Zinc and Sepsis
Abstract
1. Introduction
1.1. Sepsis
1.2. Zinc
2. Zinc Homeostasis during Sepsis
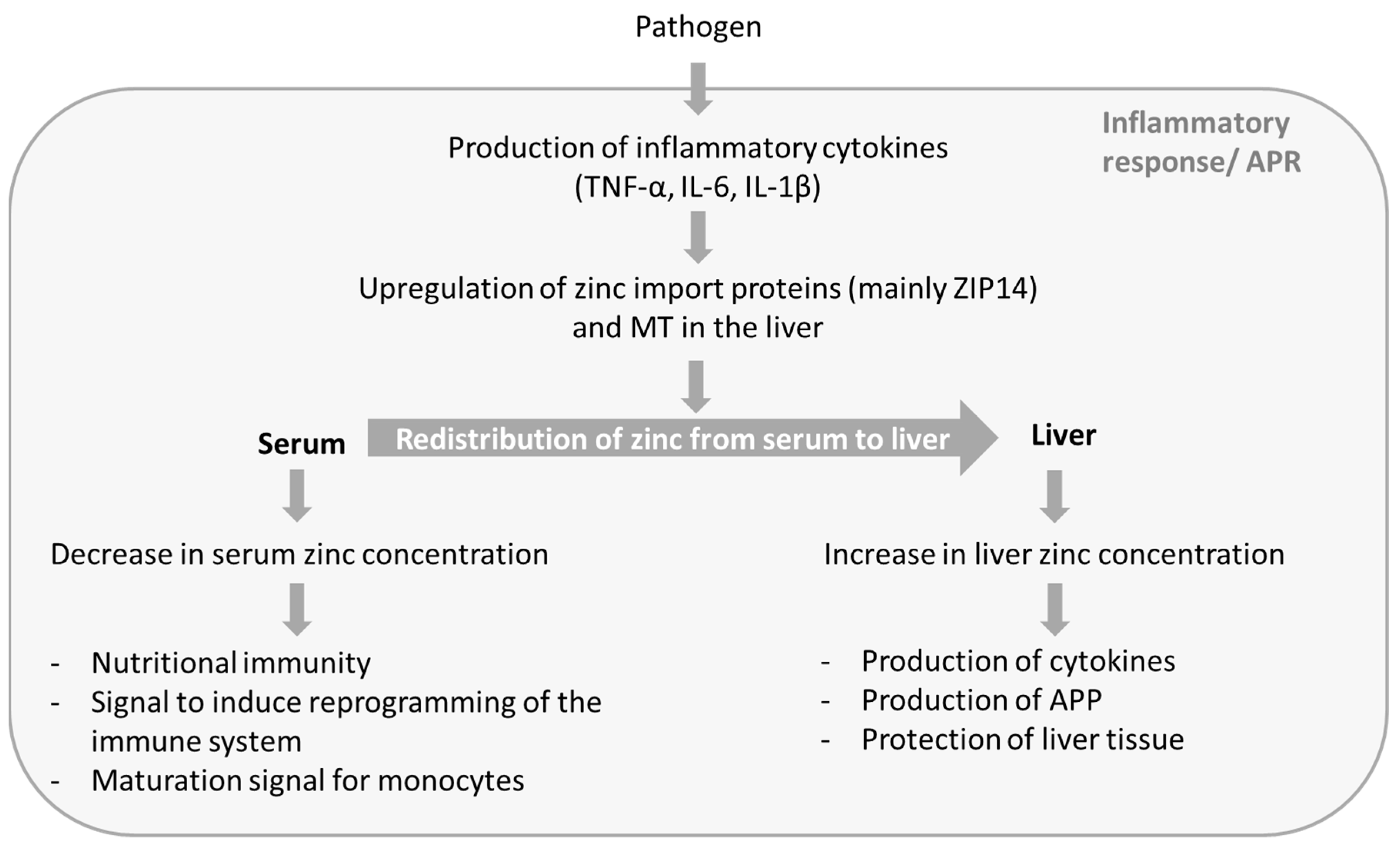
2.1. Changes in Zinc Homeostasis
2.2. Possible Reasons for the Redistribution of Zinc
2.3. Possible Adverse Effects of Zinc-Redistribution
3. Zinc Supplementation
4. Serum Zinc Concentration as a Possible Biomarker for Sepsis
5. Conclusions
Funding
Conflicts of Interest
References
- Funk, D.J.; Parrillo, J.E.; Kumar, A. Sepsis and septic shock: A history. Crit. Care Clin. 2009, 25, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- WHO (The World Health Organization). WHA Resolution A70/13—Improving the Prevention, Diagnosis and Clinical Management of Sepsis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- WHO (The World Health Organization). WHO Secretariat Report A70/13—Improving the Prevention, Diagnosis and Clinical Management of Sepsis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Vincent, J.-L.; Marshall, J.C.; Ñamendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the worldwide burden of critical illness: The Intensive Care Over Nations (ICON) audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; Mendonça, A.D.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Opal, S.M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Grodzin, C.J.; Balk, R.A. Sepsis: A New hypothesis for pathogenesis of the disease process. CHEST 1997, 112, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Dumont, T.; Francis-frank, L.; Balaan, M. Sepsis and septic shock: A review. Crit. Care Nurs. Q. 2015, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Casserly, B.; Ayala, A. The Compensatory Anti-inflammatory Response syndrome (CARS) in critically ill patients. Clin. Chest Med. 2008, 29, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef]
- Prasad, A.S. Importance of zinc in human nutrition. Am. J. Clin. Nutr. 1967, 20, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E. Zinc proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.W. Zinc and its deficiency diseases. Clin. Physiol. Biochem. 1986, 4, 94–98. [Google Scholar] [PubMed]
- King, L.E.; Frentzel, J.W.; Mann, J.J.; Fraker, P.J. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005, 24, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Meftah, S.; Abdallah, J.; Kaplan, J.; Brewer, G.J.; Bach, J.F.; Dardenne, M. Serum thymulin in human zinc deficiency. J. Clin. Investig. 1988, 82, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E. A distinct role for apoptosis in the changes in lymphopoiesis and myelopoiesis created by deficiencies in zinc. FASEB J. 2001, 15, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.S.; Uciechowski, P.; Meyer, S.; Schwerdtle, T.; Rink, L.; Haase, H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics 2014, 6, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, M.; Pléau, J.M.; Nabarra, B.; Lefrancier, P.; Derrien, M.; Choay, J.; Bach, J.F. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc. Natl. Acad. Sci. USA 1982, 79, 5370–5373. [Google Scholar] [CrossRef] [PubMed]
- Incefy, G.S.; Mertelsmann, R.; Yata, K.; Dardenne, M.; Bach, J.F.; Good, R.A. Induction of differentiation in human marrow T cell precursors by the synthetic serum thymic factor, FTS. Clin. Exp. Immunol. 1980, 40, 396–406. [Google Scholar] [PubMed]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182, S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.W.; Prasad, A.S.; Kaplan, J.; Fitzgerald, J.T.; Brewer, G.J. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. Endocrinol. Metab. 1997, 272, E1002–E1007. [Google Scholar] [CrossRef] [PubMed]
