The Effect of White Rice and White Bread as Staple Foods on Gut Microbiota and Host Metabolism
Abstract
1. Introduction
2. Method
2.1. Subjects
2.2. Study Design
2.3. Assessment of Fecal Samples
2.4. Measurement of Blood Samples
2.5. Analyses of Breath Hydrogen
2.6. Statistical Analysis
3. Results
3.1. Characteristic of Subjects
3.2. Energy Intake and Dietary Composition
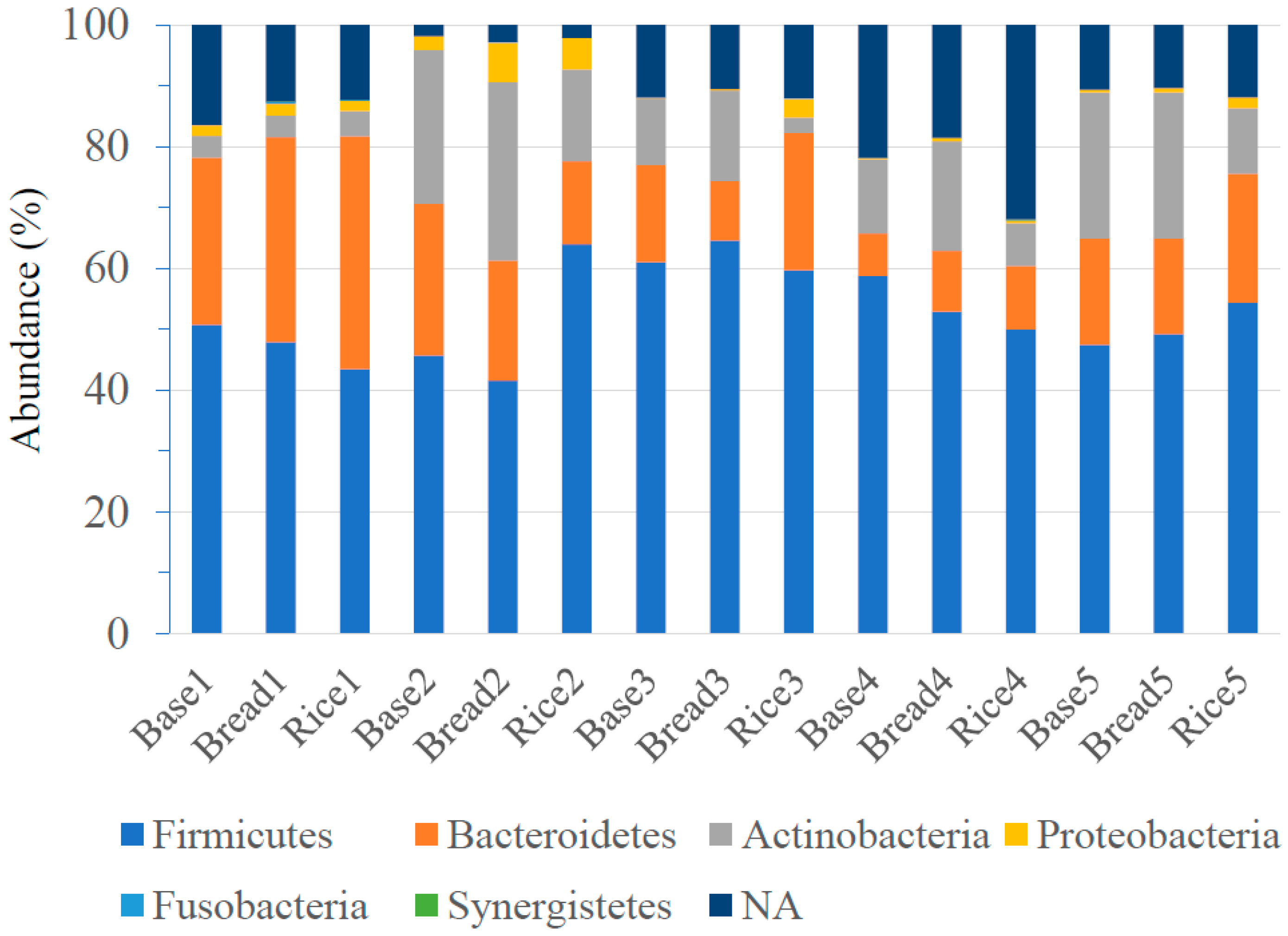
3.3. Intestinal Microbiota Composition
3.4. Hormonal and Metabolic Changes in Blood and Breath
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maff~close to your daily life. Available online: http://www.maff.go.jp/e/data/publish/maff_2016.html (accessed on 4 April 2018).
