Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis
Abstract
:1. Introduction
2. Materials and Methods
2.1. OM Induction Protocol
2.2. Glycine Supplementation
2.3. Picrosirius Staining for Collagen
2.4. Quantitative Collagen Analysis
2.5. Immunohistochemical Technique
2.6. QuantitativeAnalysis of EGF and PDGF
3. Results
3.1. Quantitative and Qualitative Analysisof Collagen Expression
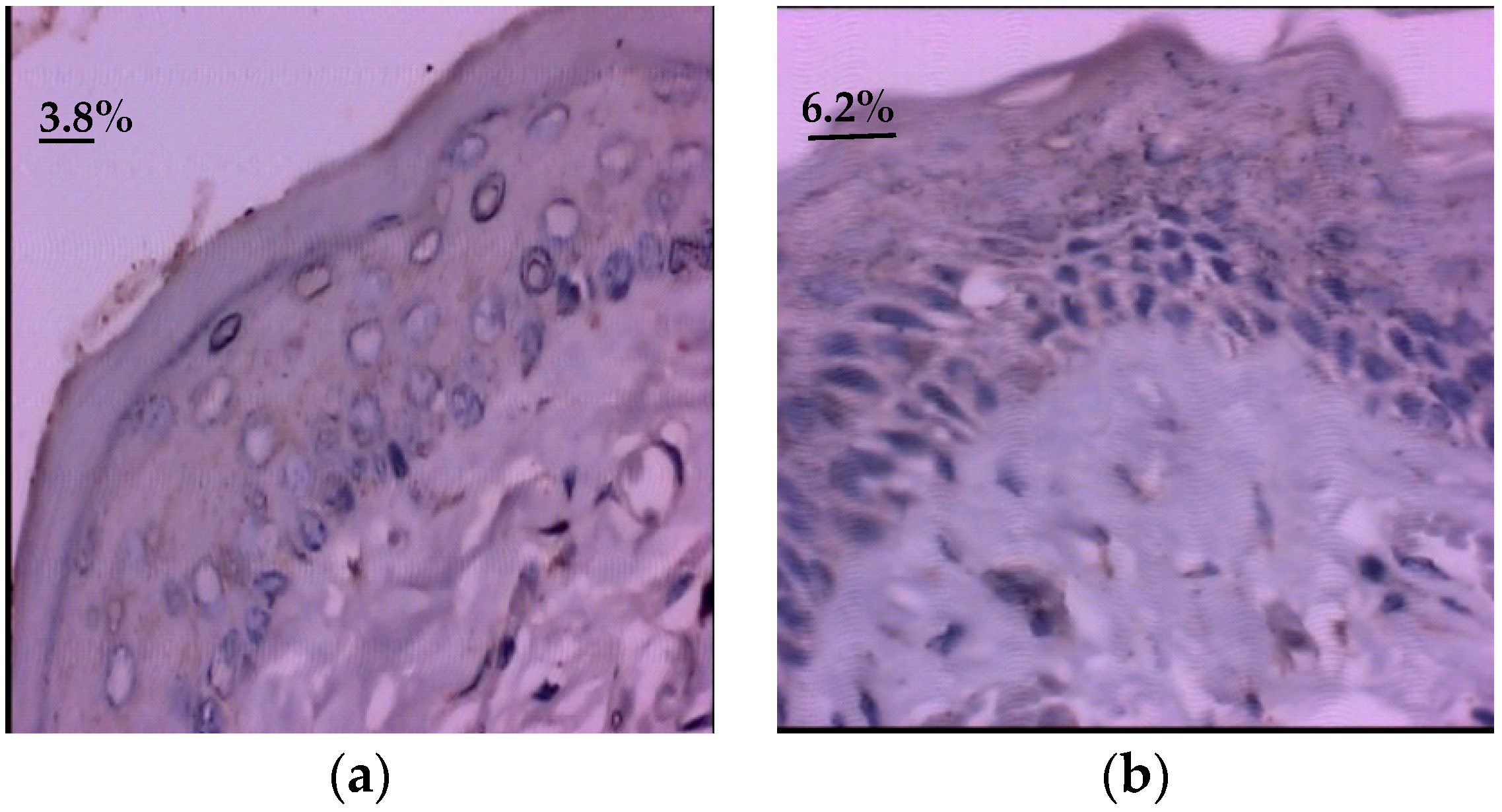
3.2. Quantitative Evaluation of Epidermal Growth Factor (EGF)
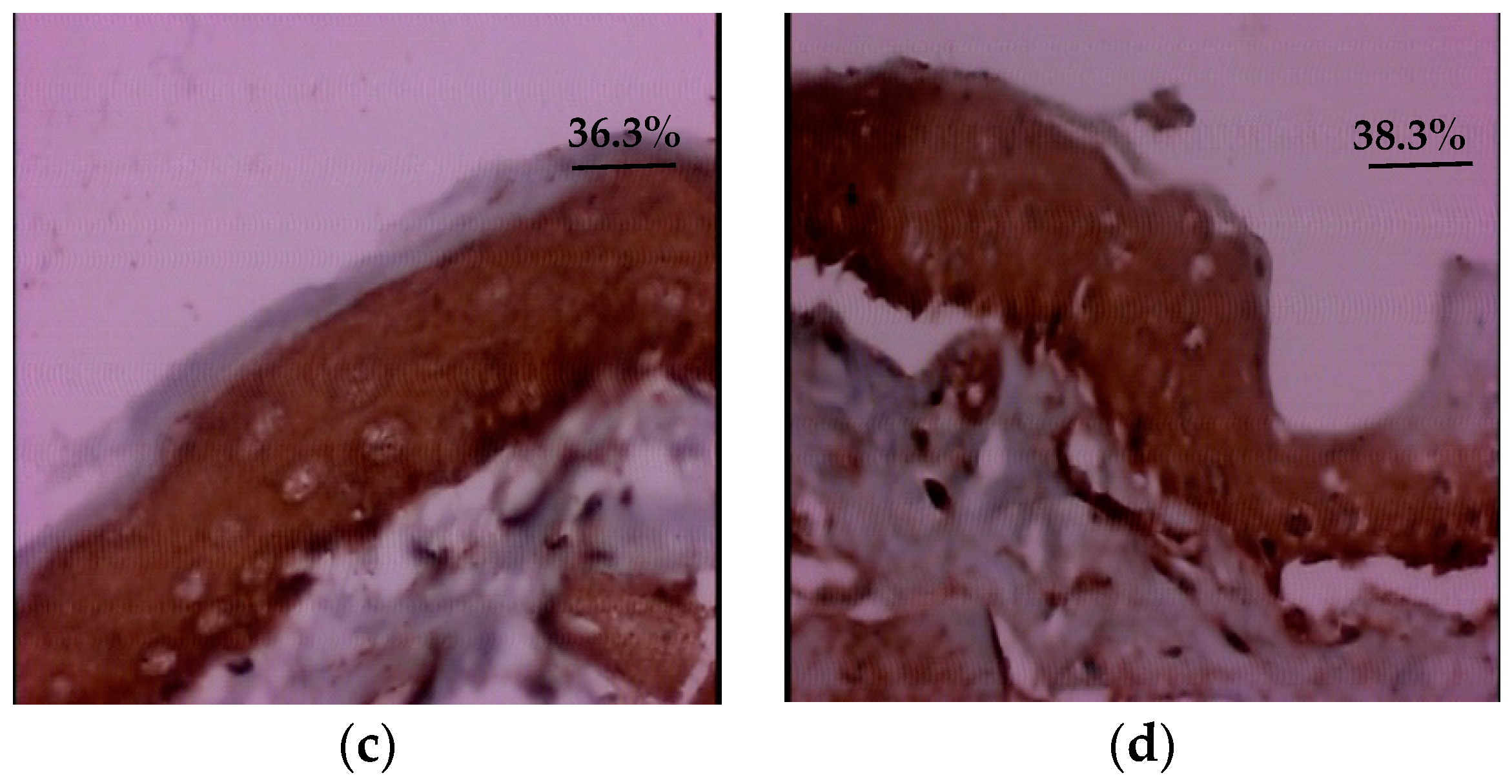
3.3. Quantitative Evaluation of Platelet Derived Growth Factor (PDGF)
3.4. Correlation between Collagen Fiber Types and Amount of Collagen and Immunoexpression Growth Factors: EGF and PDGF
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Antin, J.H.; Tedaldi, M.W.; Alterovitz, G. SNP-based Bayesian networks can predict oral mucositis risk in autologous stem cell transplant recipients. Oral Dis. 2013, 19, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Martini, S.; Muzio, J.; Cavallin, C.; Arcadipane, F.; Rampino, M.; Ostellino, O.; Pecorari, G.; Garzino Demo, P.; Fasolis, M.; et al. Prospective assessment of oral mucositis and its impacto n quality of life and patient-reported outcomes durin g radiotherapy for head and neck cancer. Med. Oncol. 2017, 34, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J. Cancer treatment-induced mucositis pain: Strategies for assessment and management. Ther. Clin. Risk Manag. 2006, 2, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Maruccii, L.; Farneti, A.; Di Ridolfi, P.; Pinnaro, P.; Pellini, R.; Giannarelli, D.; Vici, P.; Conte, M.; Landoni, V.; Sanguineti, G. Double-Blind randomized phase III study comparing a mixture of natural agents versus placebo in the prevention of acute mucositis during chemoradiotherapy for head and neck cancer. Head Neck 2017, 39, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.; Balouch, A.; Abdul, M.M.; Sedghizadeh, P.P.; Enciso, R. Efficacy of chlorhexidine