese, A.A.; Annuzzi, G.; Adiels, M.; Borén, J.; Mattila, I.; Orešič, M.; Aura, A.-M. Metabolic transformations of dietary polyphenols: Comparison between in vitro colonic and hepatic models and in vivo urinary metabolites. J. Nutr. Biochem. 2016, 33, 111–118. [Google Scholar] [CrossRef]
- Kemperman, R.; Bolca, S.; Roger, L.; Vaughan, E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L.; et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017, 551, 585. [Google Scholar] [CrossRef]
- Mell, B.; Jala, V.R.; Mathew, A.V.; Byun, J.; Waghulde, H.; Zhang, Y.; Haribabu, B.; Vijay-Kumar, M.; Pennathur, S.; Joe, B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 2015, 47, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, A.; Li, R.W.; Klimis-Zacas, D.; Kristo, A.S.; Tadepalli, S.; Krauss, E.; Young, R.; Wu, V.C.H. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS ONE 2013, 8, e67497. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.-L.; Liu, Z.; Kruger, M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World J. Microbiol. Biotechnol. 2010, 26, 1735–1743. [Google Scholar] [CrossRef]
- Molan, A.; Tiwari, Z.L.R. The ability of green tea to positively modulate key markers of gastrointestinal function in rats. Phyther. Res. 2010, 24, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases Bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Schwalm, N.D., III; Groisman, E.A. Navigating the Gut Buffet: Control of Polysaccharide Utilization in Bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef]
- Rastmanesh, R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem. Biol. Interact. 2011, 189, 1–8. [Google Scholar] [CrossRef]
- Sembries, S.; Dongowski, G.; Jacobasch, G.; Mehrländer, K.; Will, F.; Dietrich, H. Effects of dietary fibre-rich juice colloids from apple pomace extraction juices on intestinal fermentation products and microbiota in rats. Br. J. Nutr. 2003, 90, 607–615. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.A.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.O.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ 2018, 6, e4195. [Google Scholar] [CrossRef] [Green Version]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.T.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Petra, L.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2016, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jan, G.; Belzacq, A.-S.; Haouzi, D.; Rouault, A.; Métivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Miyamoto, J.; Kasubuchi, M.; Nakajima, A.; Irie, J.; Itoh, H.; Kimura, I. The role of short-chain fatty acid on blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]