- Suzuki, N.; Goto, Y.; Ota, H.; Kito, K.; Mano, F.; Joo, E.; Ikeda, K.; Inagaki, N.; Nakayama, T. Characteristics of the Japanese diet described in epidemiologic publications: A qualitative systematic review. J. Nutr. Sci. Vitaminol. 2018, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labor and Welfare. The national health nutrition survey in japan, 2016. Available online: http://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/koumoku_eiyou_chousa.html (accessed on 6 April 2018).
- Batres-Marquez, S.P.; Jensen, H.H.; Upton, J. Rice consumption in the United States: Recent evidence from food consumption surveys. J. Am. Diet. Assoc. 2009, 109, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S.; Murakami, K.; Kim, M.K.; Takahashi, Y.; Hosoi, Y.; Itabashi, M. Dietary patterns associated with functional constipation among Japanese women aged 18 to 20 years: A cross-sectional study. J. Nutr. Sci. Vitaminol. 2007, 53, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Englyst, H.N. Measurement of starch fermentation in the human large intestine. Can. J. Physiol. Pharmacol. 1991, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Knudsen, K.E.; Holst, J.J.; Bjorck, I.M. A cereal-based evening meal rich in indigestible carbohydrates increases plasma butyrate the next morning. J. Nutr. 2010, 140, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.; Grzeskowiak, L.; Franceschini, S.C.; Bressan, J.; Ferreira, C.L.; Peluzio, M.C. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br. J. Nutr. 2013, 109, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.V.; Nilsson, A.C.; Ostman, E.M.; Bjorck, I.M. Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr. J. 2013, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Holst, J.J.; Bjorck, I.M. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J. Nutr. 2008, 138, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Bjorck, I.; Backhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell MeTab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Standard tables of food composition in Japan-2015-(seventh revised version). Available online: http://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm (accessed on 4 April 2018).
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16s rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16s rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Hisada, T.; Endoh, K.; Kuriki, K. Inter- and intra-individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Arch. Microbiol. 2015, 197, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Ibrugger, S.; Vigsnaes, L.K.; Blennow, A.; Skuflic, D.; Raben, A.; Lauritzen, L.; Kristensen, M. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite 2014, 80, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Bjorck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Johansson-Boll, E.V.; Bjorck, I.M. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Nakamura, S. Evaluation of the relative available energy of several dietary fiber preparations using breath hydrogen evolution in healthy humans. J. Nutr. Sci. Vitaminol. 2014, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Tanabe, K.; Morita, S.; Hamaguchi, N.; Shimura, F.; Oku, T. Metabolism and bioavailability of newly developed dietary fiber materials, resistant glucan and hydrogenated resistant glucan, in rats and humans. Nutr. MeTab. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of bifidobacterium adolescentis and faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, S.; Suda, W.; Oshima, K.; Kim, S.; Hirose, Y.; Morita, H.; Hattori, M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The orphan g protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Douglass, J.S.; Birkett, A. Resistant starch intakes in the United States. J. Am. Diet. Assoc. 2008, 108, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhao, H.; Duan, Z.; Linlin, Z.; Wu, D. Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J. Cereal Sci. 2004, 40, 231–237. [Google Scholar] [CrossRef]
