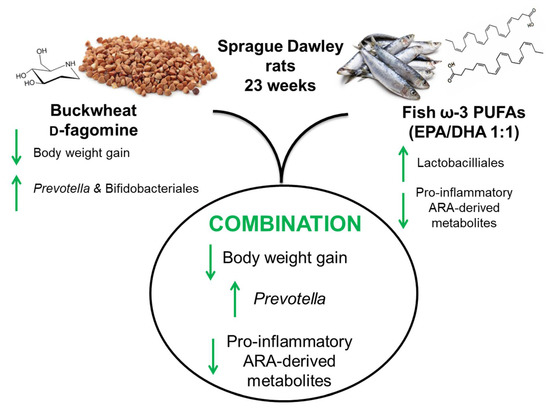
Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Data and Sample Collection
2.3. Glycemic Status
2.4. Plasma Lipid Profile
2.5. Plasma Lipid Mediators of Inflammation
2.6. Fecal Microbial Populations
2.7. Fecal Short-Chain Fatty Acids
2.8. Statistical Analysis
3. Results
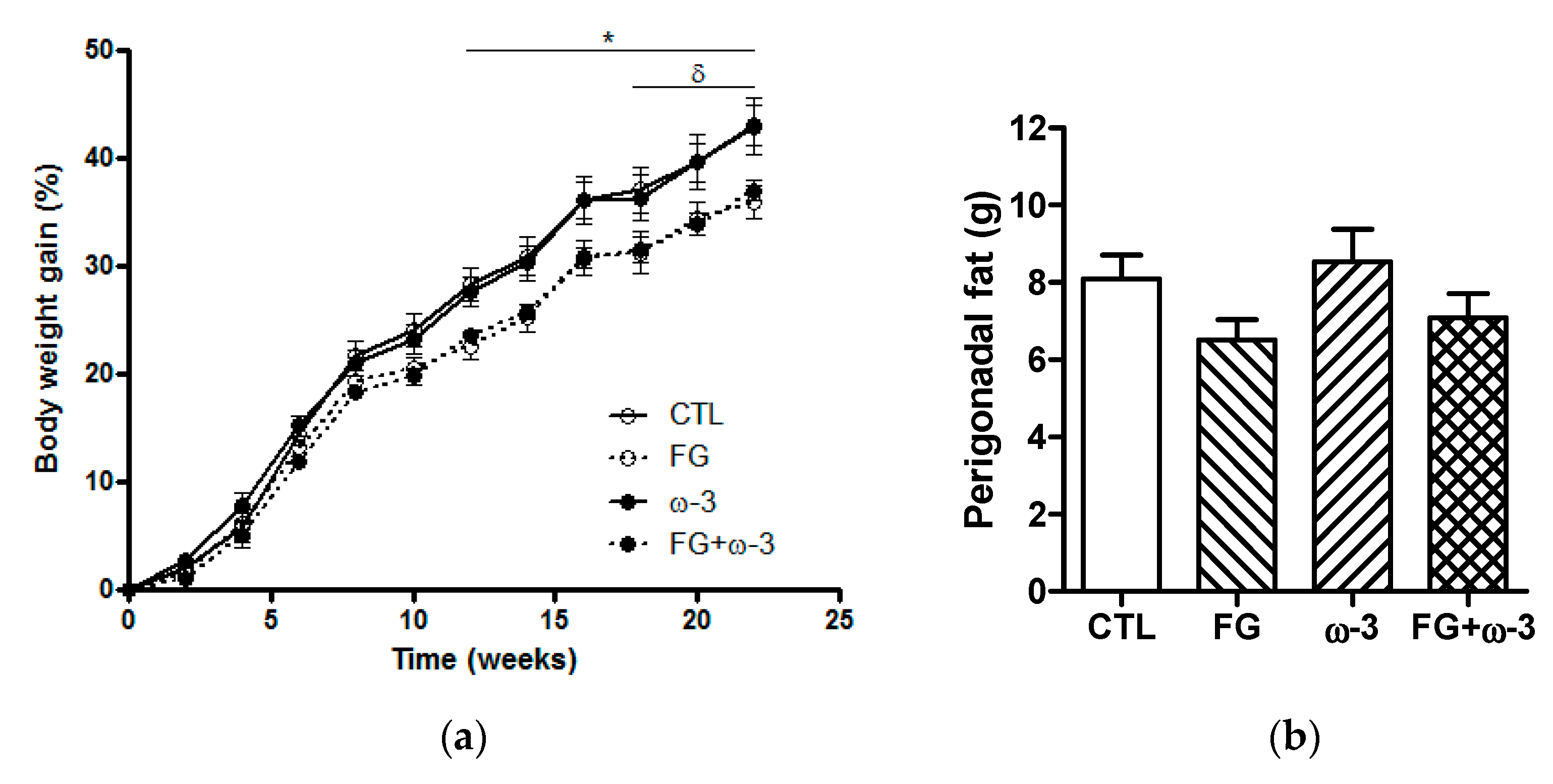
3.1. Feed and Energy Intake and Body Weight
3.2. Glycemic Status and Plasma Lipid Profile
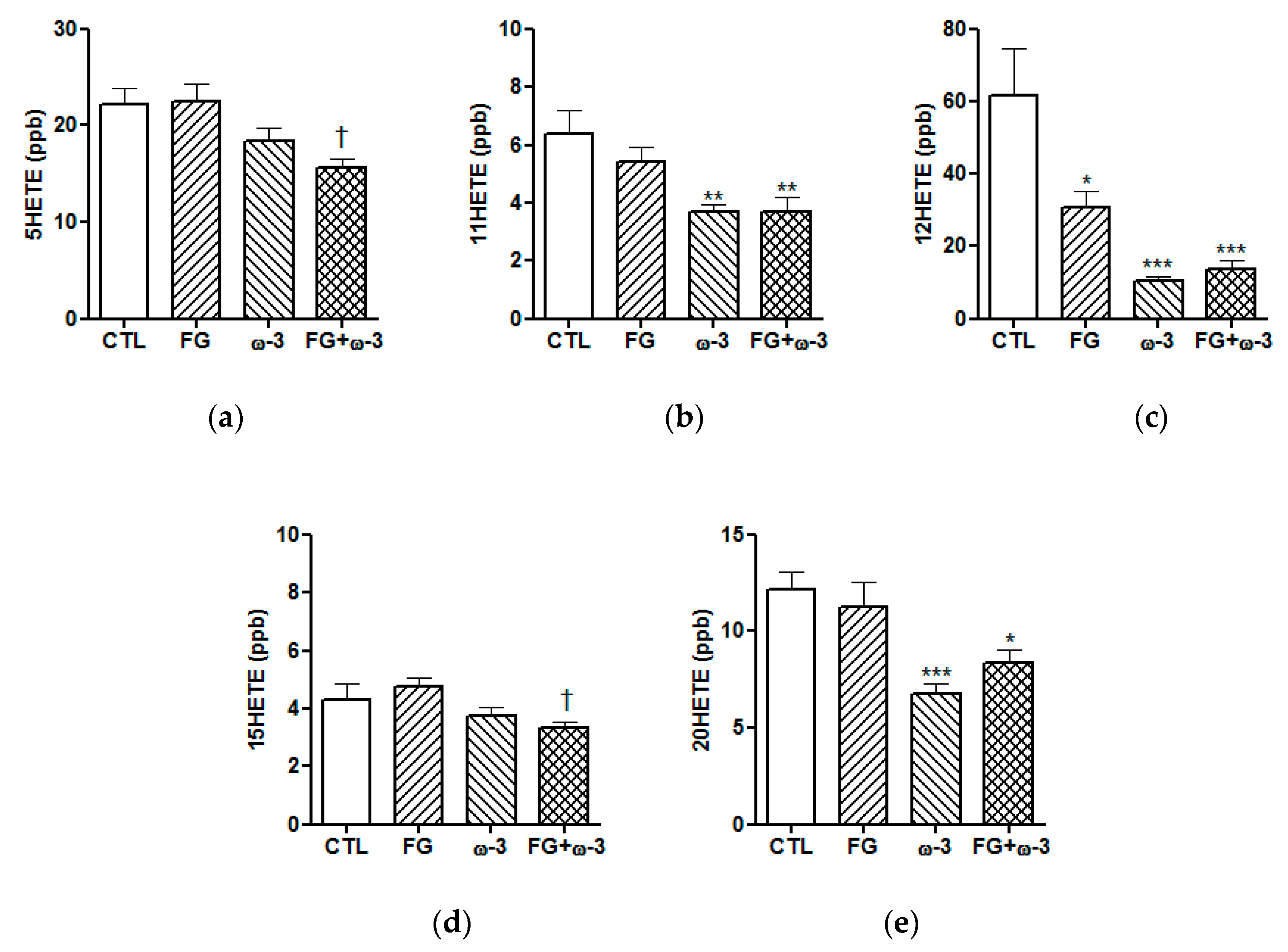
3.3. Plasma Lipid Mediators of Inflammation
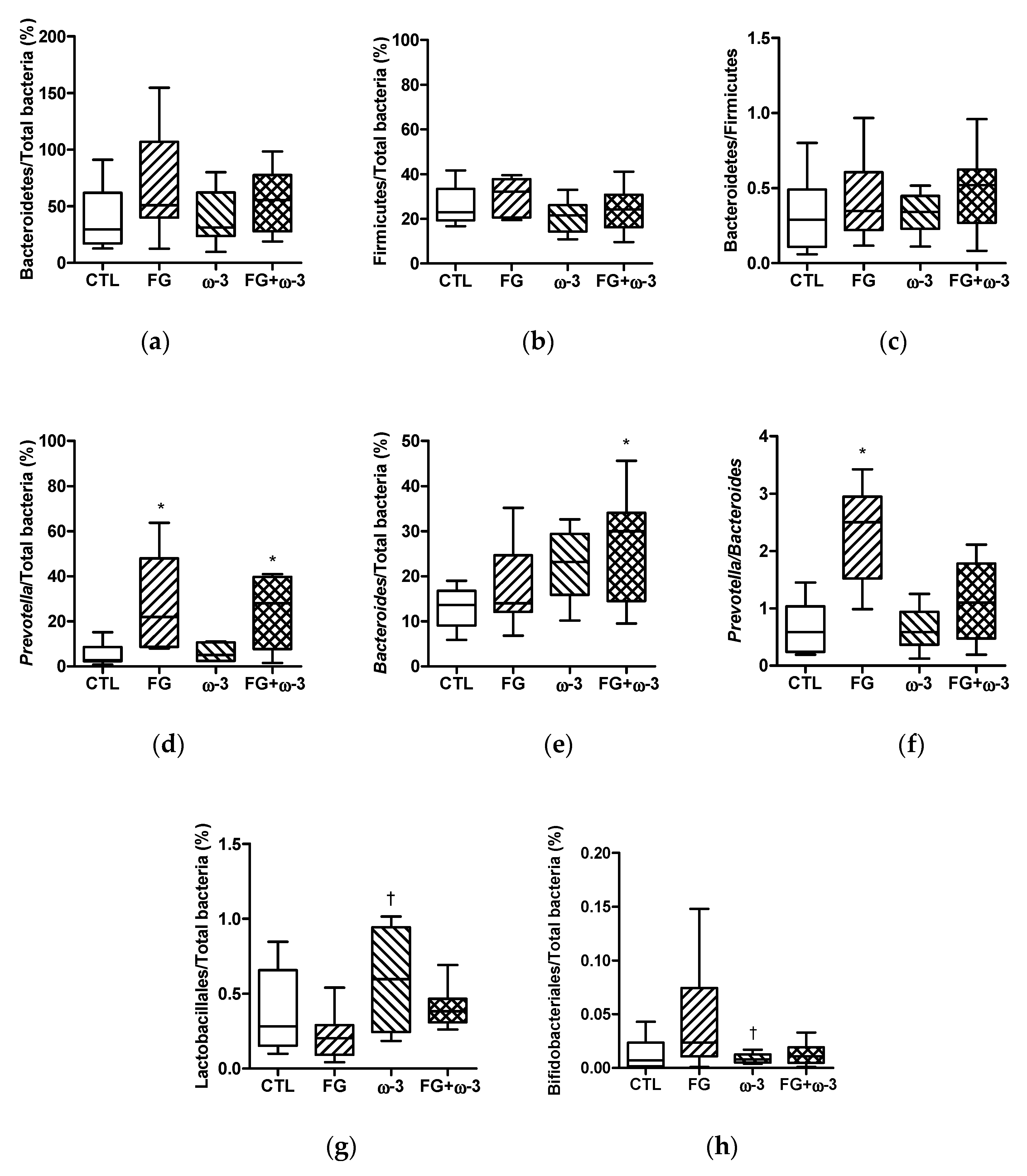
3.4. Bacterial Subgroups of Gut Microbiota
3.5. Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Nordoy, A.; Marchioli, R.; Arnesen, H.; Videbaek, J. n-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids 2001, 36, 127–129. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders. The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Sakamura, S. The Structure of a new piperidine derivative from buckwheat seeds (Fagopyrum esculentum Moench). Agric. Biol. Chem. 1974, 38, 1111–1112. [Google Scholar] [CrossRef]
- Amézqueta, S.; Galán, E.; Fuguet, E.; Carrascal, M.; Abian, J.; Torres, J.L. Determination of d-fagomine in buckwheat and mulberry by cation exchange HPLC/ESI-Q-MS. Anal. Bioanal. Chem. 2012, 402, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.Á.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. d-Fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Molinar-Toribio, E.; Gómez, L.; Pérez-Jiménez, J.; Casado, M.; Clapés, P.; Piña, B.; Torres, J.L. Effect of d-Fagomine on excreted enterobacteria and weight gain in rats fed a high-fat high-sucrose diet. Obesity 2014, 22, 976–979. