Prospective Analysis of Food Consumption and Nutritional Status and the Impact on the Dietary Inflammatory Index in Women with Breast Cancer during Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Aspects
2.2. Sample Size and Elegibility Criteria
2.3. Anthropometric Assessment
2.4. Dietary Assessment
2.5. Statistical Analysis
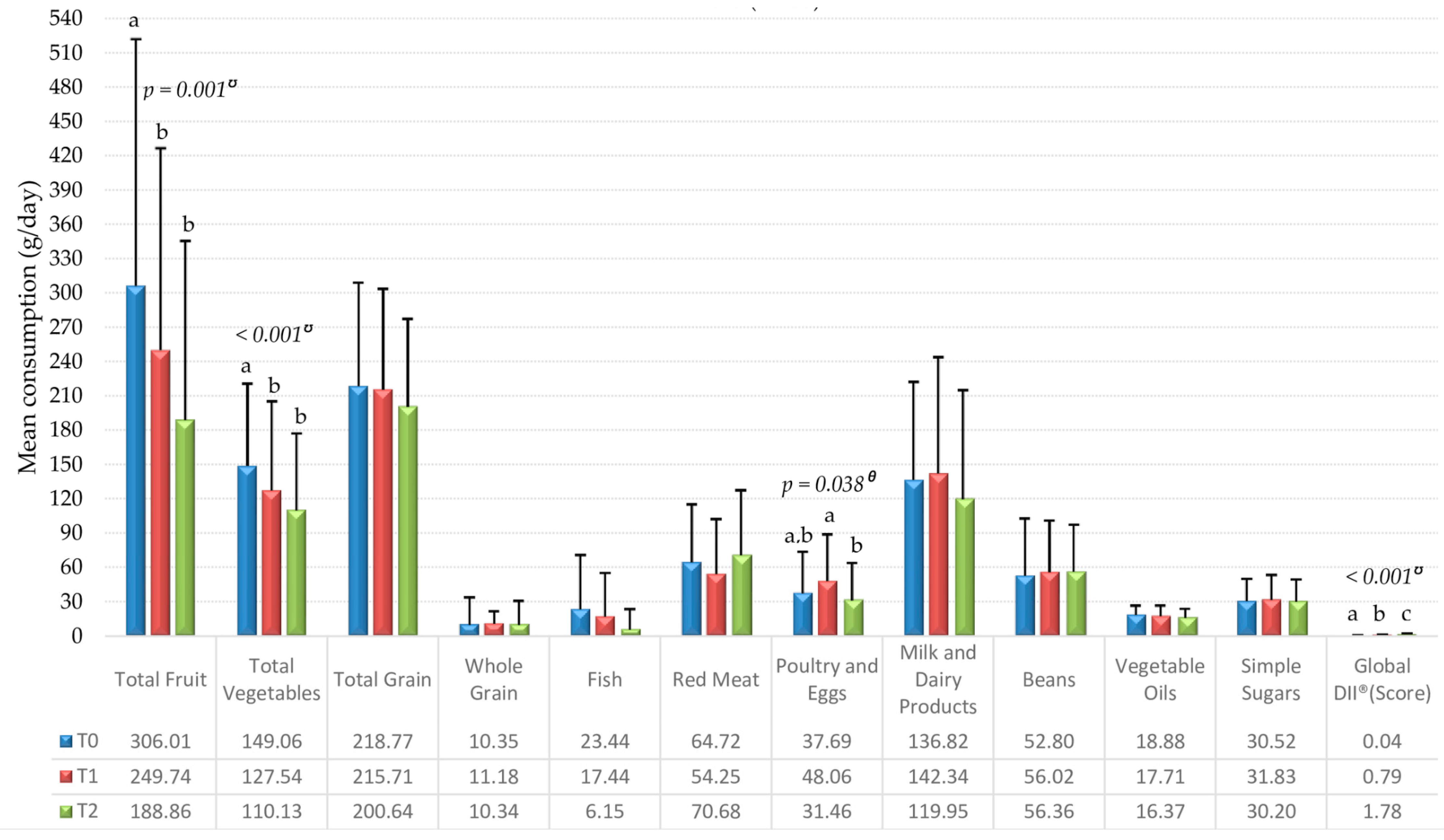
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WCRF World Cancer Research Fund. Diet, Nutrition, Physical Activity and Breast Cancer Survivors. Continuous Update Project, Analysing Research on Cancer Prevention and Survival. 2014. Available online: http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf (accessed on 30 July 2019).
