Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Study and Participants
2.2. Habitual Food Intake Recording and Caffeine-Containing Foods
2.3. Coffee Preparation
2.4. Data Collection
2.5. Race Procedures
2.6. Statistical Analyses
3. Results
3.1. General Characteristics of Runners
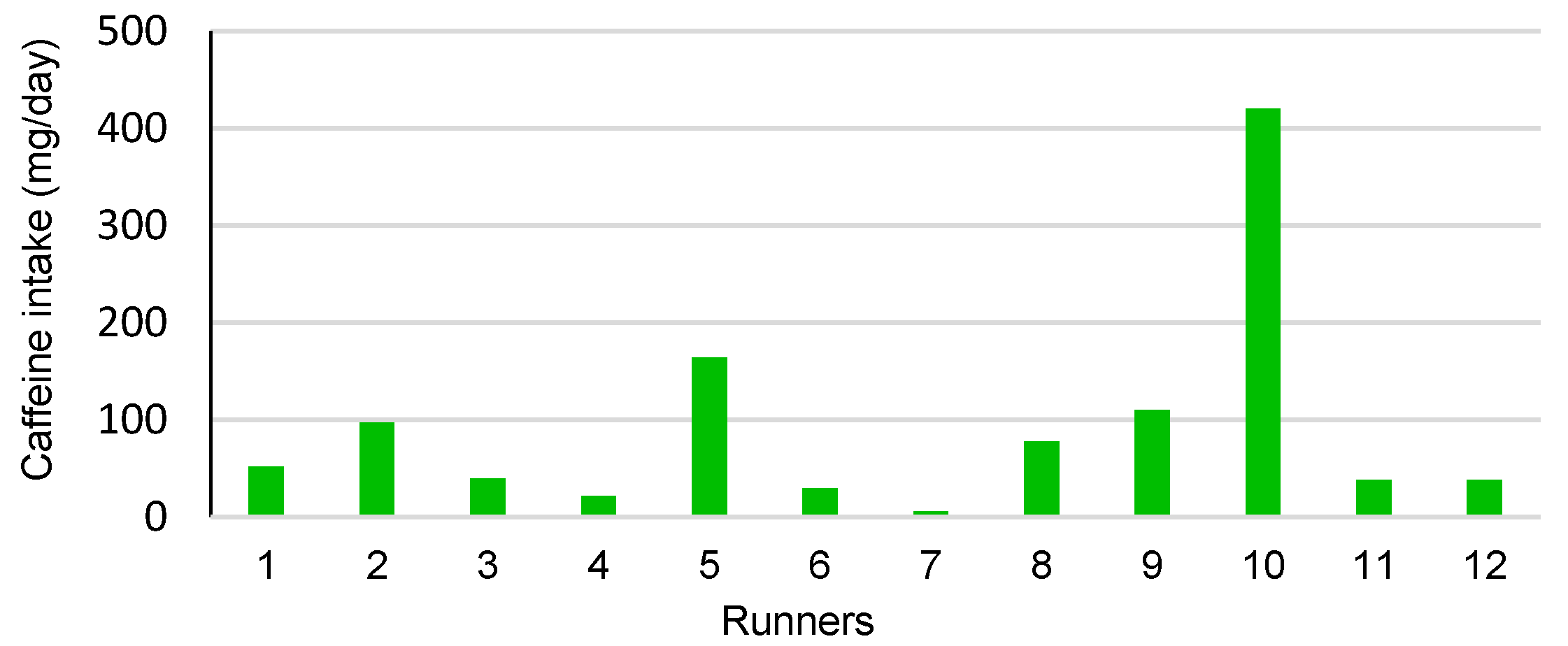
3.2. Habitual Food and Caffeine Consumption
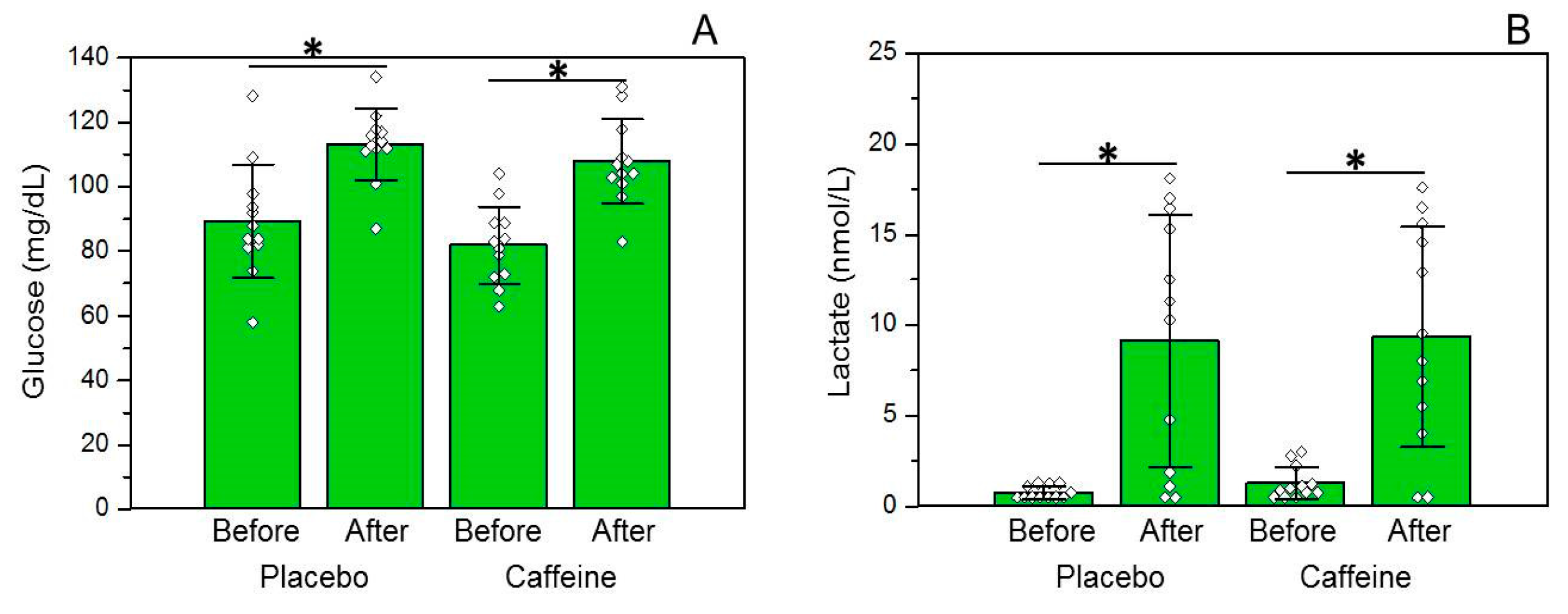
3.3. Glucose and Lactate Concentrations
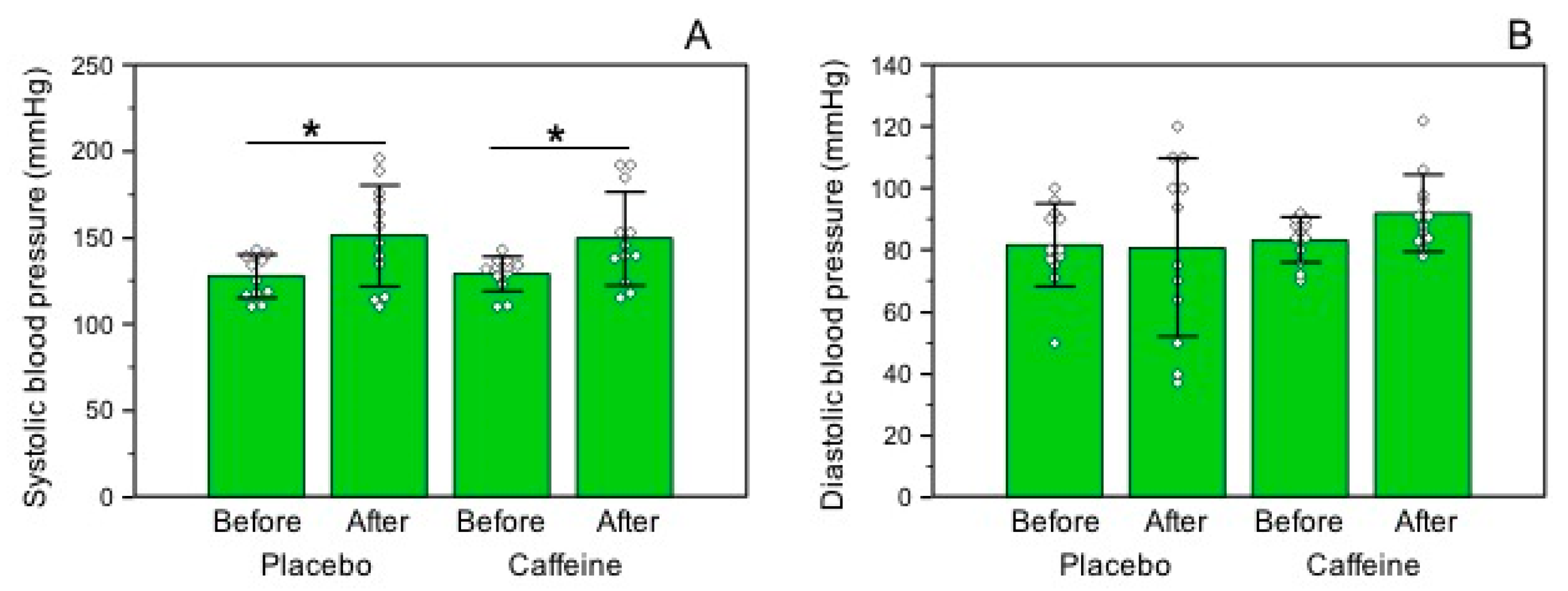
3.4. Blood Pressure Levels
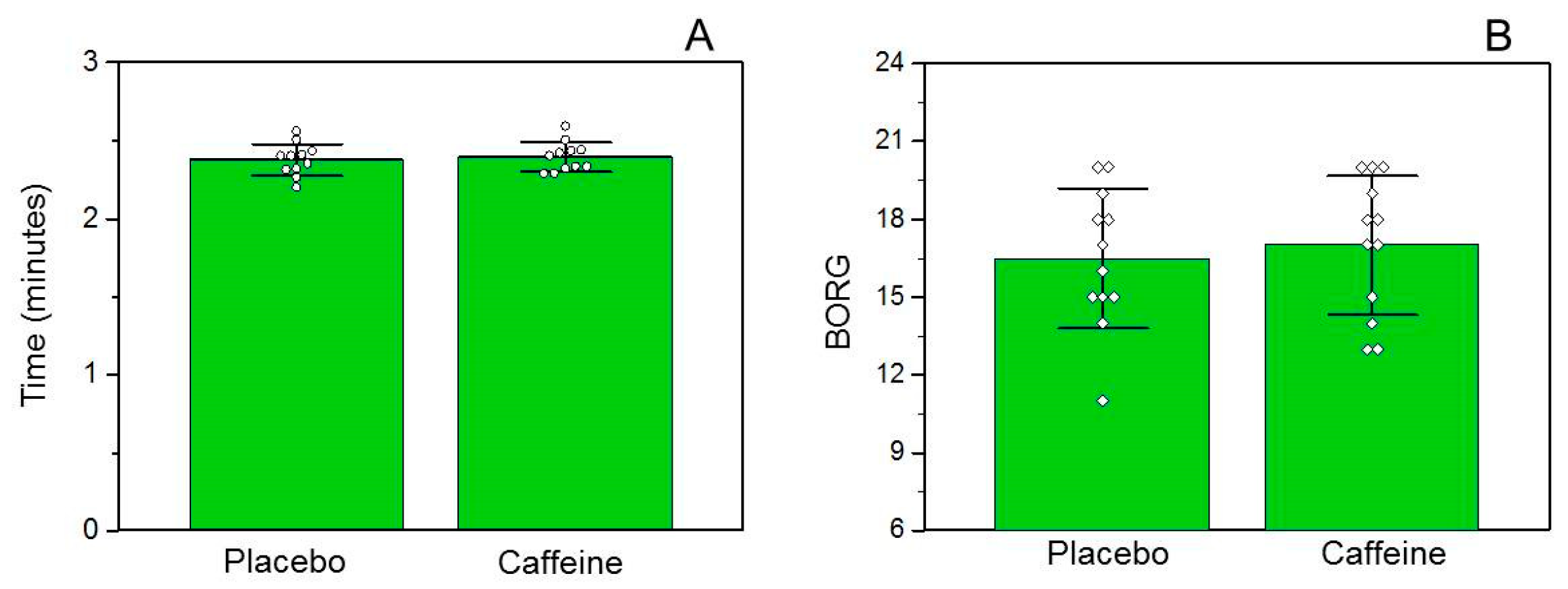
3.5. Performance and Ratings of Perceived Exertion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wickham, K.A.; Spriet, L.L. Administration of Caffeine in Alternate Forms. Sports Med. 2018, 48, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.R.; Desbrow, B.; Montgomery, P.G.; Anderson, M.E.; Bruce, C.R.; Macrides, T.A.; Martin, D.T.; Moquin, A.; Roberts, A.; Hawley, J.A.; et al. Effect of different protocols of caffeine intake on metabolism and endurance performance. J. Appl. Physiol. (1985) 2002, 93, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.D.; Richardson, D.L.; Thie, J.; Taylor, R. Coffee Ingestion Enhances One-Mile Running Race Performance. Int. J. Sports Physiol. Perform. 2017, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Hibbert, E.; Sathasivam, P. Metabolic and exercise endurance effects of coffee and caffeine ingestion. J. Appl. Physiol. (1985) 1998, 85, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.D.; Bird, S.R.; Hopkins, J.; Riley, M. Effect of caffeinated coffee on running speed, respiratory factors, blood lactate and perceived exertion during 1500-m treadmill running. Br. J. Sports Med. 1992, 26, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Camargo, M.C.R.; Toledo, M.C.F. Teor de cafeína em cafés brasileiros. Ciênc. Tecnol. Aliment. 1998, 18, 421–424. [Google Scholar] [CrossRef]
- Silva-Cavalcante, M.D.; Correia-Oliveira, C.R.; Santos, R.A.; Lopes-Silva, J.P.; Lima, H.M.; Bertuzzi, R.; Duarte, M.; Bishop, D.J.; Lima-Silva, A.E. Caffeine increases anaerobic work and restores cycling performance following a protocol designed to lower endogenous carbohydrate availability. PLoS ONE 2013, 8, e72025. [Google Scholar] [CrossRef] [PubMed]
