Assessment of the Capability of Landsat and BiodivMapR to Track the Change of Alpha Diversity in Dryland Disturbed by Mining
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data
2.2.1. Remote Sensing
2.2.2. Field Surveys
2.3. Methods
2.3.1. Estimation of Alpha Diversity
2.3.2. Accuracy Assessment
2.3.3. Change Detection
3. Results
3.1. The Effect of Parameters on Accuracy
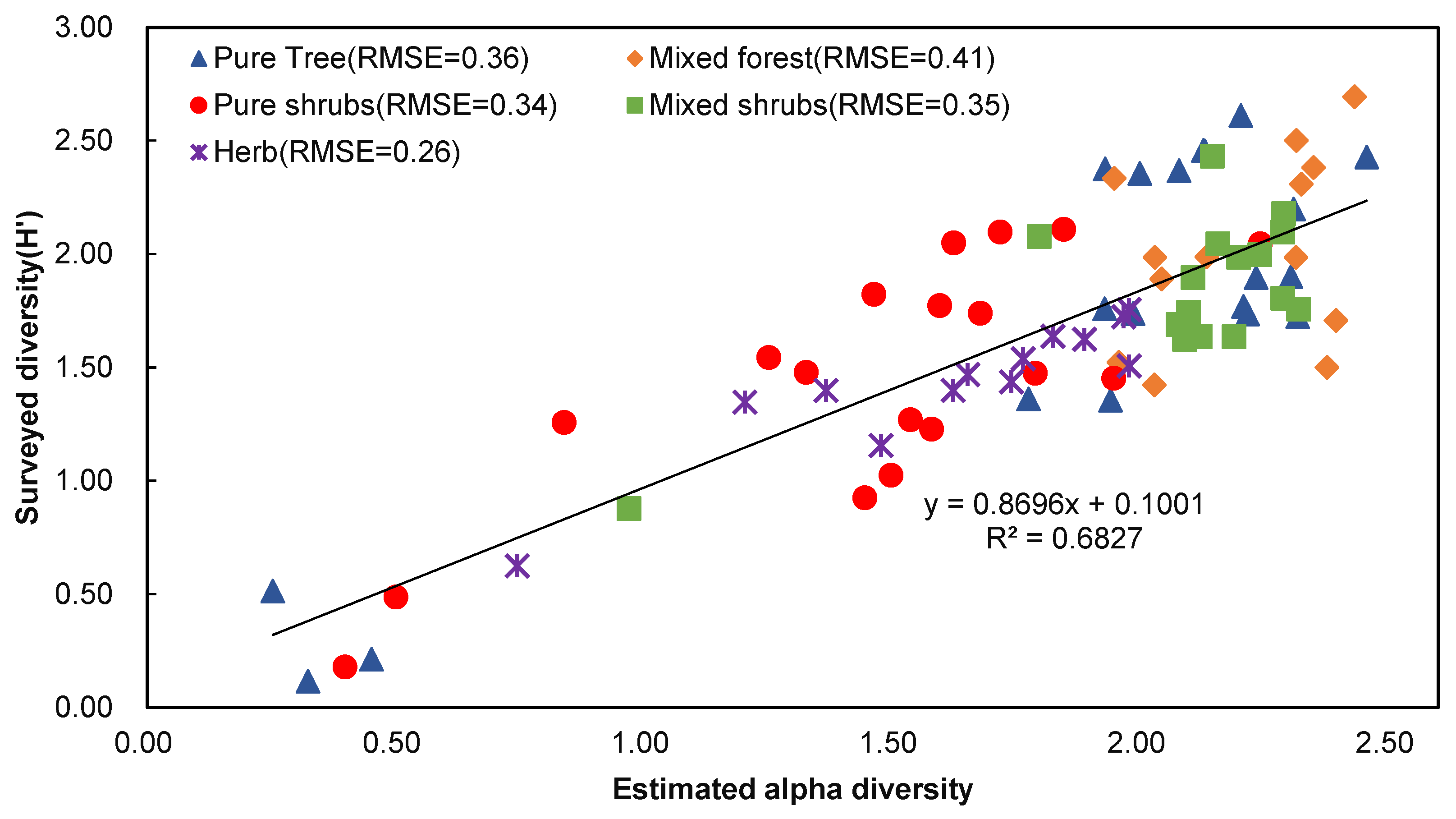
3.2. The Effect of Plant Community Types on Accuracy
3.3. Spatial and Temporal Dynamics of Alpha Diversity
3.4. Hot and Cold Spots of Diversity Change in the Study Area
3.5. Changes in Alpha Diversity of Different Plant Communities
4. Discussion
4.1. Implications for Plant Alpha Diversity Monitoring
4.2. Research Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, J.F.; Stafford Smith, D.M.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.J.; Downing, T.E.; Dowlatabadi, H.; Fernandez, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Bestelmeyer, B.T.; Okin, G.S.; Duniway, M.C.; Archer, S.R.; Sayre, N.F.; Williamson, J.C.; Herrick, J.E. Desertification, land use, and the transformation of global drylands. Front. Ecol. Environ. 2015, 13, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xia, J.Y.; Wang, Y.P.; Ahlstrom, A.; Chen, J.Q.; Cook, R.B.; Cui, E.Q.; Fang, Y.Y.; Fisher, J.B.; Huntzinger, D.N.; et al. Enhanced peak growth of global vegetation and its key mechanisms. Nat. Ecol. Evol. 2018, 2, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Erskine, P.D.; Zhang, S.L.; Wang, Y.J.; Bian, Z.F.; Lei, S.G. Effects of underground mining on vegetation and environmental patterns in a semi-arid watershed with implications for resilience management. Environ. Earth Sci. 2018, 77, 605. [Google Scholar] [CrossRef]
- Shen, Z.X.; Zhang, Q.; Piao, S.L.; Penuelas, J.; Stenseth, N.C.; Chen, D.L.; Xu, C.Y.; Singh, V.P.; Liu, T.X. Mining Can Exacerbate Global Degradation of Dryland. Geophys. Res. Lett. 2021, 48, e2021GL094490. [Google Scholar] [CrossRef]
- Gudka, M.; Davies, J.; Poulsen, L.; Schulte-Herbrüggen, B.; MacKinnon, K.; Crawhall, N.; Henwood, W.D.; Dudley, N.; Smith, J. Conserving dryland biodiversity: A future vision of sustainable dryland development. Biodiversity 2014, 15, 143–147. [Google Scholar] [CrossRef]