- King, L.E.; Osati-Ashtiani, F.; Fraker, P.J. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J. Nutr. 2002, 132, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, E.; Maywald, M.; Hilgers, R.-D.; Brieger, A.; Clarner, T.; Kipp, M.; Plümäkers, B.; Meyer, S.; Schwerdtle, T.; Rink, L. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J. Nutr. Biochem. 2016, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, B.; Choi, Y.H.; Hwang, Y.; Kim, D.H.; Cho, S.; Hong, S.J.; Lee, W. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology 2015, 146, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, C.; Fukada, T.; Kanamoto, M.; Ohashi, W.; Hojyo, S.; Atsumi, T.; Ueda, N.; Azuma, I.; Hirota, H.; Murakami, M.; et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010, 22, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Ober-Blöbaum, J.L.; Engelhardt, G.; Hebel, S.; Heit, A.; Heine, H.; Rink, L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 2008, 181, 6491–6502. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Morikawa, H.; Kamon, H.; Iguchi, M.; Hojyo, S.; Fukada, T.; Yamashita, S.; Kaisho, T.; Akira, S.; Murakami, M.; et al. Toll-like receptor–mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 2006, 7, 971. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Rink, L.; Haase, H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kaltenberg, J.; Plum, L.M.; Ober-Blöbaum, J.L.; Hönscheid, A.; Rink, L.; Haase, H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010, 40, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Souffriau, J.; Libert, C. Mechanistic insights into the protective impact of zinc on sepsis. Cytokine Growth Factor Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; McClain, C.J.; Talwalkar, R.T.; Shedlofsky, S.I. Effects of endotoxin on zinc metabolism in human volunteers. Am. J. Physiol. Endocrinol. Metab. 1997, 272, E952–E956. [Google Scholar] [CrossRef] [PubMed]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; DiSilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission123. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Mertens, K.; Lowes, D.A.; Webster, N.R.; Talib, J.; Hall, L.; Davies, M.J.; Beattie, J.H.; Galley, H.F. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br. J. Anaesth. 2015, 114, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, J.; Simon, T.-P.; Beeker, T.; Marx, G.; Haase, H.; Schuerholz, T. Persistent low serum zinc is associated with recurrent sepsis in critically ill patients—A pilot study. PLoS ONE 2017, 12, e0176069. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, J.; Simon, T.-P.; Doemming, S.; Thiele, C.; Marx, G.; Schuerholz, T.; Haase, H. Alterations in zinc binding capacity, free zinc levels and total serum zinc in a porcine model of sepsis. BioMetals 2015, 28, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Liuzzi, J.P.; Cousins, R.J. Interleukin-1β contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G860–G867. [Google Scholar] [CrossRef] [PubMed]
- Sobocinski, P.Z.; Canterbury, W.J.; Mapes, C.A.; Dinterman, R.E. Involvement of hepatic metallothioneins in hypozincemia associated with bacterial infection. Am. J. Physiol. Endocrinol. Metab. 1978, 234, E399. [Google Scholar] [CrossRef] [PubMed]
- Sobocinski, P.Z.; Canterbury, W.J. Hepatic metallothionein induction in inflammation. Ann. N. Y. Acad. Sci. 1982, 389, 354–367. [Google Scholar] [CrossRef]