- Brietzke, C.; Asano, R.Y.; De Russi de Lima, F.; Pinheiro, F.A.; Franco, A.; Ugrinowitsch, C.; Pires, F.O. Caffeine effects on VO2max test outcomes investigated by a placebo perceived-as-caffeine design. Nutr. Health 2017, 23, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.S.; Painelli, V.S.; Yamaguchi, G.; Oliveira, L.F.; Saunders, B.; da Silva, R.P.; Maciel, E.; Artioli, G.G.; Roschel, H.; Gualano, B. Dispelling the myth that habitual caffeine consumption influences the performance response to acute caffeine supplementation. J. Appl. Physiol. (1985) 2017, 123, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Kiely, J. Are the Current Guidelines on Caffeine Use in Sport Optimal for Everyone? Inter-individual Variation in Caffeine Ergogenicity, and a Move Towards Personalised Sports Nutrition. Sports Med. 2018, 48, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Exercise and sport performance with low doses of caffeine. Sports Med. 2014, 44 (Suppl. 2), 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gonglach, A.R.; Ade, C.J.; Bemben, M.G.; Larson, R.D.; Black, C.D. Muscle Pain as a Regulator of Cycling Intensity: Effect of Caffeine Ingestion. Med. Sci. Sports Exerc. 2016, 48, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M. The effects of caffeine on the maximal accumulated oxygen deficit and short-term running performance. Int. J. Sport Nutr. 1998, 8, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Landrum, R.E. College Students’ Use of Caffeine and Its Relationship to Personality. Coll. Stud. J. 1992, 26, 151–155. [Google Scholar]
- Doherty, M.; Smith, P.M. Effects of caffeine ingestion on exercise testing: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 626–646. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.B.; Randell, R.K.; Jeukendrup, A.E. The metabolic and performance effects of caffeine compared to coffee during endurance exercise. PLoS ONE 2013, 8, e59561. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [PubMed]
- USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 15 February 2018).