- Palmer, M.W.; Earls, P.G.; Hoagland, B.W.; White, P.S.; Wohlgemuth, T. Quantitative tools for perfecting species lists. Environmetrics 2002, 13, 121–137. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Colwell, R.K. III.1 Biodiversity: Concepts, Patterns, and Measurement; Princeton University Press: Princeton, NJ, USA, 2009. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M.; et al. Remotely sensed spectral heterogeneity as a proxy of species diversity: Recent advances and open challenges. Ecol. Inform. 2010, 5, 318–329. [Google Scholar] [CrossRef]
- Schmidtlein, S.; Fassnacht, F.E. The spectral variability hypothesis does not hold across landscapes. Remote Sens. Environ. 2017, 192, 114–125. [Google Scholar] [CrossRef]
- Rocchini, D.; Bacaro, G.; Chirici, G.; Da Re, D.; Feilhauer, H.; Foody, G.M.; Galluzzi, M.; Garzon-Lopez, C.X.; Gillespie, T.W.; He, K.S.; et al. Remotely sensed spatial heterogeneity as an exploratory tool for taxonomic and functional diversity study. Ecol. Indic. 2018, 85, 983–990. [Google Scholar] [CrossRef]
- Féret, J.-B.; de Boissieu, F. biodivMapR: An r package for α- and β-diversity mapping using remotely sensed images. Methods Ecol. Evol. 2020, 11, 64–70. [Google Scholar] [CrossRef]
- Gastauer, M.; Nascimento, W.R.; Caldeira, C.F.; Ramos, S.J.; Souza-Filho, P.W.M.; Féret, J.-B. Spectral diversity allows remote detection of the rehabilitation status in an Amazonian iron mining complex. Int. J. Appl. Earth Obs. Geoinf. 2022, 106, 102653. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Wan, H.-B.; Zhou, F.; Gu, P.-Y.; Xu, Q.-F.; Lu, J.-M. AIEgens-lightened Functional Polymers: Synthesis, Properties and Applications. Chin. J. Polym. Sci. 2019, 37, 302–326. [Google Scholar] [CrossRef]
- Sagang, L.T.; Ploton, P.; Viennois, G.; Feret, J.B.; Sonke, B.; Couteron, P.; Barbier, N. Monitoring vegetation dynamics with open earth observation tools: The case of fire-modulated savanna to forest transitions in Central Africa. ISPRS J. Photogramm. Remote Sens. 2022, 188, 142–156. [Google Scholar] [CrossRef]
- Liccari, F.; Sigura, M.; Bacaro, G. Use of Remote Sensing Techniques to Estimate Plant Diversity within Ecological Networks: A Worked Example. Remote. Sens. 2022, 14, 4933. [Google Scholar] [CrossRef]