- Cousins, R.J.; Leinart, A.S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1988, 2, 2884–2890. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life Sci. CMLS 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.L.; Cousins, R.J. Metallothionein expression in rat bone marrow is dependent on dietary zinc but not dependent on interleukin-1 or interleukin-6. J. Nutr. 1993, 123, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Cousins, R.J. Zinc dyshomeostasis during polymicrobial sepsis in mice involves zinc transporter Zip14 and can be overcome by zinc supplementation. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G768–G778. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.; To, L.; Tovbin, A.; Smoot, T.; Mlynarek, M. Heavy metal in the intensive care unit: A review of current literature on trace element supplementation in critically ill patients. Nutr. Clin. Pract. 2014, 29, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R. Pediatric septic shock treatment: New clues from genomic profiling. Pharmacogenomics 2007, 8, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Shanley, T.P.; Sakthivel, B.; Cvijanovich, N.; Lin, R.; Allen, G.L.; Thomas, N.J.; Doctor, A.; Kalyanaraman, M.; Tofil, N.M.; et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol. Genom. 2007, 30, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Shanley, T.P.; Wong, H.R. Molecular genetics in the pediatric intensive care unit. Crit. Care Clin. 2003, 19, 577–594. [Google Scholar] [CrossRef]
- Cvijanovich, N.; Shanley, T.P.; Lin, R.; Allen, G.L.; Thomas, N.J.; Checchia, P.; Anas, N.; Freishtat, R.J.; Monaco, M.; Odoms, K.; et al. Validating the genomic signature of pediatric septic shock. Physiol. Genom. 2008, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Moshage, H. Cytokines and the hepatic acute phase response. J. Pathol. 1997, 181, 257–266. [Google Scholar] [CrossRef]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef]
- Florea, D.; Molina-López, J.; Hogstrand, C.; Lengyel, I.; de la Cruz, A.P.; Rodríguez-Elvira, M.; Planells, E. Changes in zinc status and zinc transporters expression in whole blood of patients with Systemic Inflammatory Response Syndrome (SIRS). J. Trace Elem. Med. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rofe, A.M.; Philcox, J.C.; Coyle, P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem. J. 1996, 314, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Iizuka, Y.; Furusawa, S.; Ishikawa, M.; Satoh, S.; Takayanagi, M. Role of Zn2+ in oxidative stress caused by endotoxin challenge. Eur. J. Pharmacol. 2002, 451, 309–316. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Song, Z.; Saari, J.T.; McClain, C.J.; Kang, Y.J. Abrogation of nuclear factor-κB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-α production and liver injury. Am. J. Pathol. 2004, 164, 1547–1556. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- King, L.E.; Osati-Ashtiani, F.; Fraker, P.J. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology 1995, 85, 69–73. [Google Scholar] [PubMed]
- Dubben, S.; Hönscheid, A.; Winkler, K.; Rink, L.; Haase, H. Cellular zinc homeostasis is a regulator in monocyte differentiation of HL-60 cells by 1α,25-dihydroxyvitamin D3. J. Leukoc. Biol. 2010, 87, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.; Bao, B.; Fitzgerald, J.T.; Snell, D.C.; Steinberg, J.D.; Cardozo, L.J. Zinc supplementation decreases incidence of infections in the elderly: Effect of zinc on generation of cytokines and oxidative stress. Am. J. Clin. Nutr. 2007, 85, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Haase, H.; Engelhardt, G.; Rink, L.; Uciechowski, P. Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. J. Nutr. Biochem. 