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 30 July 2019).
- Newman, W.G.N. Pharmacogenetics: Making Cancer Treatment Safer and More Effective; Springer: London, UK, 2010; 186p, ISBN 978-90-481-8618-1. [Google Scholar]
- Boltong, A.; Aranda, S.; Keast, R.; Wynne, R.; Francis, P.A.; Chirgwin, J.; Gough, K. A Prospective Cohort Study of the Effects of Adjuvant Breast Cancer Chemotherapy on Taste Function, Food Liking, Appetite and Associated Nutritional Outcomes. PLoS ONE 2014, 9, e103512. [Google Scholar] [CrossRef] [PubMed]
- Beaver, K.; Williamson, S.; Briggs, J. Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur. J. Oncol. Nur. 2016, 20, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.B.; Marinho, E.C.; Custódio, I.D.D.; Gontijo, C.A.; Paiva, C.E.; Crispim, C.A.; Maia, Y.C.P. Food consumption and nutritional status of women in Chemotherapy. Ciênc. Saúde Coletiva 2016, 21, 2209–2218. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-81232016000702209&lng=en&nrm=iso (accessed on 30 July 2019). [CrossRef] [PubMed]
- Custódio, I.D.D.; Marinho, E.C.; Gontijo, C.A.; Pereira, T.S.S.; Paiva, C.E.; Maia, Y.C.P. Impact of Chemotherapy on Diet and Nutritional Status of Women with Breast Cancer: A Prospective Study. PLoS ONE 2016, 11, e0157113. [Google Scholar] [CrossRef]
- Marinho, E.C.; Custódio, I.D.D.; Ferreira, I.B.; Crispim, C.A.; Paiva, C.E.; Maia, Y.C.P. Impact of chemotherapy on perceptions related to food intake in women with breast cancer: A prospective study. PLoS ONE 2017, 12, e0187573. [Google Scholar] [CrossRef]
- Marinho, E.C.; Custódio, I.D.D.; Ferreira, I.B.; Crispim, C.A.; Paiva, C.E.; Maia, Y.C.P. Relationship between food perceptions and health-related quality of life in a prospective study of breast cancer patients undergoing chemotherapy. Clinics 2018, 73, e411. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322018000100290&lng=pt&nrm=iso (accessed on 30 July 2019). [CrossRef]
- Kosaka, Y.; Tanino, H.; Sengoku, N.; Minatani, N.; Kikuchi, M.; Nishimiya, H.; Waraya, M.; Katoh, H.; Enomoto, T.; Sato, T.; et al. Phase II randomized, controlled trial of 1 day versus 3 days of dexamethasone combined with palonosetron and aprepitant to prevent nausea and vomiting in Japanese breast cancer patients receiving anthracycline-based chemotherapy. Support. Care Cancer 2015, 24, 1405–1411. [Google Scholar] [CrossRef][Green Version]
- Hebert, J.R.; Augustine, A.; Barone, J.; Kabat, G.C.; Kinne, D.W.; Wynder, E.L. Weight, height and body mass index in the prognosis of breast cancer: Early results of a prospective study. Int. J. Cancer 1988, 42, 315–318. [Google Scholar] [CrossRef]
- San Felipe, M.J.R.; Martínez, A.A.; Manuel-Y.-Keenoy, B. Influencia del peso corporal en el pronóstico de las supervivientes de cáncer de mama; abordaje nutricional tras el diagnóstico. Nutr. Hosp. 2013, 28, 1829–1841. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112013000600010&lng=es&nrm=iso (accessed on 18 July 2019). [CrossRef]