for the prevention and tratment of oral mucositis in câncer patients: A systematic review with meta-analyses. J. Oral Pathol. Med. 2017, 46, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Sa, O.S.; Lopes, N.; Alves, M.; Lalla, R.; Oliva, M.; Caran, E.M.M. Glycine supplementation reduces the severity of chemotherapy-induced oral mucositis in hamsters. Nat. Sci. 2013, 5, 972–978. [Google Scholar] [CrossRef]
- Sonis, S.T.; Tracey, C.; Shlar, G.; Jenson, J.; Florine, D.; Almeida, C. An animal model for mucositis induced by cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 437–443. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Montes, G.S.; Sanchez, E.M. The influence of tissue thickness on the study of collagen by the picrosirius-polarization method. Histochemistry 1982, 74 (Suppl. 1), 153–156. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Bossi, P.; Sanguineti, G.; Trippa, F.; Ferrari, D.; Bacigalupo, A.; Ripamonti, C.; Buglione, M.; Pergolizzi, S.; Langendjik, J.A.; et al. Mucositis in head and neck câncer patients treated with radiotherapy and systemic therapies: Literature review and consensus statements. Crit. Rev. Oncol. Hematol. 2016, 100, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.; Lindquist, I.; Van Vugth, A.; Stewart, A.A.; Stam, K.; Qu, K.Y.; Iwata, K.K.; Haley, J.D. Prevention of chemotherapy-induced ulcerative mucositis by transforming growth factor beta. Cancer Res. 1994, 54, 1135–1138. [Google Scholar] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hind Hamzeh-Cognasse, H.; Cognasse, F.; Palle, S.; Chavarin, P.; Olivier, T.; Delezay, O.; Pozzetto, B.; Garraud, O. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, Z.L.; Dai, Z.L.; Yang, Y.; Wang, W.W.; Liu, C.; Wang, B.; Wang, J.J.; Yin, Y.L. Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Enock, S.; Leaper, D.J. Basic Science of wound Healing. Surgery 2008, 26, 31–37. [Google Scholar]
