Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Grape Seed Extract
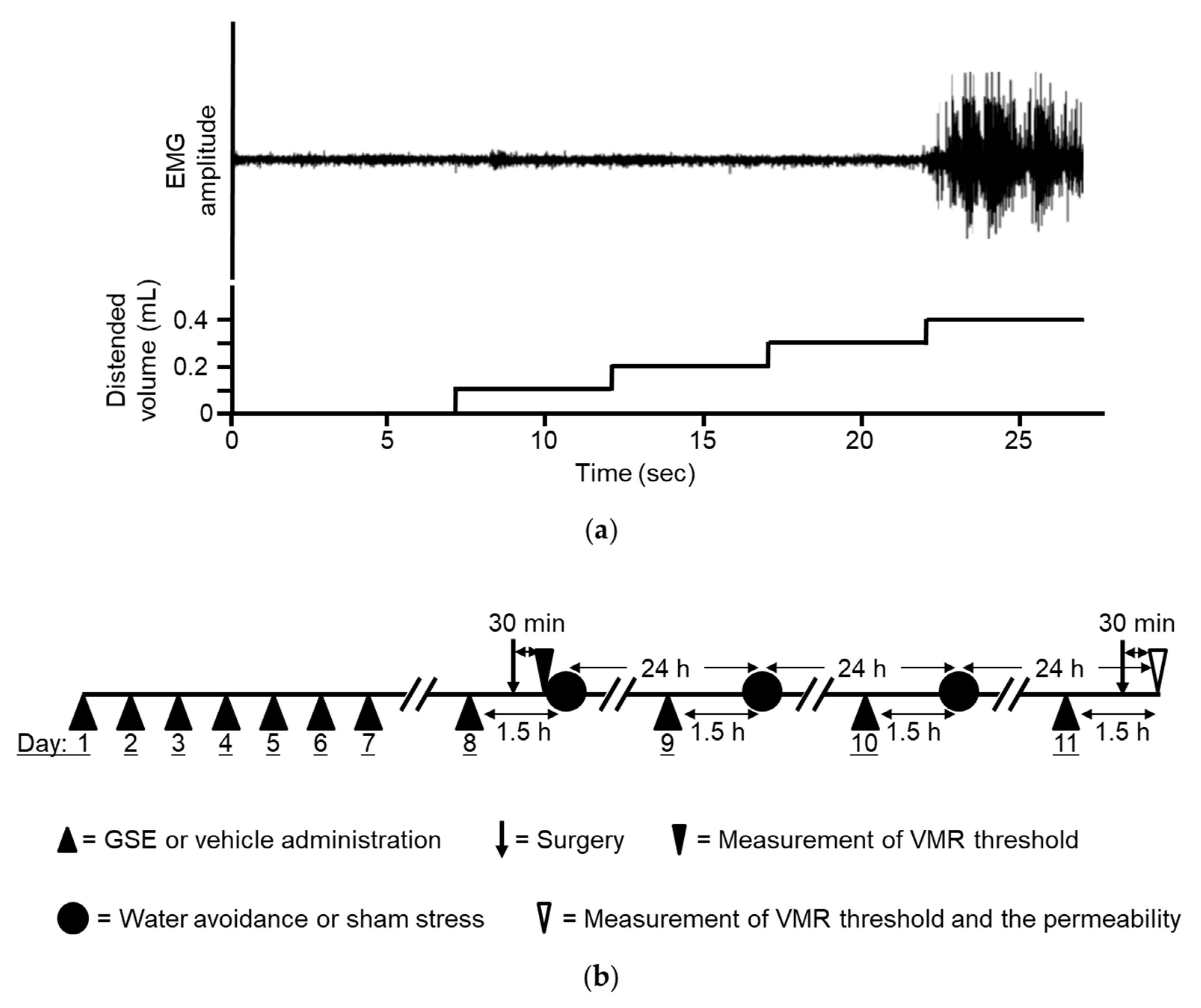
2.3. Measurement of Visceral Sensation
2.3.1. Electrode Implantation and Colonic Distention Balloon Placement
2.3.2. Colonic Distention and Measurement of Abdominal Muscle Contraction
2.4. Measurement of Colonic Permeability
2.5. Stress Protocol
2.6. Experimental Procedures
2.7. Quantification of Colonic Cytokines
2.8. Quantification of Colonic Protein Expression
2.9. Experimental Procedures in Caco-2 Cells
2.10. Statistical Analysis
3. Results
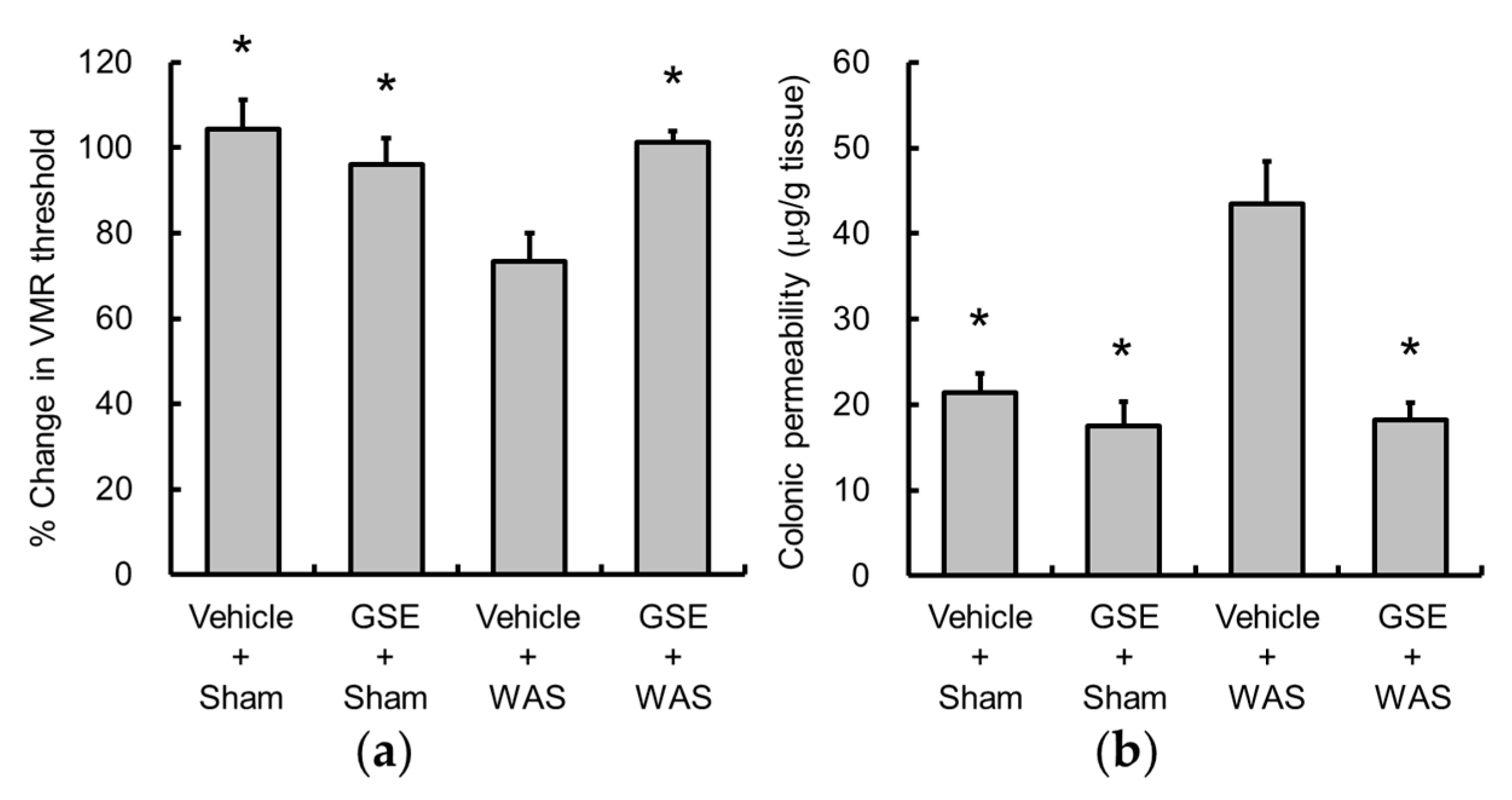
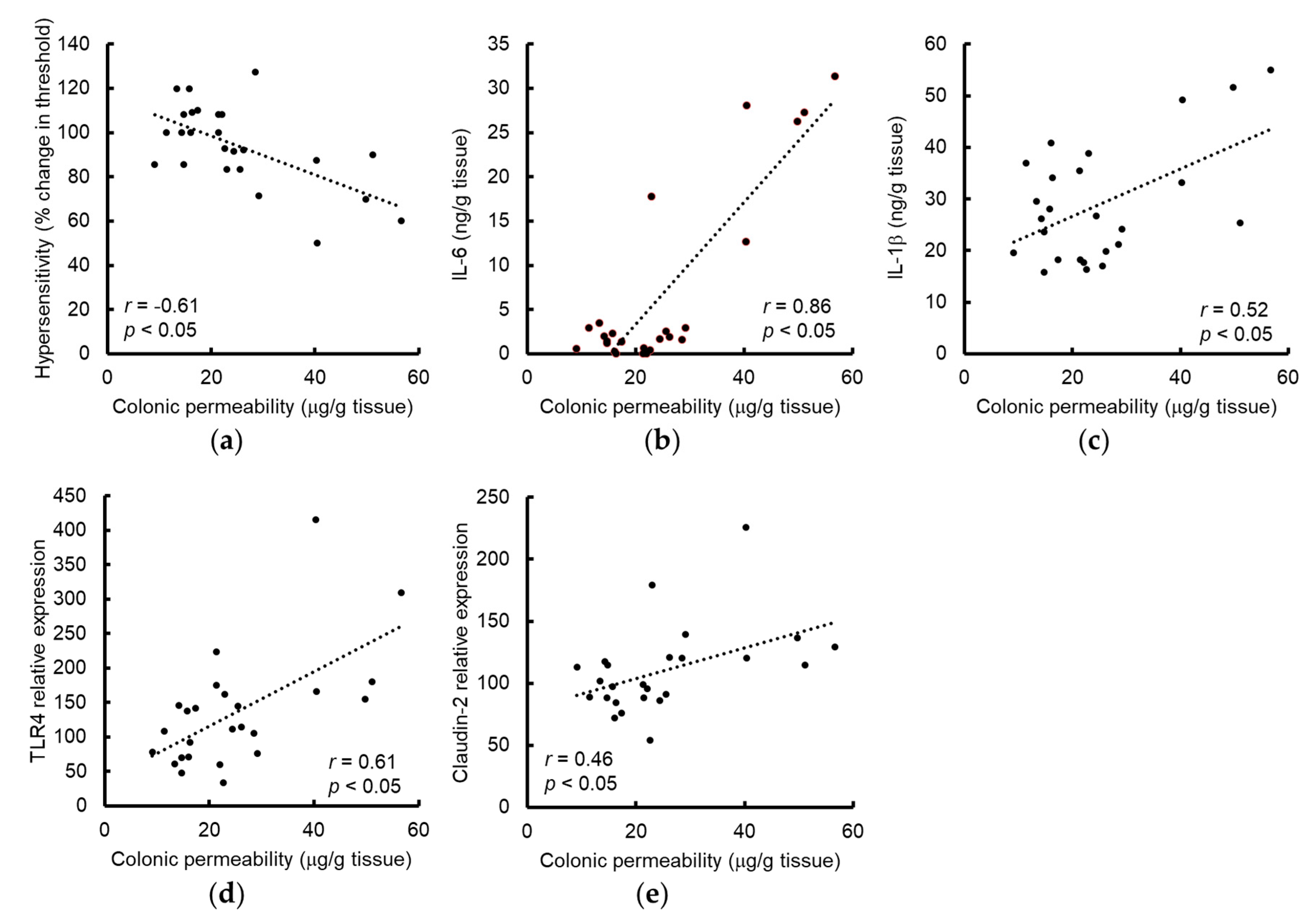
3.1. Grape Seed Extract Prevents WAS-Induced Visceral Allodynia and Colonic Hyperpermeability
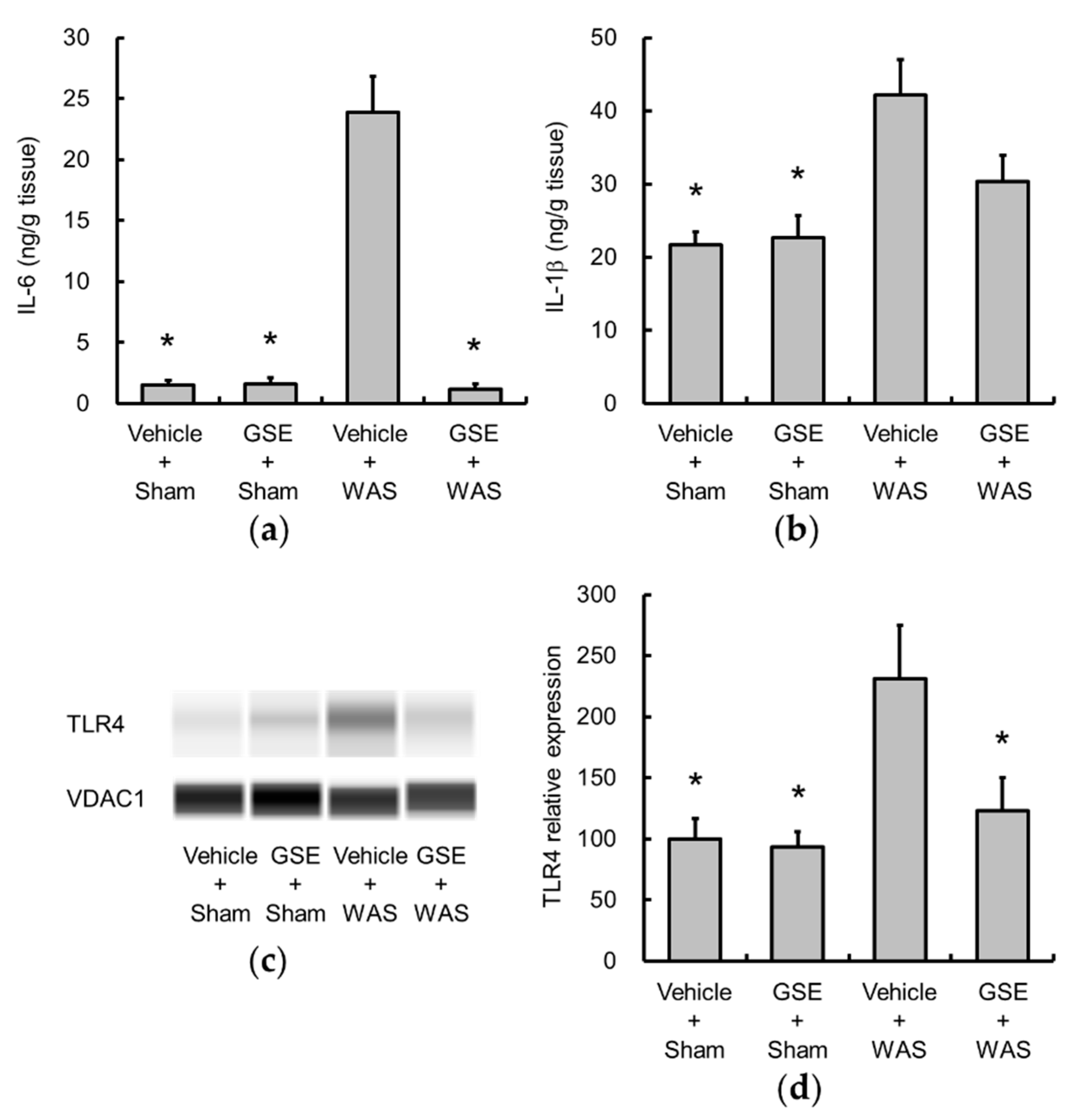
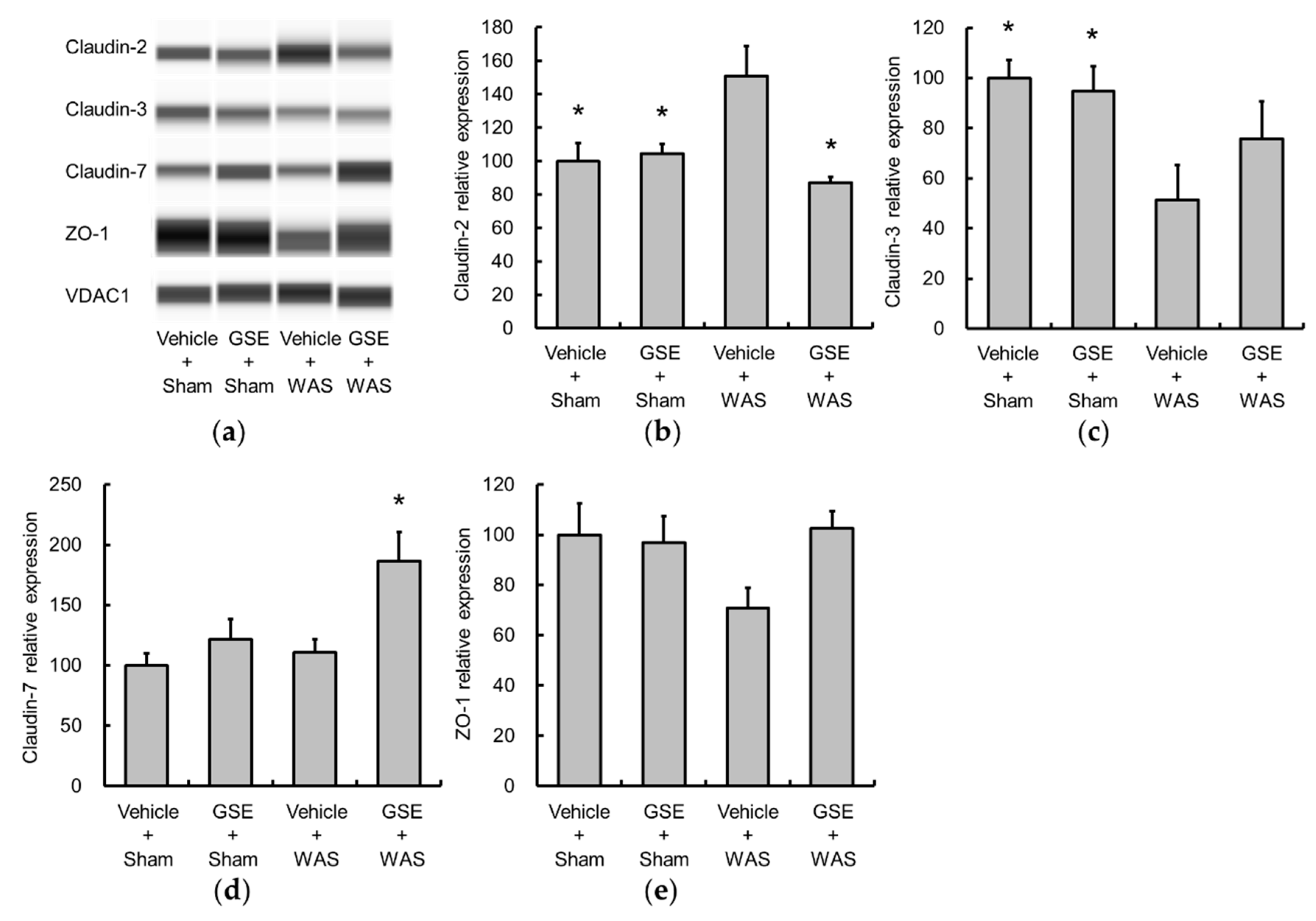
3.2. Grape Seed Extract Attenuates the Increases in Colonic Proinflammatory Cytokine and TLR4 and Restores Tight Junction Protein Expression
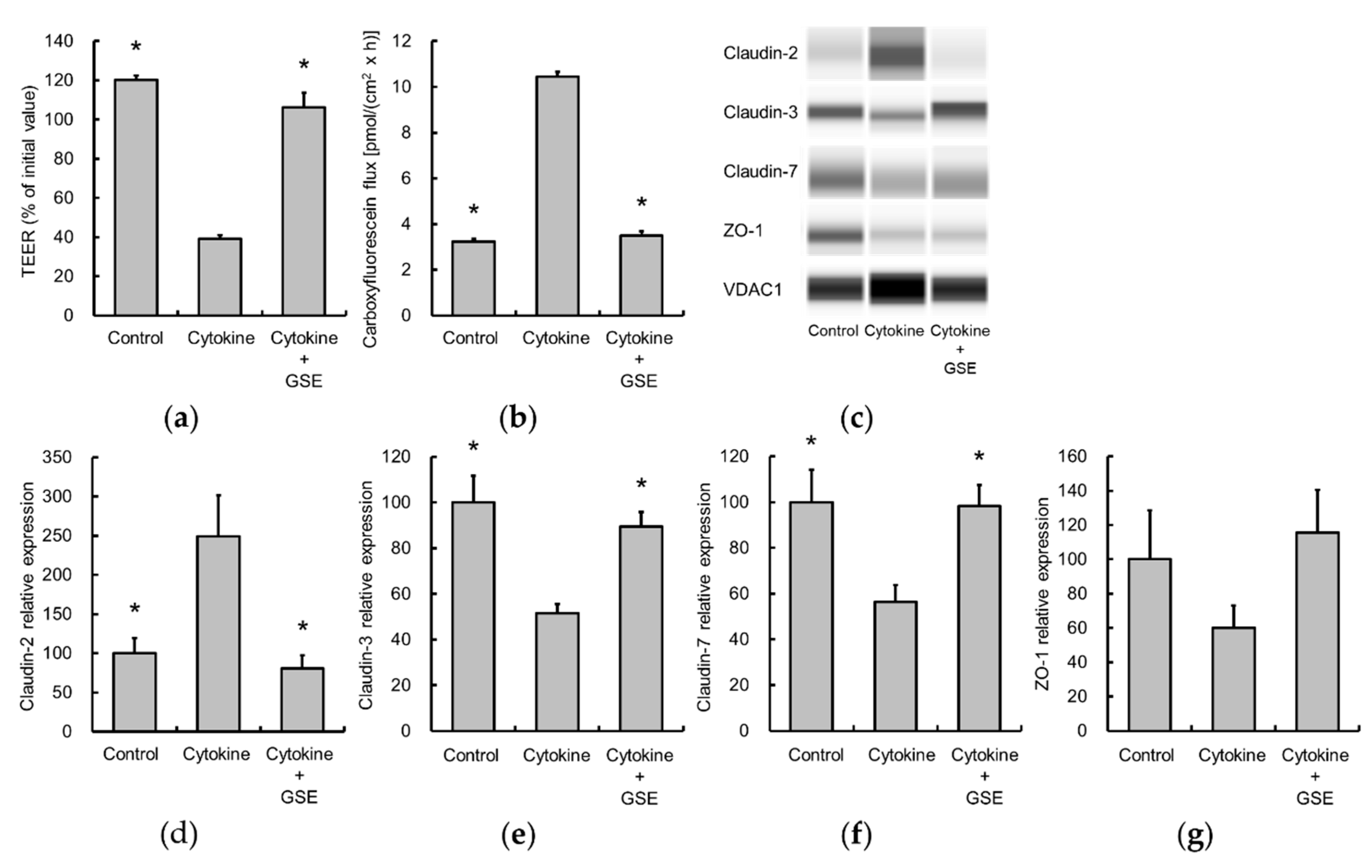
3.3. Grape Seed Extract Alleviates Cytokine-Induced Increases in Epithelial Permeability in Caco-2 Cell Monolayers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar]
- Kanazawa, M.; Hongo, M.; Fukudo, S. Visceral hypersensitivity in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2011, 26 (Suppl. 3), 119–121. [Google Scholar] [CrossRef]