- Bell, K.E.; Di Sebastiano, K.M.; Vance, V.; Hanning, R.; Mitchell, A.; Quadrilatero, J.; Russell, C.; Dubin, J.A.; Bahl, M.; Califaretti, N.; et al. A comprehensive metabolic evaluation reveals impaired glucose metabolism and dyslipidemia in breast cancer patients early in the disease trajectory. Clin. Nutr. 2014, 33, 550–557. [Google Scholar] [CrossRef]
- George, S.M.; Neuhouser, M.L.; Mayne, S.T.; Irwin, M.L.; Albanes, D.; Gail, M.H.; Alfano, C.M.; Bernstein, L.; McTiernan, A.; Reedy, J.; et al. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol. Biomarkers. Prev. 2010, 19, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular Pathways: Adipose Inflammation as a Mediator of Obesity-Associated Cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A New Dietary Inflammatory Index Predicts Interval Changes in Serum High-Sensitivity C-Reactive Protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Boltong, A.; Keast, R. The influence of chemotherapy on taste perception and food hedonics: A systematic review. Cancer Treat. Rev. 2012, 38, 152–163. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Newman, V.; Caan, B.J.; Haan, M.N.; Stefanick, M.L.; Faerber, S.; Pierce, J.P. Factors associated with weight gain in women after diagnosis of breast cancer. Women’s Healthy Eating and Living Study Group. J. Am. Diet Assoc. 1999, 99, 1212–1221. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior. Res. Method. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- WHO Expert Committee on Physical Status: The Use and Interpretation of Anthropometry (1993: Geneva, Switzerland) & World Health Organization, 1995. Physical status: The use of and interpretation of anthropometry, report of a WHO expert committee. World Health Organization, 1995. Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 29 July 2019).
- WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000; 252p, Available online: http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 29 July 2019).
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care 1994, 21, 55–67. [Google Scholar] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics: Champaign, IL, USA, 1988; 177p, ISBN 978-0873223317. [Google Scholar]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef] [PubMed]
- USDA United States Dietetic Association. Dietary Guidelines for Americans; 2005. Available online: http://health.gov/dietaryguidelines/dga2005/document/ (accessed on 6 December 2018).
- TACO. Tabela Brasileira de Composição de Alimentos, 4a ed. rev. e ampl; NEPA—UNICAMP: Campinas, Brasil, 2011; 161p, Available online: http://www.cfn.org.br/wp-content/uploads/2017/03/taco_4_edicao_ampliada_e_revisada.pdf (accessed on 18 July 2019).
- Nusser, S.M.; Carriquiry, A.L.; Dodd, K.W.; Fuller, W.A. A semiparametric transformation approach to estimating usual daily intake distributions. J. Am. Stat. Assoc. 1996, 91, 1440–1449. [Google Scholar] [CrossRef]