- Crozier, T.W.; Stalmach, A.; Lean, M.E.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Coggan, A.R.; Sidossis, L.S.; Gastaldelli, A.; Wolfe, R.R. Effect of theophylline on substrate metabolism during exercise. Metab. Clin. Exp. 1996, 45, 1153–1160. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Saris, W.H.; Brouns, F.; Kester, A.D. A new validated endurance performance test. Med. Sci. Sports Exerc. 1996, 28, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.; Corey, P.; Vescovi, J.; El-Sohemy, A. Caffeine, CYP1A2 Genotype, and Endurance Performance in Athletes. Med. Sci. Sports Exerc. 2018. [Google Scholar] [CrossRef] [PubMed]
| Variables | Mean ± SD |
|---|---|
| Age (years) | 23.50 ± 3.94 |
| Exercise frequency (times/week) | 4.92 ± 1.62 |
| Distance of race (km) | 2.83 ± 2.25 |
| Body weight (kg) | 70.38 ± 8.41 |
| Body mass index (kg/m2) | 21.83 ± 3.43 |
| Waist circumference (cm) | 75.50 ± 3.99 |
| Thigh circumference (cm) | 52.46 ± 4.71 |
| Calf circumference (cm) | 34.75 ± 3.14 |
| Triceps skinfold (mm) | 17.17 ± 6.00 |
| Subscapular skinfold (mm) | 18.17 ± 4.93 |
| Suprailiac skinfold (mm) | 17.75 ± 7.07 |
| Thigh skinfold (mm) | 23.58 ± 6.91 |
| Ambient temperature–decaffeinated group (°C) | 21.33 ± 0.49 |
| Ambient temperature–caffeinated group (°C) | 21.42 ± 0.51 |
| Ambient relative humidity–decaffeinated group (%) | 89.00 ± 3.36 |
| Ambient relative humidity–caffeinated group (%) | 87.67 ± 4.16 |
| Nutrients | Mean ± SD |
|---|---|
| Calories (kcal) | 2340.56 ± 784.20 |
| Carbohydrates (%) | 45.81 ± 12.13 |
| Carbohydrates (g/kg of b.w.) | 4.02 ± 2.18 |
| Protein (%) | 23.51 ± 6.23 |
| Protein (g/kg of b.w.) | 1.92 ± 0.81 |
| Lipids (%) | 30.61 ± 9.26 |
| Lipids (g/kg of b.w.) | 1.09 ± 0.37 |
| Foods | Daily Frequency Mean ± SD | Daily Quantity Mean (Min–Max) | Caffeine Mean (Min–Max) (mg/d) |
|---|---|---|---|
| Caffeinated coffee (mL) | 0.83 (0–3) | 137.50 (0–600) | 35.75 (0–156) |
| Chocolate (g) | 0.33 ± 0.49 | 32.75 ± 56.42 | 6.55 (0–30) |
| Ready-to-drink chocolate (mL) | 0.25 ± 0.45 | 66.66 ± 130.26 | 2.00 (0–12) |
| Cola soft drink (mL) | 0.41 ± 0.90 | 150.00 ± 306.00 | 12.00 (0–80) |
| Caffeine powder (mg) | 0.16 ± 0.38 | 35.00 ± 121.24 | 35.00 (0–420) |
| Total caffeine (mg/d) | - | 91.30 (6–420) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.C.; Jesus, A.A.; Giglio, B.M.; Marini, A.C.; Lobo, P.C.B.; Mota, J.F.; Pimentel, G.D. Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients 2018, 10, 657. https://doi.org/10.3390/nu10060657
Marques AC, Jesus AA, Giglio BM, Marini AC, Lobo PCB, Mota JF, Pimentel GD. Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients. 2018; 10(6):657. https://doi.org/10.3390/nu10060657
Chicago/Turabian StyleMarques, Alexandre C., Alison A. Jesus, Bruna M. Giglio, Ana C. Marini, Patrícia C. B. Lobo, João F. Mota, and Gustavo D. Pimentel. 2018. "Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study" Nutrients 10, no. 6: 657. https://doi.org/10.3390/nu10060657
APA StyleMarques, A. C., Jesus, A. A., Giglio, B. M., Marini, A. C., Lobo, P. C. B., Mota, J. F., & Pimentel, G. D. (2018). Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients, 10(6), 657. https://doi.org/10.3390/nu10060657