- Wu, Z.L.; Hou, Y.Q.; Hu, S.D.; Bazer, F.W.; Meininger, C.J.; McNeal, C.J.; Wu, G. Catabolism and safety of supplemental l-arginine in animals. Amino Acids 2016, 48, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.L.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Corzo, A.; Fritts, C.A.; Kidd, M.T.; Kerr, B.J. Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim. Feed Sci. Technol. 2005, 118, 319–327. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, W.; Tian, L.; Niu, J.; Liu, Y. Effect of N-acetyl cysteine and glycine supplementation on growth performance, glutathione synthesis, anti-oxidative and immune ability of Nile tilapia, Oreochromisniloticus. Fish Shellfish Immunol. 2016, 55, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Asami, N.; Kataoka, A.; Sugihara, F.; Inoue, N.; Kimira, Y.; Wada, M.; Mano, H. Oral collagen-derived dipeptides, prolylhydroxyproline and hydroxyprolyl-glycine, ameliorate skin barrier dysfunction and alter gene expression profiles in the skin. Biochem. Biophys. Res. Commun. 2015, 456, 626–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Q.; Yao, K.; Yin, Y.L.; Wu, G. Endogenous synthesis of amino acids limits growth, lactation and reproduction of animals. Adv. Nutr. 2016, 7, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Theoret, C. Physiology of wound healing. In Equine Wound Management, 3rd ed.; Theoret, C., Schumacher, J., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2017; pp. 1–13. [Google Scholar]
- Bergin, D.A.; Greene, C.M.; Sterchi, E.E.; Kenna, C.; Geraghty, P.; Belaaouaj, A.; Taggart, C.C.; O’Neill, S.J.; McElvaney, N.G. Activation of the Epidermal Growth Factor Receptor (EGFR) by a Novel Metalloprotease Pathway. J. Biol. Chem. 2008, 283, 31736–31744. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Muthukumar, T.; TirichurapalliSivagnanam, U. In vivoefficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur. J. Pharmacol. 