| 4th Week | 9th Week | |||||||
|---|---|---|---|---|---|---|---|---|
| LS | LSB | HS | HSB | LS | LSB | HS | HSB | |
| Benzoic Acid Derivatives | ||||||||
| Vanillic acid sulphate | 15.3 ± 3.2 | 94.5 ± 7.4 | 6.9 ± 3.3 | 294.0 ± 21.4 ### | 88.6 ± 35.9 | 276.0 ± 37.2 | 64.7 ± 27.8 | 288.0 ± 32.3 # |
| Protocatechuic acid sulphate 1 | 51.8 ± 11.7 | 54.0 ± 8.8 | 605 ± 200 | 779 ± 217 | 90.6 ± 42.4 | 247.0 ± 48.7 | 1038 ± 120 ** | 702.0 ± 75.6 |
| Hydroxybenzoic acid 1 | 32.7 ± 7.6 | 73.5 ± 10.6 | 206.0 ± 22.1 *** | 313.0 ± 23.0 $$$ | 134.0 ± 44.6 | 84.2 ± 25.8 | 173 ± 9.5 | 230 ± 34.7 $$$ |
| Hydroxybenzoic acid sulphate 1 | n.d. | n.d. | 2.58 ± 1.2 | 72.0 ± 8.1 $$$## | 6.2 ± 3.6 | n.d. | 138.0 ± 36.7 * | 145.0 ± 14.9 $$$ |
| Cinnamic Acid Derivatives | ||||||||
| Coumaric acid sulphate 1 | 1.8 ± 0.6 | 117.0 ± 11.7 * | 18.1 ± 2.3 | 329.0 ± 32.2 ## | n.d. | 687 ± 143 *** | 27.1 ± 3.3 | 679.0 ± 63.2 ### |
| Caffeic acid sulphate 1 | 7.0 ± 1.3 | 57.8 ± 5.0 *** | n.d. | 143.0 ± 6.5 $$$### | 10.1 ± 3.5 | 130 ± 20.5 *** | n.d. | 147.0 ± 8.6 ### |
| Caffeic acid glucuronide 1 | 1.13 ± 0.09 | 7.6 ± 0.9 | n.d. * | 13.3 ± 3.9 # | n.d. | 10.0 ± 2.2 ** | n.d. | 13.0 ± 3.4 # |
| Ferulic acid | 10.4 ± 0.8 | 31.8 ± 2.6 | 7.59 ± 1.76 | 61.9 ± 5.1 ### | 9.4 ± 2.4 | 19.5 ± 3.8 | n.d. | 1.9 ± 0.8 $ |
| Ferulic acid sulphate | n.d. | 31.2 ± 3.2 ** | 4.48 ± 2.43 | 35.9 ± 13.9 | n.d. | 87.1 ± 6.3 *** | n.d. | 32.0 ± 7.8 # |
| Ferulic acid glucuronide | 0.65 ± 0.34 | 20.7 ± 1.5 *** | n.d. | 4.2 ± 1.4 # | n.d. | 38.4 ± 7.8 *** | n.d. | 2.4 ± 0.8 $ |
| Catechol Derivatives | ||||||||
| Catechol sulphate | n.d. | 11.4 ± 7.1 | 436.0 ± 49.6 * | 781.0 ± 55.4 $$$ | n.d. | 730 ± 158 ** | 312 ± 55.4 | 1161 ± 175 $# |
| 4-methyl catechol sulphate 1 | 6.4 ± 2.9 | 4.7 ± 1.0 | n.d. | 22.6 ± 4.6 ### | 19.8 ± 5.4 | 60.2 ± 8.4 | 21.1 ± 8.1 | 52.1 ± 7.8 |
| 4-Methyl catechol glucuronide 1 | 352 ± 111 | 221.0 ± 27.6 | n.d. *** | 240.0 ± 29.5 ### | n.d. | n.d. | n.d. | n.d. |
| Pyrogallol Derivatives | ||||||||
| Pyrogallol sulphate 1 | 40.2 ± 14.3 | 2.1 ± 1.3 | 576 ± 76.4 ** | 215 ± 33.9 $$ | n.d. | n.d. | n.d | n.d. |
| Hippuric Acid Derivatives | ||||||||
| Hippuric acid | 734.0 ± 60.1 | 4225 ± 226 *** | 1494 ± 96.0 | 13416 ± 937 ### | 702 ± 135 | 4693 ± 332 * | 862 ± 73.4 | 10609 ± 864 ### |
| Propionic Acid Derivatives | ||||||||
| 3-(Hydroxyphenyl)propionic acid glucuronide 1 | n.d. | 0.09 ± 0.09 | n.d. | n.d. | n.d. | 5.4 ± 0.8 *** | 5.4 ± 0.8 | 0.04 ± 0.04 $$$ |
| 3-(4-hydroxyphenyl) propionic acid 2 | 36.5 ± 3.5 | 572 ± 75.5 | 177 ± 98.1 | 1357 ± 240 ## | 4.16 ± 1.84 | 1347 ± 312 *** | 15.7 ± 6.5 | 2068 ± 757 ### |