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Hereu, M.; Atienza, L.; Casas, J.; Taltavull, N.; Romeu, M.; Amezqueta, S.; Dasilva, G.; Medina, I.; Torres, J.L. Functional effects of the buckwheat iminosugar d-fagomine on rats with diet-induced prediabetes. Mol. Nutr. Food Res. 2018, 62, 1800373. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; García-González, N.; Amézqueta, S.; Torres, J.L. Eubiotic effect of buckwheat d-fagomine in healthy rats. J. Funct. Foods 2018, 50, 120–126. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505–1519. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Mujico, J.R.; Baccan, G.C.; Gheorghe, A.; Díaz, L.E.; Marcos, A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br. J. Nutr. 2013, 110, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.C.; Oriach, C.S.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Hereu, M.; Molinar-Toribio, E.; Almajano, M.P.; Méndez, L.; Medina, I.; Taltavull, N.; Romeu, M.; Nogués, M.R.; Torres, J.L. Effects of the combination of omega-3 PUFAs and proanthocyanidins on the gut microbiota of healthy rats. Food Res. Int. 2017, 97, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Bjorck, I.; Backhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Romeu, M.; Giralt, M.; Taltavull, N.; Muñoz-Cortés, M.; Jáuregui, O.; Méndez, L.; Medina, I.; et al. Effect of n-3 PUFA supplementation at different EPA:DHA ratios on the spontaneously hypertensive obese rat model of the metabolic syndrome. Br. J. Nutr. 2015, 113, 878–887. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar]
- Méndez, L.; Pazos, M.; Gallardo, J.M.; Torres, J.L.; Pérez-Jiménez, J.; Nogués, R.; Romeu, M.; Medina, I. Reduced protein oxidation in Wistar rats supplemented with marine omega 3 PUFAs. Free Radic. Biol. Med. 2013, 55, 8–20. [Google Scholar] [CrossRef]