2013, 24, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Clegg, M.S.; Zago, M.P.; Keen, C.L. Zinc deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Radic. Biol. Med. 2000, 28, 1091–1099. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J. Nutr. 1995, 125, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chung, C.S.; Bruno, R.S.; Traber, M.G.; Brown, K.H.; King, J.C.; Ho, E. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am. J. Clin. Nutr. 2009, 90, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Osmon, S.B.; Chang, K.C.; Wagner, T.H.; Coopersmith, C.M.; Karl, I.E. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 2005, 174, 5110–5118. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230. [Google Scholar] [CrossRef] [PubMed]
- Heidecke, C.-D.; Hensler, T.; Weighardt, H.; Zantl, N.; Wagner, H.; Siewert, J.-R.; Holzmann, B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am. J. Surg. 1999, 178, 288–292. [Google Scholar] [CrossRef]
- Andresen, M.; Regueira, T.; Bruhn, A.; Perez, D.; Strobel, P.; Dougnac, A.; Marshall, G.; Leighton, F. Lipoperoxidation and protein oxidative damage exhibit different kinetics during septic shock. Mediat. Inflamm. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, C.; Kadioglu, E.; Ozcagli, E.; Osmanoglu, G.; Izdes, S.; Agalar, C.; Basar, H.; Sardas, S. Oxidative DNA damage and total antioxidant status in rats during experimental gram-negative sepsis. Hum. Exp. Toxicol. 2008, 27, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Cander, B.; Dundar, Z.D.; Gul, M.; Girisgin, S. Prognostic value of serum zinc levels in critically ill patients. J. Crit. Care 2011, 26, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Newton, B.; Ballambattu, V.B.; Bosco, D.B.; Gopalakrishna, S.M.; Subash, C.P. Efficacy of zinc supplementation on serum calprotectin, inflammatory cytokines and outcome in neonatal sepsis—Arandomized controlled trial. J. Matern. Fetal Neonatal Med. 2017, 30, 1627–1631. [Google Scholar] [CrossRef]
- Newton, B.; Bhat, B.V.; Bosco Dhas, B.; Mondal, N.; Gopalakrishna, S.M. Effect of zinc supplementation on early outcome of neonatal sepsis—A randomized controlled trial. Indian J. Pediatr. 2016, 83, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Newton, B.; Bhat, B.V.; Bosco Dhas, B.; Christina, C.; Gopalakrishna, S.M.; Subhash Chandra, P. Short term oral zinc supplementation among babies with neonatal sepsis for reducing mortality and improving outcome—A double-blind randomized controlled trial. Indian J. Pediatr. 2018, 85, 5–9. [Google Scholar] [CrossRef]
- Mehta, K.; Bhatta, N.K.; Majhi, S.; Shrivastava, M.K.; Singh, R.R. Oral zinc supplementation for reducing mortality in probable neonatal sepsis: A double blind randomized placebo controlled trial. Indian Pediatr. 2013, 50, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, C.L.; Sowers, M.; Kovacevich, D.S.; Hill, G.M.; August, D.A. Parenteral zinc supplementation in adult humans during the acute phase response increases the febrile response. J. Nutr. 1997, 127, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, H.A.; Varisco, B.M.; Harmon, K.; Lahni, P.; Opoka, A.; Wong, H.R. Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun. 2017, 23, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.E.; Harmon, K.; Caldwell, C.C.; Wong, H.R. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2012, 13, e323–e329. [Google Scholar] [CrossRef] [PubMed]