- Rossi, C.; Kneubühler, M.; Schütz, M.; Schaepman, M.E.; Haller, R.M.; Risch, A.C. Remote sensing of spectral diversity: A new methodological approach to account for spatio-temporal dissimilarities between plant communities. Ecol. Indic. 2021, 130, 108106. [Google Scholar] [CrossRef]
- Rocchini, D.; Torresani, M.; Beierkuhnlein, C.; Feoli, E.; Foody, G.M.; Lenoir, J.; Malavasi, M.; Moudrý, V.; Šímová, P.; Ricotta, C. Double down on remote sensing for biodiversity estimation: A biological mindset. Community Ecol. 2022, 23, 267–276. [Google Scholar] [CrossRef]
- Kacic, P.; Kuenzer, C. Forest Biodiversity Monitoring Based on Remotely Sensed Spectral Diversity—A Review. Remote Sens. 2022, 14, 5363. [Google Scholar] [CrossRef]
- Viña, A.; Tuanmu, M.N.; Xu, W.; Li, Y.; Qi, J.; Ouyang, Z.; Liu, J. Relationship between floristic similarity and vegetated land surface phenology: Implications for the synoptic monitoring of species diversity at broad geographic regions. Remote Sens. Environ. 2012, 121, 488–496. [Google Scholar] [CrossRef]
- Khare, S.; Latifi, H.; Ghosh, S.K. Multi-scale assessment of invasive plant species diversity using Pléiades 1A, RapidEye and Landsat-8 data. Geocarto Int. 2018, 33, 681–698. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Da Re, D.; Chirici, G.; Galluzzi, M.; Lenoir, J.; Ricotta, C.; Torresani, M.; Ziv, G. Time-lapsing biodiversity: An open source method for measuring diversity changes by remote sensing. Remote Sens. Environ. 2019, 231, 111192. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Andréfouët, S.; Cohen, W.B.; Gómez, C.; Griffiths, P.; Hais, M.; Healey, S.P.; Helmer, E.H.; Hostert, P.; Lyons, M.B.; et al. Bringing an ecological view of change to Landsat-based remote sensing. Front. Ecol. Environ. 2014, 12, 339–346. [Google Scholar] [CrossRef]
- Lechner, A.M.; Foody, G.M.; Boyd, D.S. Applications in Remote Sensing to Forest Ecology and Management. One Earth 2020, 2, 405–412. [Google Scholar] [CrossRef]
- Masek, J.G.; Vermote, E.F.; Saleous, N.E.; Wolfe, R.E.; Hall, F.G.; Huemmrich, K.F.; Gao, F.; Kutler, J.; Lim, T.-K. A Landsat surface reflectance dataset for North America, 1990–2000. IEEE Geosci. Remote Sens. Lett. 2006, 3, 68–72. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. MSOR Connect 2014, 1, 201. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. NASA Spec. Publ. 1973, 351, 309. [Google Scholar]