| 3-(Dihydroxyphenyl) propionic acid 1 | 0.8 ± 0.2 | 14.5 ± 1.5 | n.d. | 137 ± 13.0 ### | n.d. | 30.3 ± 4.7 | 31.5 ± 14.1 | 138 ± 25.4 ### |
| 3-(Hydroxyphenyl)propionic acid sulphate 1 | 7.7 ± 1.7 | 206.0 ± 24.7 | 18.9 ± 4.9 | 1536 ± 180 ### | 7.7 ± 1.7 | 1842 ± 336 ** | 32.2 ± 8.2 | 2975 ± 299 ### |
| Dihydroferulic acid | 3.8 ± 0.3 | 19.7 ± 2.7 | n.d. | 2.1 ± 1.1 $$$ | 3.1 ± 1.2 | 16.2 ± 1.9 | 0.16 ± 0.16 | 58.2 ± 10.7 ### |
| Dihydroferulic acid sulphate | 3.3 ± 0.2 | 46.2 ± 5.8 ** | n.d. * | 5.6 ± 1.8 $$ | n.d. | 52.5 ± 9.1 *** | n.d. | 24.2 ± 5.5 ## |
| Flavanols Derivatives | ||||||||
| Methyl catechin glucuronide 1 | 1.1 ± 0.7 | 651.0 ± 54.4 *** | n.d. | 528 ± 108 ### | n.d. | 961 ± 138 *** | n.d. | 407 ± 39.8 ### |
| 4th Week | 9th Week | |||
|---|---|---|---|---|
| LSB | HSB | LSB | HSB | |
| Benzoic Acid Derivatives | ||||
| Vanillic acid | 40.1 ± 12.7 | n.d $ | 85.9 ± 17.8 *** | 19.0 ± 7.9 $ |
| Vanillic acid glucuronide ● | 7.3 ± 2.4 *** | n.d. $$$ | n.d. | n..d |
| Gallic acid ● | n.d. | n..d | 12.4 ± 7.3 ** | n.d. $$$ |
| Gallic acid glucuronide ● 1 | n.d. | n.d. | 20.2 ± 8.6 *** | n.d. $$$ |
| 4-O-methylgallic acid | 88.6 ± 7.7 ** | 97.5 ± 8.7 ### | 127.0 ± 10.7 *** | 80.7 ± 13.3 $## |
| 4-O-methylgallic acid sulphate | 38.7 ± 6.7 | 139.0 ± 11.8 ### | 177.0 ± 36.5 ** | 204.0 ± 17.1 ### |
| Syringic acid ● | 15.8 ± 1.7 *** | n.d. $$$ | 21.8 ± 2.2 *** | n.d. $$$ |
| Syringic acid sulphate ● | 2.5 ± 0.3 *** | n.d. $$$ | 9.6 ± 0.8 *** | n.d. $$$ |
| Cinnamic Acid Derivatives | ||||
| Coumaric acid 1 | 18.7 ± 2.6 | 79.9 ± 6.8 ### | 99.5 ± 18.7 ** | 99.9 ± 8.1 $$$ |
| Coumaric acid glucuronide ♦ 1 | n.d. | 1.8 ± 1.0 | n.d. | 5.8 ± 1.5 $$### |
| Phenylacetic Acids Derivatives | ||||
| 2-(Dihydroxyphenyl)acetic acid 1 | 2.1 ± 0.8 | n.d. | 5.4 ± 1.1 *** | 4.9 ± 2.5 |
| 2-(Trihydroxyphenyl)acetic acid 1 | 31.9 ± 4.0 *** | 25.6 ± 4.5 ### | 33.7 ± 4.5 ** | 26.5 ± 5.9 ## |
| Catechol Derivatives | ||||
| Catechol glucuronide | n.d. | 165.0 ± 7.9 $$$### | 46.8 ± 27.6 | 129.0 ± 21.7 $$$### |
| Valeric Acid Derivatives | ||||
| 5-(Hydroxyphenyl)valeric acid 1 | 2.8 ± 1.0 ** | n.d. $$ | 6.1 ± 2.2 *** | 0.62 ± 0.44 $$ |
| Valerolactone Derivatives | ||||
| 5-(Dihydroxypheny)l-γ-valerolactone 1 | 0.43 ± 0.22 | n.d. | 5.0 ± 1.2 * | 17.1 ± 4.4 ### |
| 5-(Dihydroxyphenyl)-γ-valerolactone sulphate 1 | 2.7 ± 0.9 | 3.1 ± 1.0 | 13.9 ± 1.2 ** | 23.3 ± 5.8 ### |
| 5-(Dihydroxyphenyl)-γ-valerolactone glucuronide ● 1 | n.d. | n.d. | 0.97 ± 0.57 *** | n.d. $$$ |
| Flavanols Derivatives | ||||
| Catechin glucuronide 1 | 105.0 ± 9.8 *** | 110.0 ± 19.3 ### | 180 ± 19.4 *** | 159.0 ± 24.1 ### |
| Methyl-O-catechin sulphate 1 | 0.24 ± 0.11 | 3.6 ± 1.8 | 8.7 ± 1.4 | 2.4 ± 1.2 |