- Krones, C.J.; Klosterhalfen, B.; Butz, N.; Hoelzl, F.; Junge, K.; Stumpf, M.; Peiper, C.; Klinge, U.; Schumpelick, V. Effect of zinc pretreatment on pulmonary endothelial cells in vitro and pulmonary function in a porcine model of endotoxemia. J. Surg. Res. 2005, 123, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Krones, C.J.; Klosterhalfen, B.; Anurov, M.; Stumpf, M.; Klinge, U.; Oettinger, A.P.; Schumpelick, V. Missing effects of zinc in a porcine model of recurrent endotoxemia. BMC Surg. 2005, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Krones, C.J.; Klosterhalfen, B.; Fackeldey, V.; Junge, K.; Rosch, R.; Schwab, R.; Stumpf, M.; Klinge, U.; Schumpelick, V. Deleterious effect of zinc in a pig model of acute endotoxemia. J. Investig. Surg. 2004, 17, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Driessen, C.; Hirv, K.; Rink, L.; Kirchner, H. Induction of cytokines by zinc ions in human peripheral blood mononuclear cells and separated monocytes. Lymphokine Cytokine Res. 1994, 13, 15–20. [Google Scholar] [PubMed]
- Foote, J.W.; Delves, H.T. Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984, 37, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Jang, J.Y.; Shim, H.; Lee, S.H.; Lee, J.G. Serum selenium and zinc levels in critically ill surgical patients. J. Crit. Care 2014, 29, 317.e5–317.e8. [Google Scholar] [CrossRef] [PubMed]
- Trame, S.; Wessels, I.; Haase, H.; Rink, L. A short 18 items food frequency questionnaire biochemically validated to estimate zinc status in humans. J. Trace Elem. Med. Biol. 2018, 49, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Sukhavasi, S.; Jothimuthu, P.; Papautsky, I.; Beyette, F.R. Development of a Point-of-Care device to quantify serum zinc to aid the diagnosis and follow-up of pediatric septic shock. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 3676–3679. [Google Scholar] [CrossRef]
- Zerhusen, B.; de Silva, G.; Pei, X.; Papautsky, I.; Beyette, F.R. Rapid quantification system for zinc in blood serum. In Proceedings of the 2013 IEEE 56th International Midwest Symposium on Circuits and Systems (MWSCAS), Columbus, OH, USA, 4–7 August 2013; pp. 400–403. [Google Scholar]
- Maret, W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 2015, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Kipp, A.P.; Haase, H.; Meyer, S.; Schwerdtle, T. The crux of inept biomarkers for risks and benefits of trace elements. TrAC Trends Anal. Chem. 2017. [Google Scholar] [CrossRef]
| Study Population | Intervention/Zn-Supply | Observation Time Points | Results (Zinc Group vs. Control Group) | Reference |
|---|---|---|---|---|
| Neonates with clinical signs suggestive of sepsis and at least two screening tests positive | Zinc group *: Antibiotic treatment, dose of 3 mg/kg bodyweight (BW) zinc sulfate monohydrate twice a day for 10 days (corresponding to 2.1 mg/kg BW Zn2+ per day) Control group: Antibiotic treatment | Measurement of blood samples from base line (BL) and after 10 days | • Significant increase in serum zinc concentrations compared to BL • Significant decrease in TNF-α compared to BL • Lower mortality rate, but not reaching significance (7.4% compared to 16.4%) • Similar duration of hospitalization | [78] |
| Neonates with clinical features of sepsis and positive blood culture or positive sepsis screening tests | Zinc group *: Antibiotic treatment, dose of 3 mg/kg BW zinc sulfate monohydrate twice a day for 10 days (corresponding to 2.1 mg/kg BW Zn2+ per day) Control group: Antibiotic treatment | Measurement of blood samples from BL and after 10 days | • Increase in serum zinc concentrations compared to BL, but not reaching significance • Lower mortality rate, but not reaching significance (4.5% compared to 13.6%) • Better neurological status (chance of having abnormalities is 70% less) at one month of age • Similar duration of hospitalization | [79] |