- Willet, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nut. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory Changes Experienced by Patients Undergoing Cancer Chemotherapy: A Qualitative Interview Study. J. Pain Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Hummel, T.; Böhner, C.; Berktold, S.; Hundt, W.; Kriner, H.; Heinrich, P.; Sommer, H.; Hanusch, C.; Prechtl, U. Qualitative and Quantitative Assessment of Taste and Smell Changes in Patients Undergoing Chemotherapy for Breast Cancer or Gynecologic Malignancies. J. Clin. Oncol. 2009, 27, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Bonassa, E.M.A.; Gato, M.I.R. Terapêutica Oncológica para Enfermeiros e Farmacêuticos, 4th ed.; Atheneu: São Paulo, Brasil, 2012; 650p, ISBN 978-8538802846. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Wang, A.; Neal, B.; Jun, M.; Wong, M.G.; Jardine, M.; Hillis, G.S.; Perkovic, V. Effects of the Mediterranean Diet on Cardiovascular Outcomes—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0159252. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional Recommendations for Cardiovascular Disease Prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A.; et al. Elevated Biomarkers of Inflammation Are Associated With Reduced Survival Among Breast Cancer Patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.; Palermo, C.; Boltong, A. Dietary experiences and support needs of women who gain weight following chemotherapy for breast cancer. Support Care Cancer 2015, 23, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- George, S.M.; Ballard-Barbash, R.; Shikany, J.M.; Caan, B.J.; Freudenheim, J.L.; Kroenke, C.H.; Vitolins, M.Z.; Beresford, S.A.; Neuhouser, M.L. Better Postdiagnosis Diet Quality Is Associated with Reduced Risk of Death among Postmenopausal Women with Invasive Breast Cancer in the Women’s Health Initiative. Cancer Epidemiol. Biomarkers. Prev. 2014, 23, 575–583. [Google Scholar] [CrossRef]
- Pereira, P.F.; Serrano, H.M.S.; Carvalho, G.Q.; Ribeiro, S.M.R.; Peluzio, M.C.G.; Franceschini, S.C.C.; Priore, S.E. Medidas de localização da gordura corporal: Uma avaliação da colinearidade com massa corporal, adiposidade e estatura em adolescentes do sexo feminino. Rev. Paul. Pediatr. 2015, 33, 63–71. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-05822015000100063&lng=en&nrm=iso (accessed on 9 July 2019). [CrossRef]
- Guo, W.; Key, T.J.; Reeves, G.K. Adiposity and breast cancer risk in postmenopausal women: Results from the UK Biobank prospective cohort. Int. J. Cancer 2018, 143, 1037–1046. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Vance, V.; Mourtzakis, M.; Mccargar, L.; Hanning, R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obes. Rev. 2011, 12, 282–294. [Google Scholar] [CrossRef]
- Trédan, O.; Bajard, A.; Meunier, A.; Roux, P.; Fiorletta, I.; Gargi, T.; Bachelot, T.; Guastalla, J.P.; Lallemand, Y.; Faure, C.; et al. Body weight change in women receiving adjuvant chemotherapy for breast cancer: A French prospective study. Clin. Nutr. 2010, 29, 187–191. [Google Scholar] [CrossRef]
- Irwin, M.L.; McTiernan, A.; Bernstein, L.; Gilliland, F.D.; Baumgartner, R.; Baumgartner, K.; Ballard-Barbash, R. Physical activity levels among breast cancer survivors. Med. Sci. Sports Exerc. 2004, 36, 1484–1491. [Google Scholar] [PubMed]