- Ali, I.; Rehman, A.U.; Khan, D.M.; Khan, Z.; Shafiq, M.; Choi, J.-G. Model Selection Using K-Means Clustering Algorithm for the Symmetrical Segmentation of Remote Sensing Datasets. Symmetry 2022, 14, 1149. [Google Scholar] [CrossRef]
- Yu, P.; Zhou, T.; Luo, H.; Liu, X.; Shi, P.; Zhang, J.; Zhang, Y.; Zhou, P.; Xu, Y. Global Pattern of Ecosystem Respiration Tendencies and Its Implications on Terrestrial Carbon Sink Potential. Earth’s Future 2022, 10, e2022EF002703. [Google Scholar] [CrossRef]
- Fairbanks, D.H.K.; McGwire, K.C. Patterns of floristic richness in vegetation communities of California: Regional scale analysis with multi-temporal NDVI. Glob. Ecol. Biogeogr. 2004, 13, 221–235. [Google Scholar] [CrossRef]
- Gould, W. Remote Sensing of Vegetation, Plant Species Richness, and Regional Biodiversity Hotspots. Ecol. Appl. 2000, 10, 1861–1870. [Google Scholar] [CrossRef]
- He, K.S.; Zhang, J.; Zhang, Q. Linking variability in species composition and MODIS NDVI based on beta diversity measurements. Acta Oecologica 2009, 35, 14–21. [Google Scholar] [CrossRef]
- Oindo, B.O.; Skidmore, A.K. Interannual variability of NDVI and species richness in Kenya. Int. J. Remote Sens. 2002, 23, 285–298. [Google Scholar] [CrossRef]
- Parviainen, M.; Luoto, M.; Heikkinen, R.K. NDVI-based productivity and heterogeneity as indicators of plant-species richness in boreal landscapes. Boreal Environ. Res. 2010, 15, 301–318. [Google Scholar]
- Mpakairi, K.S.; Dube, T.; Dondofema, F.; Dalu, T. Spatio–temporal variation of vegetation heterogeneity in groundwater dependent ecosystems within arid environments. Ecol. Inform. 2022, 69, 101667. [Google Scholar] [CrossRef]
- Ebrahimi-Khusfi, Z.; Mirakbari, M.; Khosroshahi, M. Vegetation response to changes in temperature, rainfall, and dust in arid environments. Environ. Monit. Assess. 2020, 192, 691. [Google Scholar] [CrossRef]
- Tao, S.; Fang, J.; Zhao, X.; Zhao, S.; Shen, H.; Hu, H.; Tang, Z.; Wang, Z.; Guo, Q. Rapid loss of lakes on the Mongolian Plateau. Proc. Natl. Acad. Sci. USA 2015, 112, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, M.D. China’s Coal Production Goes West: Assessing Recent Geographical Restructuring and Industrial Transformation. Prof. Geogr. 2015, 67, 630–640. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Tian, X. Vegetation change detection research of Dunhuang city based on GF-1 data. Int. J. Coal Sci. Technol. 2018, 5, 105–111. [Google Scholar] [CrossRef]