- Dasilva, G.; Pazos, M.; Gallardo, J.M.; Rodríguez, I.; Cela, R.; Medina, I. Lipidomic analysis of polyunsaturated fatty acids and their oxygenated metabolites in plasma by solid-phase extraction followed by LC-MS. Anal. Bioanal. Chem. 2014, 406, 2827–2839. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef] [Green Version]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Haakensen, M.; Dobson, C.M.; Deneer, H.; Ziola, B. Real-time PCR detection of bacteria belonging to the Firmicutes Phylum. Int. J. Food Microbiol. 2008, 125, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2009, 18, 190–195. [Google Scholar] [CrossRef]
- Koletsky, S. Pathologic findings and laboratory data in a new strain of obese hypertensive rats. Am. J. Pathol. 1975, 80, 129–142. [Google Scholar]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef]
- Molinar-Toribio, E.; Pérez-Jiménez, J.; Ramos-Romero, S.; Gómez, L.; Taltavull, N.; Nogués, M.R.; Adeva, A.; Jaúregui, O.; Joglar, J.; Clapés, P.; et al. d-Fagomine attenuates metabolic alterations induced by a high-energy-dense diet in rats. Food Funct. 2015, 6, 2614–2619. [Google Scholar] [CrossRef]
- Lombardo, Y.B.; Chicco, A.G. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J. Nutr. Biochem. 2006, 17, 1–13. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Gordon, J.I. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2018, 42, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Soliman, M.L.; Combs, C.K.; Rosenberger, T.A. Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J. Neuroimmune Pharm. 2013, 8, 287–300. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-alpha-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Vogt, J.A.; Wolever, T.M. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J. Nutr. 2003, 133, 3145–3148. [Google Scholar] [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.T.; Álvarez-Quintero, R.; Velásquez-Mejía, E.P.; Sierra, J.A.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