- Hebert, J.R.; Ebbeling, C.B.; Matthews, C.E.; Hurley, T.G.; MA, Y.; Druker, S.; Clemow, L. Systematic errors in middle-aged women’s estimates of energy intake: Comparing three self-report measures to total energy expenditure from doubly labeled water. Ann. Epidemiol. 2002, 12, 577–586. [Google Scholar] [CrossRef]
- Steck, S.E.; Shivappa, N.; Tabung, F.K.; Harmon, B.E.; Wirth, M.D.; Hurley, T.G.; Hebert, J.R. The Dietary Inflammatory Index: A New Tool for Assessing Diet Quality Based on Inflammatory Potential. [Acad. Nutr. Diet.] Digest. 2014, 49, 1–9. [Google Scholar]
- Harmon, B.E.; Carter, M.; Hurley, T.G.; Shivappa, N.; Teas, J.; Hebert, J.R. Nutrient Composition and Anti-inflammatory Potential of a Prescribed Macrobiotic Diet. Nutr. Cancer 2015, 67, 933–940. [Google Scholar] [CrossRef]
- Kushi, L.H.; Cunningham, J.; Hebert, J.R.; Lerman, R.; Bandera, E.V.; Teas, J. The macrobiotic diet in cancer. J. Nutr. 2001, 131, 3056S–3064S. [Google Scholar] [CrossRef]
| Variable | n or Mean ± SD | % |
|---|---|---|
| Age | 51.5 ± 10.1 | 100.0 |
| Marital status | ||
| With partner | 33 | 60.0 |
| Without partner | 22 | 40.0 |
| Income (R$1) | ||
| ≤2 minimum wage | 29 | 52.7 |
| >2 minimum wage | 26 | 47.3 |
| Education | ||
| ≤9 years of study | 24 | 43.6 |
| 9 to 12 years of study | 18 | 32.7 |
| >12 years of study | 12 | 21.8 |
| Tumoral Subtype | ||
| Invasive ductal carcinoma | 53 | 96.4 |
| Invasive lobular carcinoma | 2 | 3.6 |
| Menopausal Status | ||
| Pre-menopausal | 21 | 38.2 |
| Post-menopausal | 34 | 61.8 |
| Surgery | ||
| Conservative | 24 | 43.6 |
| Mastectomy | 8 | 14.6 |
| Did not undergo surgery (neoadjuvant) | 23 | 41.8 |
| Chemotherapy | ||
| Adjuvant | 32 | 58.2 |
| Neoadjuvant | 23 | 41.8 |
| Chemotherapy Regimen | ||
| AC → Docetaxel | 33 | 60.0 |
| AC → Paclitaxel | 8 | 14.6 |
| FAC | 9 | 16.4 |
| CMF | 5 | 9.1 |
| Variables | Median DII® T0 (0.141) | p | Median DII® T1 (0.864) | p | Median DII® T2 (1.888) | p | T0 | T1 | T2 | p | DII® | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Below | Equal/Above | Below | Equal/Above | Below | Equal/Above | T0 | T1 | T2 | ||||||||
| Weight (kg) | 62.5 (56.1–80.6) | 73.2 (60.1–85.3) | 0.08 | 65.2 (58.7–86.3) | 67.3 (58.2–83.9) | 0.97 | 64.6 (58.2–85.1) | 68.0 (59.8–81.5) | 0.66 | 66.1 (58.5–84.1) | 67.2 (58.6–83.9) a.b | 66.4 (58.5–83.1) b | 0.008 ᶷ | 0.205 † | 0.068 † | 0.143 † |
| BMI (kg/m2) | 27.0 ± 6.7 | 29.7 ± 5.9 | 0.12 | 25.8 (23.7–32.4) | 27.6 (23.7–35.0) | 0.71 | 25.9 (23.7–33.6) | 26.6 (24.1–34.2) | 0.64 | 26.4 (23.5–33.7) a | 26.3 (23.8–33.6) a.b | 26.5 (23.9–33.3) b | 0.009 ᶷ | 0.190 † | 0.159 † | 0.209 † |
| WC (cm) | 87.1 ± 16.4 | 94.2 ± 14.4 | 0.09 | 90.2 ± 16.5 | 92.2 ± 14.8 | 0.63 | 90.1 ± 16.3 | 92.0 ± 14.4 | 0.64 | 86.5 (78.5–105.0) a | 88.0 (79.0–103.0) a | 87.0 (80.0–103.5) a | 0.030 ᶷ | 0.165 † | 0.217 ‡ | 0.218 † |
| WHR | 0.84 ± 0.1 | 0.87 ± 0.1 | 0.10 | 0.84 ± 0.07 | 0.86 ± 0.07 | 0.40 | 0.84 ± 0.07 | 0.86 ± 0.07 | 0.35 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.221 ᶿ | 0.165 † | 0.252 ‡ | 0.298 ‡ * |