| Neonates with clinical manifestations of sepsis who exhibited two positive screening tests | Zinc group *: Antibiotic treatment, dose of 3 mg/kg BW zinc sulfate monohydrate twice a day for 10 days (corresponding to 2.1 mg/kg BW Zn2+ per day) Control group: Antibiotic treatment | Measurement of blood samples from BL and after 10 days | • Significant increase in serum zinc concentrations • Significantly lower mortality rate (6.6% compared to 17.3%) • Better neurodevelopment (significantly better Mental Development Quotient) at 12 month of age | [80] |
| Neonates with probable sepsis | Zinc group *: Antibiotic treatment, dose of 1 mg/kg BW zinc sulfate per day until the final outcome (discharge/death) (corresponding to 0.4 mg/kg BW Zn2+ per day) Control group Antibiotic treatment, dose of placebo until the final outcome (discharge/death) | Final outcome at discharge/death | • No significant differences in mortality rate • No significant differences in duration of hospital stay • No significant differences in requirement of antibiotic treatment | [81] |
| Patients with pancreatitis or catheter sepsis | Zinc group *: Total parental nutrition, 30 mg zinc sulfate per day for 3 days (corresponding to 12.1 mg Zn2+ per day) Control group: Total parental nutrition, 0 mg zinc sulfate for 3 days | Measurement of blood samples from BL, day 1, 2, 3; highest temperatures from patients’ bedside charts from day 1, 2, 3 | • Higher temperatures, reaching significance on day 3 • No difference in serum IL-6 and ceruloplasmin | [82] |
| Animals | Sepsis Model | Intervention/Zn-Supply | Results (Zinc Group vs. Control Group) | Reference |
|---|---|---|---|---|
| Male mice (C57BL/6) | Intraperitoneal (i. p.) fecal slurry injection; sacrifice of mice at 24 h to conduct assays or observed 72 h for survival study | Zinc group: Injection of 10 mg/kg BW zinc gluconate every 24 h for 3 days prior to induction of sepsis, injection continued every 24 h after induction of peritonitis (corresponding to 1.4 mg/kg BW Zn2+ per day) Control group: Injection of equal volume of saline at the same time points as for the zinc group | • Significantly improved survival following sepsis at 72 h after induction • Significantly lower myeloperoxidase activity in lung tissue (at 24 h) • Significantly lower bacterial burden in blood and spleen (at 24 h) • Significantly lower serum keratinocyte chemoattractant concentration (at 24 h) • No significant difference between serum concentration of IL6, IL-1β, IL-10 | [84] |
| Male and female mice (C57BL/6) | Cecal ligation and puncture; sacrifice of mice at 24 h | Zinc group: High-zinc diet (180 mg/kg) for 7 days prior to induction of sepsis Control group: Zinc-adequate diet (30 mg/kg) for 7 days prior to induction of sepsis | • Significantly lower IL-6 mRNA expression in hepatocytes • Significantly lower TNF-α mRNA expression in hepatic leukocytes • Significantly lower S100A9 mRNA expression in white blood cells • Significantly lower serum concentrations of TNF-α, S100A8 and S100A9 • Significantly lower serum concentration of plasma alanine aminotransferase • Significantly lower bacterial burden in blood and spleen | [50] |