| Target Bacteria | Annealing Temperature (°C) | Sequences (5′-3′) | Positive Control DNA | Reference |
|---|---|---|---|---|
| Total Bacteria | 65 | F: ACT CCT ACG GGA GGC AGC AGT R: ATT ACC GCG GCT GCT GGC | (a) | [30] |
| Bacteroidetes | 62 | F: ACG CTA GCT ACA GGC TTA A R: ACG CTA CTT GGC TGG TTC A | Bacteroides fragilis | [31] |
| Firmicutes | 52 | F: CTG ATG GAG CAA CGC CGC GT R: ACA CYT AGY ACT CAT CGT TT | Ruminococcus productus | [32] |
| Lactobacillales | 60 | F: AGC AGT AGG GAA TCT TCC A R: CAC CGC TAC ACA TGG AG | Lactobacillus acidophylus | [33] |
| Bifidobacteriales | 55 | F: CTC CTG GAA ACG GGT GG R: GGT GTT CTT CCC GAT ATC TAC A | Bifidobacterium longum | [34] |
| Bacteroides | 60 | F: GGT TCT GAG AGG AGG TCC C R: GCT GCC TCC CGT AGG AGT | Bacteroides fragilis | [35] |
| Prevotella | 60 | F: CAG CAG CCG CGG TAA TA R: GGC ATC CAT CGT TTA CCG T | Prevotella copri | [35] |
| Variables | CTL | FG | ω-3 | FG + ω-3 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Feed intake (g/day/100 g body weight) | 4.6 | 0.5 | 4.8 | 0.4 | 4.6 | 0.5 | 4.9 | 0.4 |
| Energy intake δ (kcal/day/100 g body weight) | 13.3 | 1.4 | 13.8 | 1.2 | 13.4 | 1.3 | 14.1 | 1.3 |
| Initial body weight (g) | 373 | 7 | 360 | 3 | 363 | 7 | 360 | 7 |
| Final body weight (g) | 540 | 16 | 493* | 5 | 523 | 13 | 497 * | 11 |
| Fasting glucose & (mg/dL) | 65 | 2 | 62 | 1 | 67 | 2 | 63 | 2 |
| Fasting insulin & (ng/mL) | 0.56 | 0.10 | 0.34 | 0.03 | 0.65† | 0.07 | 0.43 | 0.06 |
| AUC from OGTT | 3750 | 246.4 | 3212 | 246.1 | 3117 | 152.4 | 2674 | 107.5 |
| Triglycerides (mmol/L) | 0.69 | 0.02 | 0.61* | 0.02 | 0.56***† | 0.01 | 0.53*** | 0.02 |
| Cholesterol (mmol/L) | 3.61 | 0.04 | 3.30** | 0.03 | 3.23*** | 0.08 | 3.24*** | 0.06 |