- Gastauer, M.; Silva, J.R.; Caldeira Junior, C.F.; Ramos, S.J.; Souza Filho, P.W.M.; Furtini Neto, A.E.; Siqueira, J.O. Mine land rehabilitation: Modern ecological approaches for more sustainable mining. J. Clean. Prod. 2018, 172, 1409–1422. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, J.; Zhang, Y.; Zhang, S.; Zhou, Y.; Hou, H.; Liu, R. Reforestation improves vegetation coverage and biomass, but not spatial structure, on semi-arid mine dumps. Ecol. Eng. 2022, 175, 106508. [Google Scholar] [CrossRef]
- Krzyszowska Waitkus, A. Sustainable reclamation practices for a large surface coal mine in shortgrass prairie, semiarid environment (Wyoming, USA): Case study. Int. J. Coal Sci. Technol. 2022, 9, 32. [Google Scholar] [CrossRef]
- Bruno Rocha Martins, W.; Douglas Roque Lima, M.; de Oliveira Barros Junior, U.; Sousa Villas-Boas Amorim, L.; de Assis Oliveira, F.; Schwartz, G. Ecological methods and indicators for recovering and monitoring ecosystems after mining: A global literature review. Ecol. Eng. 2020, 145, 105707. [Google Scholar] [CrossRef]
- Ren, H.; Zhao, Y.; Xiao, W.; Hu, Z. A review of UAV monitoring in mining areas: Current status and future perspectives. Int. J. Coal Sci. Technol. 2019, 6, 320–333. [Google Scholar] [CrossRef]
- Almeida, D.R.A.; Broadbent, E.N.; Zambrano, A.M.A.; Wilkinson, B.E.; Ferreira, M.E.; Chazdon, R.L.; Meli, P.; Görgens, E.B.; Silva, C.A.; Stark, S.C.; et al. Monitoring the structure of forest restoration plantations with a drone-lidar system. Int. J. Appl. Earth Obs. Geoinf. 2019, 79, 192–198. [Google Scholar] [CrossRef]
- Szostak, M.; Pietrzykowski, M.; Likus-Cieślik, J. Reclaimed Area Land Cover Mapping Using Sentinel-2 Imagery and LiDAR Point Clouds. Remote. Sens. 2020, 12, 261. [Google Scholar] [CrossRef]
- Tang, J.; Liang, J.; Yang, Y.; Zhang, S.; Hou, H.; Zhu, X. Revealing the Structure and Composition of the Restored Vegetation Cover in Semi-Arid Mine Dumps Based on LiDAR and Hyperspectral Images. Remote Sens. 2022, 14, 978. [Google Scholar] [CrossRef]