| WHtR | 0.56 ± 0.1 | 0.59 ± 0.1 | 0.16 | 0.57 ± 0.1 | 0.59 ± 0.1 | 0.43 | 0.57 ± 0.1 | 0.59 ± 0.1 | 0.49 | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.761 ᶷ | 0.153 † | 0.263 ‡ ** | 0.247 † |
| Food Groups (g/Day) | T0 | T1 | T2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | B | SE | p | n | B | SE | p | n | B | SE | p | |
| Total Fruit | 52 | −0.003 | 0.001 | 0.001 | 54 | −0.002 | 0.001 | 0.02 | 52 | −0.002 | 0.001 | 0.01 |
| Total Vegetables | 52 | −0.009 | 0.002 | <0.001 | 51 | −0.011 | 0.002 | <0.001 | 52 | −0.010 | 0.001 | <0.001 |
| Total Grains | 52 | 0.004 | 0.002 | 0.06 | 54 | 0.004 | 0.002 | 0.033 | 52 | 0.005 | 0.002 | 0.01 |
| Whole Grains | 47 | −0.017 | 0.030 | 0.57 | 49 | −0.028 | 0.019 | 0.16 | 47 | −0.029 | 0.016 | 0.08 |
| Fish | 47 | −0.014 | 0.010 | 0.15 | 44 | −0.015 | 0.017 | 0.39 | 46 | * | * | * |
| Red meat | 54 | 0.001 | 0.003 | 0.66 | 51 | 0.003 | 0.004 | 0.53 | 52 | 0.000 | 0.003 | 0.92 |
| Poultry and Eggs | 51 | 0.005 | 0.008 | 0.52 | 54 | −0.003 | 0.004 | 0.41 | 50 | 0.001 | 0.006 | 0.84 |
| Milk and Dairy Products | 54 | −0.003 | 0.002 | 0.07 | 55 | 0.001 | 0.001 | 0.34 | 52 | −0.001 | 0.001 | 0.31 |
| Beans | 52 | 0.005 | 0.003 | 0.15 | 55 | −0.004 | 0.003 | 0.17 | 53 | 0.000 | 0.003 | 0.93 |
| Vegetable Oils | 54 | −0.006 | 0.019 | 0.75 | 51 | 0.000 | 0.026 | 0.99 | 51 | −0.010 | 0.021 | 0.65 |
| Simple Sugars | 53 | 0.022 | 0.008 | 0.006 | 54 | 0.020 | 0.007 | 0.008 | 52 | 0.009 | 0.007 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custódio, I.D.D.; Franco, F.d.P.; Marinho, E.d.C.; Pereira, T.S.S.; Lima, M.T.M.; Molina, M.d.C.B.; Shivappa, N.; Hebert, J.R.; Paiva, C.E.; Maia, Y.C.d.P. Prospective Analysis of Food Consumption and Nutritional Status and the Impact on the Dietary Inflammatory Index in Women with Breast Cancer during Chemotherapy. Nutrients 2019, 11, 2610. https://doi.org/10.3390/nu11112610
Custódio IDD, Franco FdP, Marinho EdC, Pereira TSS, Lima MTM, Molina MdCB, Shivappa N, Hebert JR, Paiva CE, Maia YCdP. Prospective Analysis of Food Consumption and Nutritional Status and the Impact on the Dietary Inflammatory Index in Women with Breast Cancer during Chemotherapy. Nutrients. 2019; 11(11):2610. https://doi.org/10.3390/nu11112610
Chicago/Turabian StyleCustódio, Isis Danyelle Dias, Fernanda de Paula Franco, Eduarda da Costa Marinho, Taísa Sabrina Silva Pereira, Mariana Tavares Miranda Lima, Maria del Carmen Bisi Molina, Nitin Shivappa, James R. Hebert, Carlos Eduardo Paiva, and Yara Cristina de Paiva Maia. 2019. "Prospective Analysis of Food Consumption and Nutritional Status and the Impact on the Dietary Inflammatory Index in Women with Breast Cancer during Chemotherapy" Nutrients 11, no. 11: 2610. https://doi.org/10.3390/nu11112610
APA StyleCustódio, I. D. D., Franco, F. d. P., Marinho, E. d. C., Pereira, T. S. S., Lima, M. T. M., Molina, M. d. C. B., Shivappa, N., Hebert, J. R., Paiva, C. E., & Maia, Y. C. d. P. (2019). Prospective Analysis of Food Consumption and Nutritional Status and the Impact on the Dietary Inflammatory Index in Women with Breast Cancer during Chemotherapy. Nutrients, 11(11), 2610. https://doi.org/10.3390/nu11112610