- Tache, Y.; Kiank, C.; Stengel, A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr. Gastroenterol. Rep. 2009, 11, 270–277. [Google Scholar] [CrossRef]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Toshikatsu, O. Altered colonic sensory and barrier functions by CRF: Roles of TLR4 and IL-1. J. Endocrinol. 2018, 239, 241–252. [Google Scholar] [CrossRef]
- Dlugosz, A.; Nowak, P.; D’Amato, M.; Mohammadian Kermani, G.; Nystrom, J.; Abdurahman, S.; Lindberg, G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2015, 27, 1747–1754. [Google Scholar] [CrossRef]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin. Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Lipopolysaccharide induces visceral hypersensitivity: Role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J. Gastroenterol. 2017, 52, 72–80. [Google Scholar] [CrossRef]
- Scully, P.; McKernan, D.P.; Keohane, J.; Groeger, D.; Shanahan, F.; Dinan, T.G.; Quigley, E.M. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am. J. Gastroenterol. 2010, 105, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Quigley, E.M.; Ahmed, S.M.; Scully, P.; O’Brien, S.; O’Mahony, L.; O’Mahony, S.; Shanahan, F.; Keeling, P.W. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology 2006, 130, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lucas, M.; Saz-Peiro, P.; Sebastian-Domingo, J.J. Irritable bowel syndrome immune hypothesis. Part two: The role of cytokines. Rev. Esp. Enferm. Dig. 2010, 102, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Tache, Y. Stress and visceral pain: From animal models to clinical therapies. Exp. Neurol. 2012, 233, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozu, T.; Miyagishi, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Repeated water avoidance stress induces visceral hypersensitivity: Role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor. J. Gastroenterol. Hepatol. 2017, 32, 1958–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebregts, T.; Adam, B.; Bredack, C.; Roth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar]
- Weseler, A.R.; Bast, A. Masquelier’s grape seed extract: From basic flavonoid research to a well-characterized food supplement with health benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Zhang, H.; Huang, Y.; Yang, G.; Du, M.; Zhu, M.J. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol. Nutr. Food Res. 2013, 57, 2253–2257. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xue, Y.; Zhang, H.; Du, M.; Zhu, M.J. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice. Br. J. Nutr. 2015, 114, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Gines, I.; Pinent, M.; Ardevol, A.; Arola, L.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal alterations in obese rats. Mol. Nutr. Food Res. 2017, 61, 1601039. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Tokutate, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and fecal odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- Nozu, T.; Miyagishi, S.; Kumei, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Lovastatin inhibits visceral allodynia and increased colonic permeability induced by lipopolysaccharide or repeated water avoidance stress in rats. Eur. J. Pharmacol. 2018, 818, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.J.; Beard, R.S., Jr.; Chen, L.; Eitnier, R.A.; Wu, M.H. Interleukin-1beta Mediates beta-Catenin-Driven Downregulation of Claudin-3 and Barrier Dysfunction in Caco2 Cells. Dig. Dis. Sci. 2016, 61, 2252–2261. [Google Scholar] [CrossRef]
- Yu, D.; Marchiando, A.M.; Weber, C.R.; Raleigh, D.R.; Wang, Y.; Shen, L.; Turner, J.R. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA 2010, 107, 8237–8241. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Takechi, M.; Kiyonari, H.; Shioi, G.; Tamura, A.; Tsukita, S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 2015, 64, 1529–1538. [Google Scholar] [CrossRef]
- Bradesi, S.; Eutamene, H.; Garcia-Villar, R.; Fioramonti, J.; Bueno, L. Acute and chronic stress differently affect visceral sensitivity to rectal distension in female rats. Neurogastroenterol. Motil. 2002, 14, 75–82. [Google Scholar] [CrossRef]
- Kocak, E.; Akbal, E.; Koklu, S.; Ergul, B.; Can, M. The Colonic Tissue Levels of TLR2, TLR4 and Nitric Oxide in Patients with Irritable Bowel Syndrome. Intern. Med. 2016, 55, 1043–1048. [Google Scholar] [Green Version]
- Belmonte, L.; Beutheu Youmba, S.; Bertiaux-Vandaele, N.; Antonietti, M.; Lecleire, S.; Zalar, A.; Gourcerol, G.; Leroi, A.M.; Dechelotte, P.; Coeffier, M.; et al. Role of toll like receptors in irritable bowel syndrome: Differential mucosal immune activation according to the disease subtype. PLoS ONE 2012, 7, e42777. [Google Scholar] [CrossRef]
- Obreja, O.; Rathee, P.K.; Lips, K.S.; Distler, C.; Kress, M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: Involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002, 16, 1497–1503. [Google Scholar] [CrossRef]
