Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Biological Samples
2.2. Biopsy Processing and Culture
2.3. Human Intestinal Cytokine Milieu
2.4. Blood Processing and Culture
2.5. Antibody Labelling and Flow Cytometry
2.6. Statistical Analysis
3. Results
3.1. Differential Profile of Mucosal Cytokine Production in HC and IBD Patients
3.2. Bacterial Peptide Conditioning over the IBD Mucosa
3.3. Characterization of Circulating APC in HC and IBD Patients
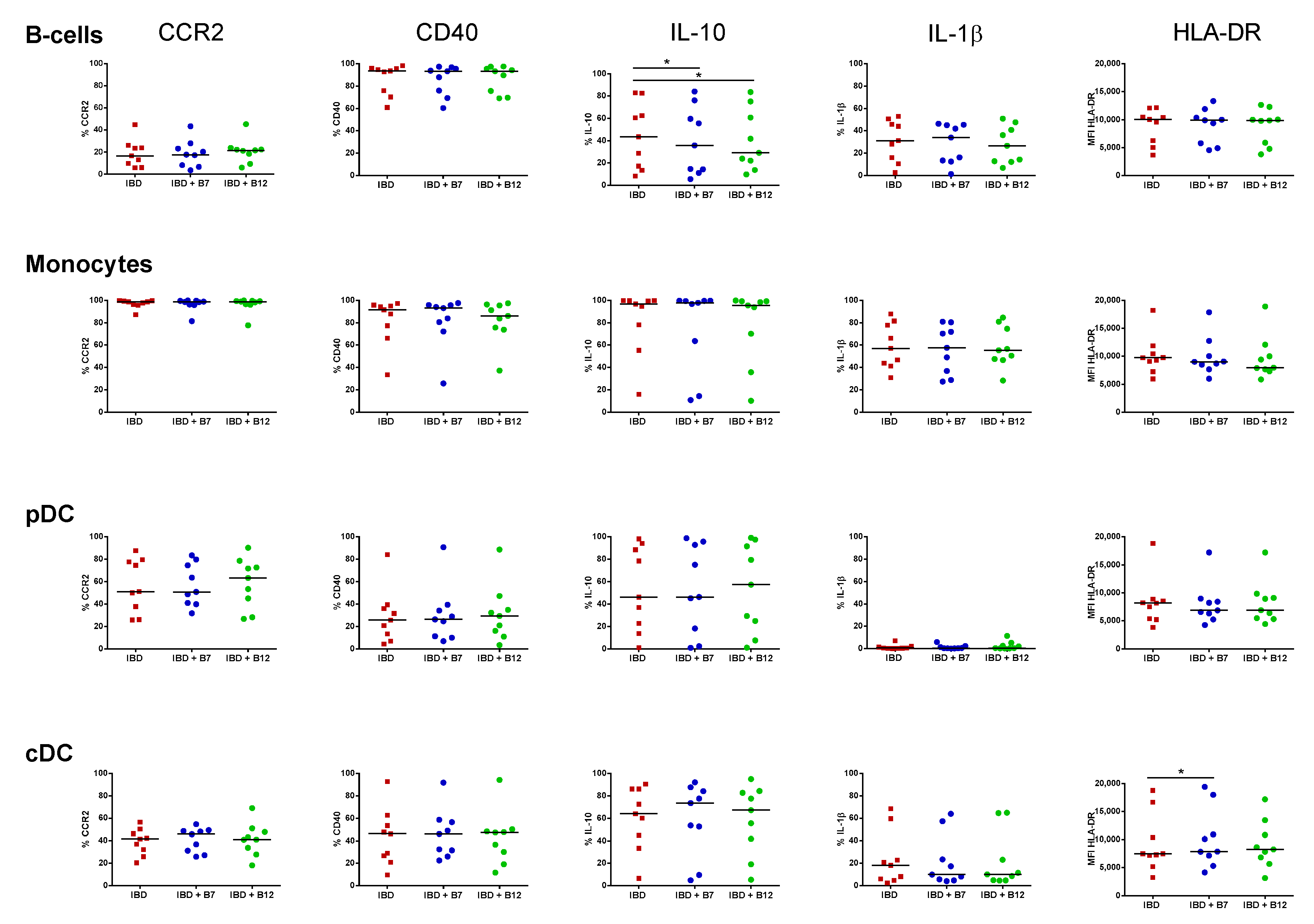
3.4. Immunomodulatory Effect of Bacterial Peptides over Circulating APC from HC and IBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Jones, G.R.; Lyons, M.; Plevris, N.; Jenkinson, P.W.; Bisset, C.; Burgess, C.; Din, S.; Fulforth, J.; Henderson, P.; Ho, G.T.; et al. IBD prevalence in Lothian, Scotland, derived by capture–recapture methodology. Gut 2019, 68, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M. To respond or not to respond - a personal perspective of intestinal tolerance. Nat. Rev. Immunol. 2018, 18, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Dai, C.; Zheng, C.Q.; Meng, F.J.; Zhou, Z.; Sang, L.X.; Jiang, M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell. Biochem. 2013, 374, 1–11. [Google Scholar]
- Chiba, Y.; Shida, K.; Nagata, S.; Wada, M.; Bian, L.; Wang, C.; Shimizu, T.; Yamashiro, Y.; Kiyoshima-Shibata, J.; Nanno, M.; et al. Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology 2010, 130, 352–362. [Google Scholar] [CrossRef]
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyana, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516. [Google Scholar] [CrossRef]
- Belkaid, Y.; Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immun. 2013, 14, 646–653. [Google Scholar] [CrossRef]
- Ruiz, L.; Hevia, A.; Bernardo, D.; Margolles, A.; Sanchez, B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol. Lett. 2014, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Miguez, A.; Gutierrez-Jacome, A.; Fdez-Riverola, F.; Lourenco, A.; Sanchez, B. MAHMI database: A comprehensive MetaHit-based resource for the study of the mechanism of action of the human microbiota. Database (Oxford) 2017, 2017, 157. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Moro-Garcia, M.A.; Blanco-Miguez, A.; Fdez-Riverola, F.; Lourenco, A.; Alonso-Arias, R.; Sanchez, B. In Silico screening of the human gut metaproteome identifies th17-promoting peptides encrypted in proteins of commensal bacteria. Front. Microbiol 2017, 8, 1726. [Google Scholar] [CrossRef]
- Cambeiro-Pérez, N.; Hidalgo-Cantabrana, C.; Moro-García, M.A.; Alonso-Arias, R.; Simal-Gándara, J.; Sánchez, B.; Martínez-Carballo, E. A metabolomics approach reveals immunomodulatory effects of proteinaceous molecules derived from gut bacteria over human peripheral blood mononuclear cells. Front. Microbiol. 2018, 9, 2701. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Fdez-Riverola, F.; Lourenço, A.; Sánchez, B. In silico prediction reveals the existence of potential bioactive neuropeptides produced by the human gut microbiota. Food Res. Int. 2019, 119, 221–226. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Montalban-Arques, A.; Díaz-Guerra, A.; Galvan-Roman, J.M.; Marin, A.C.; Mora-Gutiérrez, I.; Ortega Moreno, L.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Peptides encrypted in the human intestinal microbial-exoproteome as novel biomarkers and immunomodulatory compounds in the gastrointestinal tract. J. Funct. Foods 2019, 52, 459–468. [Google Scholar] [CrossRef]