2017, 814, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Anbarasu, K.; Prakash, D.; Sastry, T.P. Effect of growth factors and pro-inflammatory cytokines by the collagen biocomposite dressing material containing Macrotylomauniflorum plant extract-In vivo wound healing. Colloids Surf. B Biointerfaces 2014, 121, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugrillon, A.; Klüter, H. Current Use of Platelet Concentrates for Topical Application in Tissue Repair. Transfus. Med. Hemother. 2017, 29, 67–70. [Google Scholar] [CrossRef]
- Epstein, J.B.; Emerton, S.; Guglietta, A.; Le, N. Assessment of epidermal growth factor in oral secretions of patients receiving radiation therapy for cancer. Oral Oncol. 1997, 33, 359–363. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Sotiriou, C.; Cockman, M.E.; Ratcliffe, P.J.; Maxwell, P.; Liu, E.; Harris, A.L. Gene array of VHL mutation and hypoxia shows novel hypoxia-induced genes and that cyclin D1 is a VHL target gene. Br. J. Cancer 2004, 90, 1235–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Kang, Y. Epidermal growth factor signaling and bone metastasis. Br. J. Cancer 2010, 102, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Konkinalla, V.; Efferth, T. Inhibition of epidermal gowth factor receptor-overexpressing cancer cells by camptothecin, 20-(N,N-diethyl) glycinate. Biochem. Pharmacol. 2010, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifatorious Beneficial effect of nonessential amino acid, glycine: A review. Oxid. Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.N.F.; Plapler, H.; Lalla, R.H.V.; Chavantes, M.C.; Yoshimura, E.M.; Silva, M.A.; Alves, M.T. Effects of Low-Level Laser Therapy on Collagen Expression and Neutrophil Infiltrate in 5-Fluorouracil-Induced Oral Mucositis in Hamsters. Lasers Surg. Med. 2010, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, T.; Kraus, D.; Winter, J.; Wolf, M.; Deschner, J.; Jäger, A. Potential Immune Modularly Role of Glycine in Oral Gingival Inflammation. Clin. Dev. Immunol. 2013, 2013, 808367. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
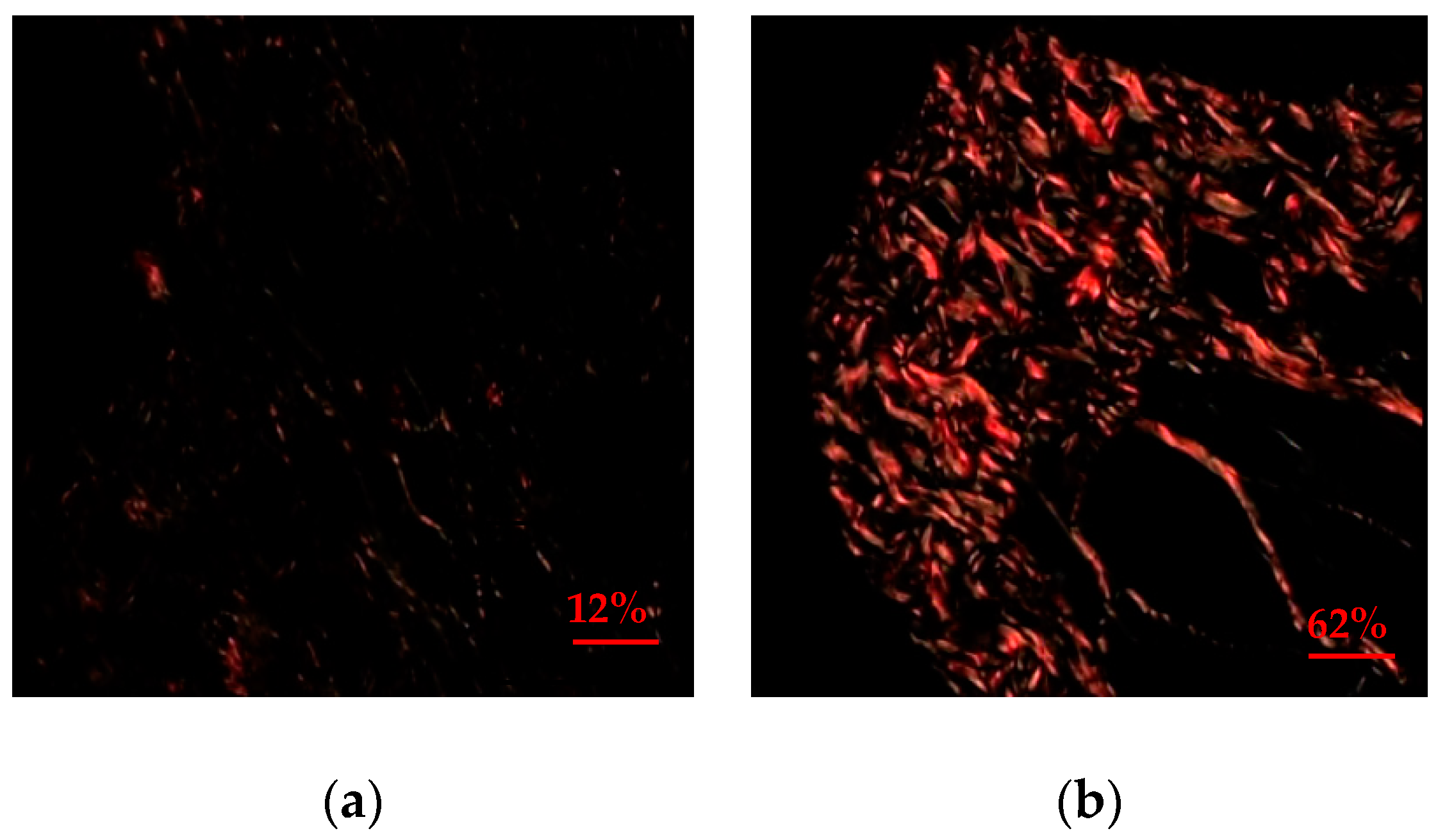