- von Banchet, G.S.; Kiehl, M.; Schaible, H.G. Acute and long-term effects of IL-6 on cultured dorsal root ganglion neurones from adult rat. J. Neurochem. 2005, 94, 238–248. [Google Scholar] [CrossRef]
- Creekmore, A.L.; Hong, S.; Zhu, S.; Xue, J.; Wiley, J.W. Chronic stress-associated visceral hyperalgesia correlates with severity of intestinal barrier dysfunction. Pain 2018, 159, 1777–1789. [Google Scholar] [CrossRef]
- Ishimoto, H.; Oshima, T.; Sei, H.; Yamasaki, T.; Kondo, T.; Tozawa, K.; Tomita, T.; Ohda, Y.; Fukui, H.; Watari, J.; et al. Claudin-2 expression is upregulated in the ileum of diarrhea predominant irritable bowel syndrome patients. J. Clin. Biochem. Nutr. 2017, 60, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Yadav, H. Bacterial Translocation from the Gut to the Distant Organs: An Overview. Ann. Nutr. Metab. 2017, 71 (Suppl. 1), 11–16. [Google Scholar] [CrossRef]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Nozu, T.; Okumura, T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J. Gastroenterol. 2015, 50, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Tsatsanis, C.; Androulidaki, A.; Alissafi, T.; Charalampopoulos, I.; Dermitzaki, E.; Roger, T.; Gravanis, A.; Margioris, A.N. Corticotropin-releasing factor and the urocortins induce the expression of TLR4 in macrophages via activation of the transcription factors PU.1 and AP-1. J. Immunol. 2006, 176, 1869–1877. [Google Scholar] [CrossRef]