- Banerjee, B.P.; Raval, S. Mapping Sensitive Vegetation Communities in Mining Eco-space using UAV-LiDAR. Int. J. Coal Sci. Technol. 2022, 9, 40. [Google Scholar] [CrossRef]
- Nasiri, V.; Beloiu, M.; Asghar Darvishsefat, A.; Griess, V.C.; Maftei, C.; Waser, L.T. Mapping tree species composition in a Caspian temperate mixed forest based on spectral-temporal metrics and machine learning. Int. J. Appl. Earth Obs. Geoinf. 2023, 116, 103154. [Google Scholar] [CrossRef]
- Thouverai, E.; Marcantonio, M.; Bacaro, G.; Re, D.D.; Iannacito, M.; Marchetto, E.; Ricotta, C.; Tattoni, C.; Vicario, S.; Rocchini, D. Measuring diversity from space: A global view of the free and open source rasterdiv R package under a coding perspective. Community Ecol. 2021, 22, 1–11. [Google Scholar] [CrossRef]
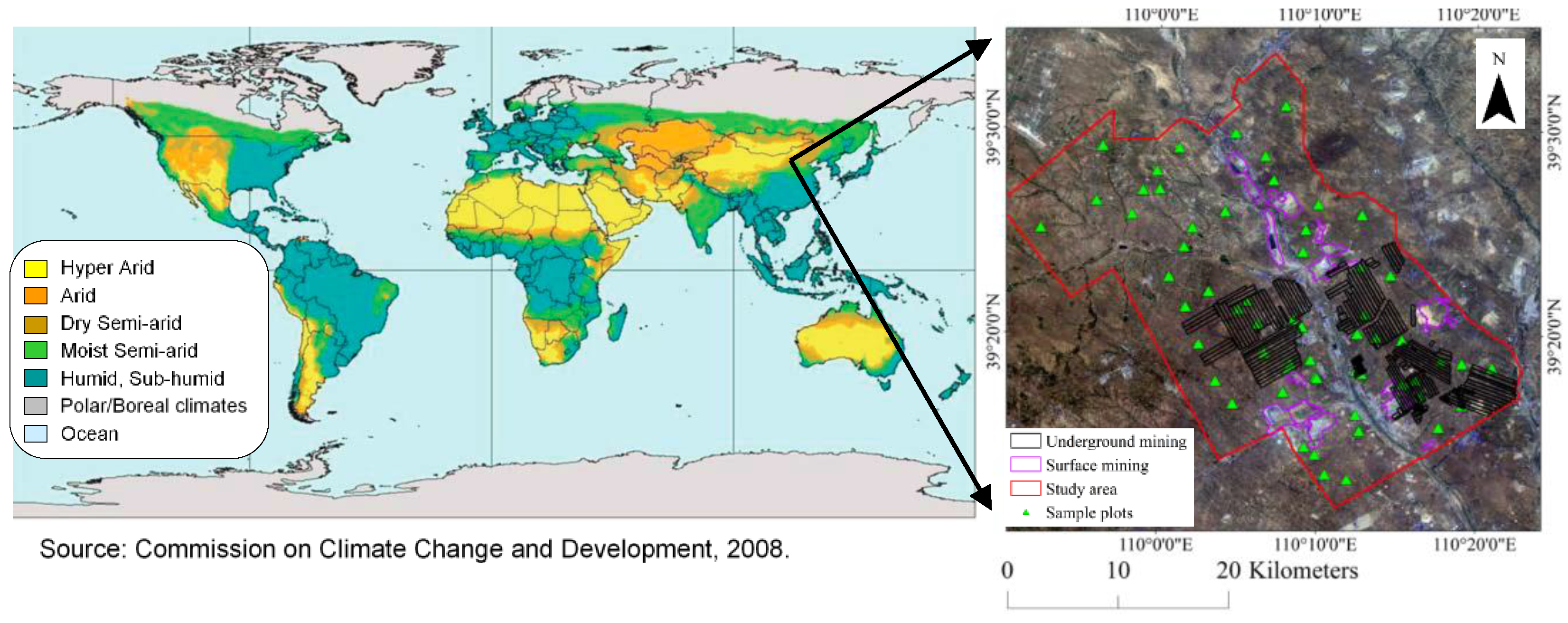






| Name | Date Acquired | Cloud Coefficient |
|---|---|---|
| LT51270331990241BJC00 | 19900829 | 0.3% |
| LT51270332000237BJC00 | 20000824 | 0.1% |
| LT51270332010258IKR00 | 20100915 | 0.2% |
| LE71270332020252EDC00 | 20200908 | 0.1% |
| Estimated Value | Surveyed Value | Correlation Coefficient (R2) | p-Value | RMSE |
|---|---|---|---|---|
| Alpha diversity | Shannon–Wiener index | 0.6827 | 0.002 | 5.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tang, J.; Wu, Q.; Huang, S.; Yao, X.; Dong, J. Assessment of the Capability of Landsat and BiodivMapR to Track the Change of Alpha Diversity in Dryland Disturbed by Mining. Remote Sens. 2023, 15, 1554. https://doi.org/10.3390/rs15061554
Zhang Y, Tang J, Wu Q, Huang S, Yao X, Dong J. Assessment of the Capability of Landsat and BiodivMapR to Track the Change of Alpha Diversity in Dryland Disturbed by Mining. Remote Sensing. 2023; 15(6):1554. https://doi.org/10.3390/rs15061554
Chicago/Turabian StyleZhang, Yan, Jiajia Tang, Qinyu Wu, Shuai Huang, Xijun Yao, and Jing Dong. 2023. "Assessment of the Capability of Landsat and BiodivMapR to Track the Change of Alpha Diversity in Dryland Disturbed by Mining" Remote Sensing 15, no. 6: 1554. https://doi.org/10.3390/rs15061554
APA StyleZhang, Y., Tang, J., Wu, Q., Huang, S., Yao, X., & Dong, J. (2023). Assessment of the Capability of Landsat and BiodivMapR to Track the Change of Alpha Diversity in Dryland Disturbed by Mining. Remote Sensing, 15(6), 1554. https://doi.org/10.3390/rs15061554