| Epicatechin glucuronide 1 | 56.4 ± 6.5 * | 77.6 ± 11.0 ### | 96.3 ± 23.9 ** | 99.2 ± 9.9 ### |
| Methyl-O-epicatechin sulphate 1 | 14.2 ± 2.7 | 51.4 ± 12.0 ### | 30.2 ± 2.5 ** | 37.0 ± 13.2 |
| Methyl-O-epicatechin glucuronide 1 | 114.0 ± 17.5 *** | 67.7 ± 14.5 # | 221 ± 73.1 *** | 67.3 ± 19.2 |
| 4th Week | 9th Week | |||||||
|---|---|---|---|---|---|---|---|---|
| LS | LSB | HS | HSB | LS | LSB | HS | HSB | |
| Benzoic Acid Derivatives | ||||||||
| Vanillic acid | 1.3 ± 0.8 | 4.3 ± 1.7 ** | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Vanillic acid sulphate | 3.7 ± 1.6 | 4.1 ± 1.8 | n.d. ** | n.d. $ | n.d. | n.d. | n.d. | n.d. |
| Gallic acid | 16.6 ± 6.2 | 102.0 ± 25.1 | n.d. | 10.7 ± 0.8 ### | 0.79 ± 0.55 | 12.5 ± 4.3 | 0.77 ± 0.49 | 3.5 ± 0.4 |
| Gallic acid sulphate 1 | 48.9 ± 27.9 | 33.1 ± 14.6 | n.d. ** | n.d. $ | n.d. | n.d. | n.d. | n.d. |
| 4-O-methyl gallic acid sulphate | 1.2 ± 0.7 | 5.0 ± 2.3 | n.d. | n.d. $ | n.d. | 0.06 ± 0.05 | n.d. | n.d. |
| Protocatechuic acid sulphate 1 | 8.0 ± 3.1 | 3.5 ± 1.4 | n.d. *** | n.d. | 0.32 ± 0.12 | 0.35 ± 0.14 | n.d. | n.d. |
| Hydroxybenzoic acid 1 | 40.2 ± 15.3 | 17.7 ± 5.2 | 3.2 ± 1.2 | 3.6 ± 1.0 | 4.5 ± 1.2 | 4.4 ± 1.4 | 0.37 ± 0.24 | 3.5 ± 1.8 |
| Hydroxybenzoic acid sulphate 1 | 51.2 ± 29.4 | 31.8 ± 12.2 ** | n.d. | 0.10 ± 0.07 $$ | 0.00 ± 0.00 | 0.52 ± 0.26 | n.d. | n.d. |
| Cinnamic Acid Derivatives | ||||||||
| Coumaric acid 1 | 3.5 ± 0.8 | 4.8 ± 1.9 | 0.34 ± 0.10 | 0.40 ± 0.07 | n.d. | 0.41 ± 0.15 | n.d. | 0.12 ± 0.08 |
| Coumaric acid sulphate 1 | 14.1 ± 7.9 | 54.2 ± 19.9 | n.d. ** | 2.1 ± 0.5 # | n.d. | 2.2 ± 0.6 * | n.d. | 1.4 ± 0.3 |
| Caffeic acid | 27.6 ± 20.7 | 53.5 ± 24.4 | n.d. | n.d. $$$ | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid | 7.2 ± 3.0 | 6.5 ± 2.9 | 0.12 ± 0.02 | 0.14 ± 0.03 | 0.28 ± 0.05 | 0.50 ± 0.16 | 0.02 ± 0.02 | 0.09 ± 0.05 |
| Ferulic acid sulphate | 10.1 ± 5.6 | 12.4 ± 5.3 | n.d. * | n.d. | n.d. | 0.35 ± 0.10 * | n.d. | n.d. $ |
| Phenylacetic Acid Derivatives | ||||||||
| 2-(Phenyl)acetic acid | 3039 ± 1541 | 1209 ± 225 | 87.4 ± 20.8 | 59.0 ± 16.0 $$ | 117 ± 27.8 | 87.8 ± 15.9 | 38.1 ± 7.7 | 9.2 ± 2.6 |
| 2-(Hydroxyphenyl)acetic acid 1 | 197 ± 124 | 291.0 ± 37.3 | 7.0 ± 1.5 * | 19.3 ± 3.8 $ | 31.9 ± 5.3 | 51.1 ± 11.7 | 3.7 ± 0.6 * | 8.2 ± 2.1 |
| Catechol Derivatives | ||||||||
| Catechol sulphate | 8.32 ± 1.46 | 7.26 ± 3.25 | n.d. *** | 0.41 ± 0.14 | 0.46 ± 0.17 | 0.71 ± 0.29 | n.d. | 0.19 ± 0.12 |
| 4-methyl catechol sulphate 1 | 5.65 ± 2.21 | 3.51 ± 1.38 | n.d. * | 0.01 ± 0.01 | 0.18 ± 0.12 | 0.16 ± 0.11 | n.d. | n.d. |
| Propionic acid Derivatives | ||||||||