- Agelaki, S.; Tsatsanis, C.; Gravanis, A.; Margioris, A.N. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infect. Immun. 2002, 70, 6068–6074. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.Q.; Liu, X.Y.; Yang, L.; Geng, X.R.; Yang, G.; Liu, Z.G.; Zheng, P.Y.; Yang, P.C. Stress-Derived Corticotropin Releasing Factor Breaches Epithelial Endotoxin Tolerance. PLoS ONE 2013, 8, e65760. [Google Scholar] [CrossRef]
- Terra, X.; Valls, J.; Vitrac, X.; Merrillon, J.M.; Arola, L.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; et al. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-kappab signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar] [CrossRef]
- Wan, J.; Shan, Y.; Fan, Y.; Fan, C.; Chen, S.; Sun, J.; Zhu, L.; Qin, L.; Yu, M.; Lin, Z. NF-kappaB inhibition attenuates LPS-induced TLR4 activation in monocyte cells. Mol. Med. Rep. 2016, 14, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Overman, E.L.; Rivier, J.E.; Moeser, A.J. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS ONE 2012, 7, e39935. [Google Scholar] [CrossRef] [PubMed]
- Piche, T. Tight junctions and IBS--the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol. Motil. 2014, 26, 296–302. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ge, B.; Yang, X.L.; Zhai, J.; Yang, L.N.; Wang, X.X.; Liu, X.; Shi, J.C.; Wu, Y.J. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int. Immunopharmacol. 2011, 11, 1620–1627. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arie, H.; Nozu, T.; Miyagishi, S.; Ida, M.; Izumo, T.; Shibata, H. Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats. Nutrients 2019, 11, 2646. https://doi.org/10.3390/nu11112646
Arie H, Nozu T, Miyagishi S, Ida M, Izumo T, Shibata H. Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats. Nutrients. 2019; 11(11):2646. https://doi.org/10.3390/nu11112646
Chicago/Turabian StyleArie, Hideyuki, Tsukasa Nozu, Saori Miyagishi, Masayuki Ida, Takayuki Izumo, and Hiroshi Shibata. 2019. "Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats" Nutrients 11, no. 11: 2646. https://doi.org/10.3390/nu11112646
APA StyleArie, H., Nozu, T., Miyagishi, S., Ida, M., Izumo, T., & Shibata, H. (2019). Grape Seed Extract Eliminates Visceral Allodynia and Colonic Hyperpermeability Induced by Repeated Water Avoidance Stress in Rats. Nutrients, 11(11), 2646. https://doi.org/10.3390/nu11112646