- Nakamura, S.; Satoh, H.; Ohtsubo, K. Development of formulae for estimating amylose content, amylopectin chain length distribution, and resistant starch content based on the iodine absorption curve of rice starch. Biosci. Biotechnol. Biochem. 2015, 79, 443–455. [Google Scholar] [CrossRef] [PubMed]


| Subjects (n) | 7 |
| Glucose (mg/dL) | 91.2 ± 2.9 |
| Insulin (µIU/mL) | 5.2 ± 1.6 |
| GIP (pg/mL) | 56.6 ± 31.6 |
| GLP-1 (pg/mL) | 15.7 ± 7.5 |
| TG (mg/dL) | 64.4 ± 22.3 |
| FFA (µEq/L) | 646.3 ± 250.0 |
| Short-chain fatty acids | |
| Acetate (µg/mL) | 6.64 ± 6.14 |
| Propionate (µg/mL) | 0.07 ± 0.02 |
| Butyrate (µg/mL) | 0.04 ± 0.02 |
| Breath H2 (ppm) | 15.8 ± 12.0 |
| Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) | Fiber (g) | |
|---|---|---|---|---|---|
| Bread (n = 7) | 270.6 ± 43.5 | 9.5 ± 1.5 | 47.9 ± 7.7 | 4.5 ± 0.7 | 2.4 ± 0.4 |
| Rice (n = 7) | 272.7 ± 32.9 | 4.1 ± 0.5 | 60.2 ± 7.3 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Side dish (n = 7) | 294.7 ± 66.8 | 13.7 ± 3.2 | 28.5 ± 7.4 | 14.0 ± 5.0 | 4.3 ± 1.0 |
| Baseline (n = 7) | Bread (n = 7) | Rice (n = 7) | |
|---|---|---|---|
| Bifidobacterium (%) | 15.3 ± 13.2 | 19.2 ± 14.5 * | 6.2 ± 6.6 |
| Collinsella (%) | 3.2 ± 3.9 | 3.5 ± 4.5 | 2.3 ± 2.8 |
| Eggerthella (%) | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.3 ± 0.3 |
| Actinomyces (%) | 0.1 ± 0.05 | 0.1 ± 0.1 | 0.1 ± 0.05 |
| Bread (n = 7) | Rice (n = 7) | p Value | |
|---|---|---|---|
| Glucose (mg/dL) | 86.2 ± 5.0 | 87.4 ± 6.9 | 0.52 |
| Insulin (µIU/mL) | 4.0 ± 0.9 | 3.5 ± 1.1 | 0.34 |
| GIP (pg/mL) | 55.2 ± 18.9 | 43.8 ± 21.1 | 0.11 |
| GLP-1 (pg/mL) | 13.6 ± 2.0 | 10.5 ± 2.9 | 0.03 * |
| TG (mg/dL) | 58.4 ± 16.4 | 73.9 ± 29.4 | 0.20 |
| FFA (µEq/L) | 619.6 ± 149.1 | 561.6 ± 281.6 | 0.63 |
| Short-chain fatty acids | |||
| Acetate (µg/mL) | 5.34 ± 4.08 | 4.12 ± 3.06 | 0.70 |
| Propionate (µg/mL) | 0.11 ± 0.09 | 0.06 ± 0.03 | 0.16 |
| Butyrate (µg/mL) | 0.06 ± 0.04 | 0.02 ± 0.01 | 0.12 |
| Breath H2 (ppm) | 23.4 ± 9.9 | 8.2 ± 5.2 | 0.02 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mano, F.; Ikeda, K.; Joo, E.; Fujita, Y.; Yamane, S.; Harada, N.; Inagaki, N. The Effect of White Rice and White Bread as Staple Foods on Gut Microbiota and Host Metabolism. Nutrients 2018, 10, 1323. https://doi.org/10.3390/nu10091323
Mano F, Ikeda K, Joo E, Fujita Y, Yamane S, Harada N, Inagaki N. The Effect of White Rice and White Bread as Staple Foods on Gut Microbiota and Host Metabolism. Nutrients. 2018; 10(9):1323. https://doi.org/10.3390/nu10091323
Chicago/Turabian StyleMano, Fumika, Kaori Ikeda, Erina Joo, Yoshihito Fujita, Shunsuke Yamane, Norio Harada, and Nobuya Inagaki. 2018. "The Effect of White Rice and White Bread as Staple Foods on Gut Microbiota and Host Metabolism" Nutrients 10, no. 9: 1323. https://doi.org/10.3390/nu10091323
APA StyleMano, F., Ikeda, K., Joo, E., Fujita, Y., Yamane, S., Harada, N., & Inagaki, N. (2018). The Effect of White Rice and White Bread as Staple Foods on Gut Microbiota and Host Metabolism. Nutrients, 10(9), 1323. https://doi.org/10.3390/nu10091323