| Group | Quantitative Evaluation of Collagen Expression (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Medium | SD | IC 95% | Minimum | Maximum | Valor p | |
| Group I (Control) | 25.0 | 25.3 | 3.8 | 23.2–26.8 | 20.4 | 30.4 | 0.0002 * |
| Group II (Glycine) | 61.6 | 63.2 | 9.4 | 57.2–66.0 | 36.2 | 73.2 | |
| Group | Qualitative Evaluation of Collagen Expression | ||
|---|---|---|---|
| Type I N (%) | Type III N (%) | Valor p | |
| Group I (Control) | 1 (5) | 19 (95) | 0.0001 * |
| Group II (Glycine) | 19 (95) | 1 (5) | |
| Group | Quantitative Evaluation of EGF Immunoexpression (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Medium | SD | IC 95% | Minimum | Maximum | Valor p | |
| Group I (Control) | 40.3 | 40.2 | 11.4 | 34.9–45.7 | 9.4 | 65.4 | 0.0001 * |
| Group II (Glycine) | 7.3 | 6.5 | 7.2 | 3.9–10.7 | 1.9 | 35.5 | |
| Group | Quantitative Evaluation of PDGF Immunoexpression (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Medium | SD | IC 95% | Minimum | Maximum | Valor p | |
| Group I (Control) | 31.3 | 35.1 | 16.2 | 23.7–38.9 | 1.3 | 49.6 | 0.0001 * |
| Group II (Glycine) | 10.8 | 1.5 | 6.4 | 1.3–11.5 | 1.1 | 40.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, O.M.d.S.; Lopes, N.N.F.; Alves, M.T.S.; Caran, E.M.M. Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis. Nutrients 2018, 10, 1485. https://doi.org/10.3390/nu10101485
Sá OMdS, Lopes NNF, Alves MTS, Caran EMM. Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis. Nutrients. 2018; 10(10):1485. https://doi.org/10.3390/nu10101485
Chicago/Turabian StyleSá, Odara Maria de Sousa, Nilza Nelly Fontana Lopes, Maria Teresa Seixas Alves, and Eliana Maria Monteiro Caran. 2018. "Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis" Nutrients 10, no. 10: 1485. https://doi.org/10.3390/nu10101485
APA StyleSá, O. M. d. S., Lopes, N. N. F., Alves, M. T. S., & Caran, E. M. M. (2018). Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis. Nutrients, 10(10), 1485. https://doi.org/10.3390/nu10101485