| 3-(Phenyl)propionic acid | n.d. | 3.3 ± 1.7 | 1.0 ± 0.3 | 2.3 ± 0.3 | 2.7 ± 0.9 | 3.2 ± 1.0 | 1.2 ±0.4 | 1.4 ± 0.3 |
| 3-(hydroxyphenyl) propionic acid 1 | 55.0 ± 17.9 | 353 ± 125 | 2.7 ± 0.4 | 10.0 ± 2.3 | n.d. | 53.7 ± 12.1 | 0.87 ± 0.55 | 5.9 ± 1.7 $$$ |
| (Hydroxyphenyl)propionic acid sulphate 1 | 62.1 ± 35.2 | 299 ± 128 | n.d. ** | 0.66 ± 0.23 | 10.0 ± 4.3 | 11.6 ± 3.8 | n.d. | 0.20 ± 0.13 |
| Dihydroferrulic acid | 4.2 ± 2.7 | 3.2 ± 1.2 | n.d. | n.d. $ | 0.28 ± 0.08 | 0.39 ± 0.16 | n.d. | n.d. |
| Hippuric Acid Derivatives | ||||||||
| Hippuric acid | 546 ± 326 | 1567 ± 701 | 0.41 ± 0.27 ** | 2.9 ± 0.5 | 2.4 ± 0.6 | 64.9 ± 21.4 | 0.48 ± 0.30 | 0.94 ± 0.39 $$ |
| 4th Week | 9th Week | ||||
|---|---|---|---|---|---|
| LSB | HSB | LSB | HSB | ||
| Benzoic Acid Derivatives | |||||
| Protocatechuic acid | 73.2 ± 22.2 *** | 6.6 ± 1.2 ### | n.d. | n.d. | |
| 4-O-methyl gallic acid ● | 10.0 ± 1.3 *** | n.d. $$$ | 1.7 ± 0.2 ** | n.d. $$ | |
| Syringic acid | 19.5 ± 2.6 *** | 2.8 ± 0.5 ## | 4.5 ± 0.8 ** | 1.6 ± 0.3 | |
| Propionic Acid Derivatives | |||||
| 3-(dihydroxyphenyl) propionic acid ● 1 | n.d. | n.d. | 1.5 ± 0.8 ** | n.d. $$ | |
| Valeric Acid Derivatives | |||||
| 5-(Hydroxyphenyl)valeric acid 1 | 84.9 ± 16.8 *** | 3.9 ± 0.7 # | 25.0 ± 9.2 *** | 1.7 ± 0.5 | |
| Flavanols Derivatives | |||||
| Catechin | n.d. | 1.7 ± 0.6 | 0.34 ± 0.23 | 6.5 ± 4.2 # | |
| Epicatechin | 13.0 ± 8.5 | 7.1 ± 2.4 ### | n.d. | 3.3 ± 0.9 | |
| Diarylpropan-2-ol 1 | 2.9 ± 1.3 | 0.22 ± 0.15 | 0.94 ± 0.37 * | 0.21 ± 0.14 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.; Oudot, C.; Macià, A.; Foito, A.; Carregosa, D.; Stewart, D.; Van de Wiele, T.; Berry, D.; Motilva, M.-J.; Brenner, C.; et al. Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota. Nutrients 2019, 11, 2634. https://doi.org/10.3390/nu11112634
Gomes A, Oudot C, Macià A, Foito A, Carregosa D, Stewart D, Van de Wiele T, Berry D, Motilva M-J, Brenner C, et al. Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota. Nutrients. 2019; 11(11):2634. https://doi.org/10.3390/nu11112634
Chicago/Turabian StyleGomes, Andreia, Carole Oudot, Alba Macià, Alexandre Foito, Diogo Carregosa, Derek Stewart, Tom Van de Wiele, David Berry, Maria-José Motilva, Catherine Brenner, and et al. 2019. "Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota" Nutrients 11, no. 11: 2634. https://doi.org/10.3390/nu11112634
APA StyleGomes, A., Oudot, C., Macià, A., Foito, A., Carregosa, D., Stewart, D., Van de Wiele, T., Berry, D., Motilva, M.-J., Brenner, C., & Nunes dos Santos, C. (2019). Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota. Nutrients, 11(11), 2634. https://doi.org/10.3390/nu11112634