| Male and female juvenile mice (C57BL/6) | I. p. cecal-slurry injection and measurement of blood samples at 6 h and 12 h; mice were sacrificed at 6 h or 12 h or observed for 72 h for survival study | Zinc group: Injection of 10 mg/kg BW zinc gluconate once a day for 3 days prior to induction of sepsis (corresponding to 1.4 mg/kg BW Zn2+ per day) Control group: Injection of equal volume of saline for 3 days prior to induction of sepsis | • Significantly improved survival of following sepsis at 72 h after induction • Significantly lower myeloperoxidase activity in lung tissue (at 12 h) • Significantly lower bacterial burden in peritoneal fluid (at 12 h) • Significantly lower serum concentrations of IL-2 (at 6 h, 12 h), IL-6 (at 6 h, 12 h), IL-1β (at 6 h), and keratinocyte-derived chemokines (at 12 h) | [83] |
| Female farm pigs (Deutsche Landrasse) | Intravenous infusion of LPS and measurement of the parameters for a duration of 300 min after infusion of LPS; pigs were sacrificed at 500 min of total registration time | Zinc group: Infusion of 25 mg/kg BW zinc-bis-(dl-hydrogenaspartate) 24 h prior to infusion of LPS (corresponding to 5 mg/kg BW Zn2+ per day) Control group: Infusion of saline 24 h prior to infusion of LPS | • Increased arterial and venous oxygen pressure (reaching significance at 45 min or 210 min) • Increased arterial and venous oxygen saturation (reaching significance at 210 min) • Stable intrapulmonary shunt (instead of an increase in the control group) • Stable extravascular lung water (EVLW) (instead of an increase in the control group) | [85] |
| Female farm pigs (Deutsche Landrasse) | Intravenous infusion of LPS and measurement of parameters for a duration of 60 min after infusion of LPS; pigs were sacrificed at 60 min and organs removed for analysis | Zinc group: Infusion of 25 mg/kg BW zinc-bis-(dl-hydrogenaspartate) 2 h prior to infusion of LPS (corresponding to 5 mg/kg BW Zn2+ per day) Control group: Infusion of saline 2 h prior to infusion of LPS | • Decrease in arterial and venous oxygen pressure (reaching significance at 30 min) • Decrease in arterial and venous oxygen saturation(reaching significance at 30 min or 15 min) • Increase in intrapulmonary shunt (reaching significance at 30 min) • Increase in EVLW (reaching significance at 45 min) • Increase in mean hemoglobin (reaching significance at 30 min) • Increase in IL-6 and TNF-α plasma concentrations (reaching significance at 0 min or 45 min) • Significant higher weights of lungs, width of alveolar septae and rate of paracentral liver necrosis | [87] |
| Female farm pigs (Deutsche Landrasse) | Intravenous infusion of LPS and measurement of parameters for a duration of 1020 min, with infusion of zinc from 600 to 720 min; pigs were sacrificed at the end of the study period and a necropsy carried out | Zinc group: Infusion of LPS at 0 h, 5 h and 12 h, infusion of 25 mg/kg BW zinc-bis-(dl-hydrogenaspartate)(corresponding to 5 mg/kg BW Zn2+ per day) at 10 h during sepsis Control group: Infusion of LPS at 0 h, 5 h and 12 h, infusion of saline at 10 h during sepsis | • Trend to higher arterial and venous oxygen pressure • Trend to higher arterial and venous oxygen saturation • No significant differences in intrapulmonary shunt • No significant differences in EVLW • Different courses in IL-6 and TNF-α plasma concentrations, at the end almost similar levels | [86] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. https://doi.org/10.3390/nu10080976
Alker W, Haase H. Zinc and Sepsis. Nutrients. 2018; 10(8):976. https://doi.org/10.3390/nu10080976
Chicago/Turabian StyleAlker, Wiebke, and Hajo Haase. 2018. "Zinc and Sepsis" Nutrients 10, no. 8: 976. https://doi.org/10.3390/nu10080976
APA StyleAlker, W., & Haase, H. (2018). Zinc and Sepsis. Nutrients, 10(8), 976. https://doi.org/10.3390/nu10080976