| HDL/LDL | 2.82 | 0.08 | 2.13*** | 0.06 | 2.34*** | 0.03 | 2.11***φ | 0.04 |
| SCFAs | CTL | FG | ω-3 | FG + ω-3 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Acetic acid | 115 | 13 | 125 | 14 | 23*** | 6 | 19*** | 4 |
| Propionic acid | 13.5 | 0.9 | 15.7 | 3.6 | 9.2 | 1.6 | 10 | 2 |
| Isobutyric acid | 2.7 | 0.2 | 0.9*** | 0.1 | 0.9*** | 0.2 | 0.9*** | 0.1 |
| Butyric acid | 17 | 2 | 27 | 9 | 12 | 3 | 16 | 3 |
| Isovaleric acid | 1.8 | 0.2 | 1.3 | 0.3 | 1.2 | 0.3 | 1.2 | 0.1 |
| Valeric acid | 1.4 | 0.1 | 1.7 | 0.5 | 1.3 | 0.3 | 1.6 | 0.2 |
| Total SCFAs | 152 | 9 | 158 | 19 | 48*** | 11 | 49*** | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hereu, M.; Ramos-Romero, S.; Marín-Valls, R.; Amézqueta, S.; Miralles-Pérez, B.; Romeu, M.; Méndez, L.; Medina, I.; Torres, J.L. Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats. Nutrients 2019, 11, 2606. https://doi.org/10.3390/nu11112606
Hereu M, Ramos-Romero S, Marín-Valls R, Amézqueta S, Miralles-Pérez B, Romeu M, Méndez L, Medina I, Torres JL. Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats. Nutrients. 2019; 11(11):2606. https://doi.org/10.3390/nu11112606
Chicago/Turabian StyleHereu, Mercè, Sara Ramos-Romero, Roser Marín-Valls, Susana Amézqueta, Bernat Miralles-Pérez, Marta Romeu, Lucía Méndez, Isabel Medina, and Josep Lluís Torres. 2019. "Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats" Nutrients 11, no. 11: 2606. https://doi.org/10.3390/nu11112606
APA StyleHereu, M., Ramos-Romero, S., Marín-Valls, R., Amézqueta, S., Miralles-Pérez, B., Romeu, M., Méndez, L., Medina, I., & Torres, J. L. (2019). Combined Buckwheat d-Fagomine and Fish Omega-3 PUFAs Stabilize the Populations of Gut Prevotella and Bacteroides While Reducing Weight Gain in Rats. Nutrients, 11(11), 2606. https://doi.org/10.3390/nu11112606