- Lefort, N.; LeBlanc, R.; Surette, M.E. Dietary Buglossoides Arvensis oil increases circulating n-3 polyunsaturated fatty acids in a dose-dependent manner and enhances lipopolysaccharide-stimulated whole blood interleukin-10—A randomized placebo-controlled trial. Nutrients 2017, 9, 261. [Google Scholar] [CrossRef]
- Bernardo, D.; Chaparro, M.; Gisbert, J.P. Human Intestinal Dendritic Cells in Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2018, 62, e1700931. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Hidalgo, C.; Blanco-Miguez, A.; Lourenco, A.; Sanchez, B.; Margolles, A. Tackling probiotic and gut microbiota functionality through proteomics. J. Proteom. 2016, 147, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines and inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Matsuno, H.; Kayama, H.; Nishimura, J.; Sekido, Y.; Osawa, H.; Barman, S.; Ogino, T.; Takahashi, H.; Haraguchi, N.; Hata, T.; et al. CD103+ Dendritic Cell Function Is Altered in the Colons of Patients with Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1524–1534. [Google Scholar] [CrossRef]
- Bernardo, D.; Marin, A.C.; Fernández-Tomé, S.; Montalban-Arques, A.; Carrasco, A.; Tristán, E.; Ortega-Moreno, L.; Mora-Gutiérrez, I.; Díaz-Guerra, A.; Caminero-Fernández, R.; et al. Human intestinal pro-inflammatory CD11chighCCR2+CX3CR1+ macrophages, but not their tolerogenic CD11c-CCR2-CX3CR1- counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018, 11, 1114–1126. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Metzke, D.; Schmitz, J.; Scheffold, A.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut 2005, 54, 228–236. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Gren, S.T.; Grip, O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1992–1998. [Google Scholar] [CrossRef]
- Vuckovic, S.; Florin, T.H.J.; Khalil, D.; Zhang, M.F.; Patel, K.; Hamilton, I.; Hart, D.N.J. CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases. Am. J. Gastroenterol. 2001, 96, 2946–2956. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; Kirjavainen, P.; Wong, V.H.Y.; El-Nezami, H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J. Funct. Foods 2015, 13, 71–79. [Google Scholar] [CrossRef]
- Chang, Y.L.; Rossetti, M.; Vlamakis, H.; Casero, D.; Sunga, G.; Harre, N.; Miller, S.; Humphries, R.; Stappenbeck, T.; Simpson, K.W.; et al. A screen of Crohn’s disease-associated microbial metabolites identifies ascorbate as a novel metabolic inhibitor of activated human T cells. Mucosal Immunol. 2019, 12, 457–467. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Mallet, J.F.; Graham, E.; Matar, C. Role of probiotics and prebiotics in immunomodulation. Curr. Opin. Food Sci. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Van Bergenhenegouwen, J.; Overbeek, S.; Van De Kant, H.J.G.; Garssen, J.; Folkerts, G.; Vos, P.; Morgan, M.E.; Kraneveld, A.D. Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Zheng, B.; van Bergenhenegouwen, J.; van de Kant, H.J.G.; Folkerts, G.; Garssen, J.; Vos, A.P.; Morgan, M.E.; Kraneveld, A.D. Specific probiotic dietary supplementation leads to different effects during remission and relapse in murine chronic colitis. Benef. Microbes 2016, 7, 205–213. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- Koretz, R.L. Probiotics in Gastroenterology: How Pro Is the Evidence in Adults? Am. J. Gastroenterol. 2018, 113, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Durant, L.; Mann, E.R.; Bassity, E.; Montalvillo, E.; Man, R.; Vora, R.; Reddi, D.; Bayiroglu, F.; Fernandez-Salazar, L.; et al. Chemokine (C-C Motif) Receptor 2 Mediates Dendritic Cell Recruitment to the Human Colon but Is Not Responsible for Differences Observed in Dendritic Cell Subsets, Phenotype, and Function Between the Proximal and Distal Colon. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 22–39. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Gueimonde, M.; Margolles, A.; Suárez, A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int. J. Food Microbiol. 2010, 138, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Blandford, L.E.; Johnston, E.L.; Sanderson, J.D.; Wade, W.G.; Lax, A.J. Promoter orientation of the immunomodulatory Bacteroides fragilis capsular polysaccharide A (PSA) is off in individuals with inflammatory bowel disease (IBD). Gut Microbes 2019, 10, 569–577. [Google Scholar] [CrossRef] [PubMed]



| Cytokines | IBD | IBD + B7 | IBD + B12 | |||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| IL-1β | 123.0 | 43.4 | 120.3 | 43.4 | 142.8 | 41.2 |
| IFN-α2 | 3.4 | 0.7 | 3.4 | 0.6 | 3.2 | 0.6 |
| IFN-γ | 21.3 | 6.9 | 13.0 | 5.5 | 29.9 | 12.7 |
| TNF-α | 24.7 | 4.9 | 22.0 | 7.3 | 22.2 | 7.9 |
| CCL-2 | 677.3 | 235.6 | 1032.0 | 212.4 | 726.3 | 215.4 |
| IL-6 | 980.2 | 104.4 | 891.2 | 141.5 | 979.2 | 127.8 |
| IL-10 | 13.5 | 2.5 | 10.0 | 3.5 * | 9.4 | 2.4 * |
| IL-17A | 2.2 | 0.9 | 3.3 | 2.1 | 4.8 | 1.8 |
| IL-18 | 7.8 | 1.3 | 8.6 | 1.5 | 6.7 | 1.5 |
| IL-23 | 9.8 | 1.7 | 19.3 | 6.6 | 25.9 | 8.5 |
| IL-33 | 23.8 | 4.7 | 22.7 | 5.7 | 22.0 | 6.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Tomé, S.; Marin, A.C.; Ortega Moreno, L.; Baldan-Martin, M.; Mora-Gutiérrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sánchez, B.; Chaparro, M.; et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 2605. https://doi.org/10.3390/nu11112605
Fernández-Tomé S, Marin AC, Ortega Moreno L, Baldan-Martin M, Mora-Gutiérrez I, Lanas-Gimeno A, Moreno-Monteagudo JA, Santander C, Sánchez B, Chaparro M, et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients. 2019; 11(11):2605. https://doi.org/10.3390/nu11112605
Chicago/Turabian StyleFernández-Tomé, Samuel, Alicia C. Marin, Lorena Ortega Moreno, Montserrat Baldan-Martin, Irene Mora-Gutiérrez, Aitor Lanas-Gimeno, José Andrés Moreno-Monteagudo, Cecilio Santander, Borja Sánchez, María Chaparro, and et al. 2019. "Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease" Nutrients 11, no. 11: 2605. https://doi.org/10.3390/nu11112605
APA StyleFernández-Tomé, S., Marin, A. C., Ortega Moreno, L., Baldan-Martin, M., Mora-Gutiérrez, I., Lanas-Gimeno, A., Moreno-Monteagudo, J. A., Santander, C., Sánchez, B., Chaparro, M., Gisbert, J. P., & Bernardo, D. (2019). Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients, 11(11), 2605. https://doi.org/10.3390/nu11112605





