Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae
Abstract
1. Introduction
- (i)
- Compare the performance of Sentinel-2 and Landsat 8 satellites in the surveillance of damage caused by CBW larvae in maize;
- (ii)
- Find the optimal (highest correlated) time periods and maize phenological phases for satellite-based CBW damage surveillance;
- (iii)
- Identify a spectral band or vegetation index that reliably estimates the damage (r ≥ 0.4 Pearson correlation, consistently) under the optimal phenological phases and is robust against various circumstances (showing similar correlation coefficients);
- (iv)
- Identify other agronomic factors that influence satellite imagery performance (resulting in inconsistent correlations) in predicting and monitoring the cotton bollworm.
2. Materials and Methods
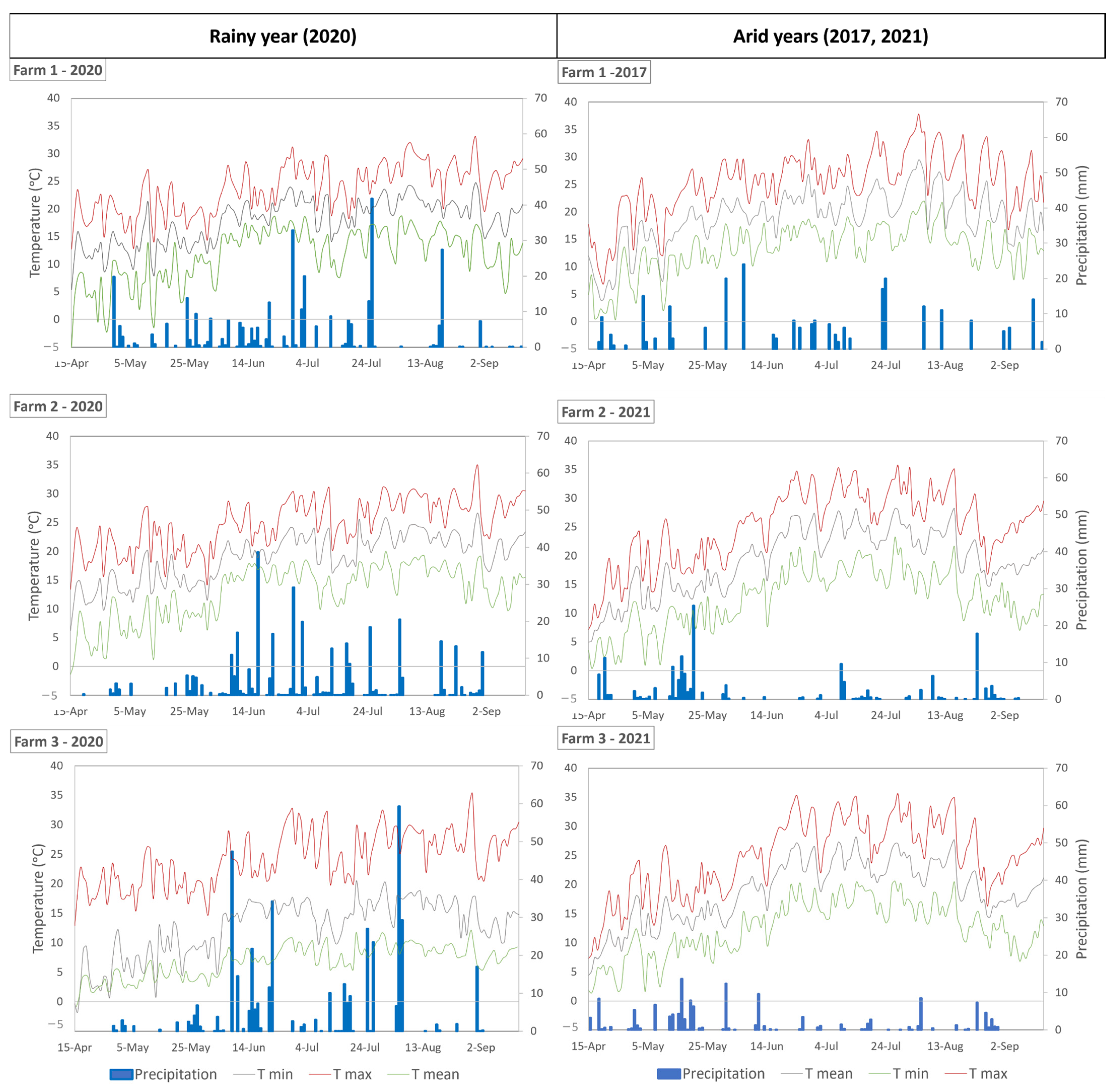
2.1. Study Area
2.2. Satellite Remote Sensing Imagery Retrieving
2.3. Vegetation Index Selection and Calculation
2.4. Cotton Bollworm Larval Damage Observations
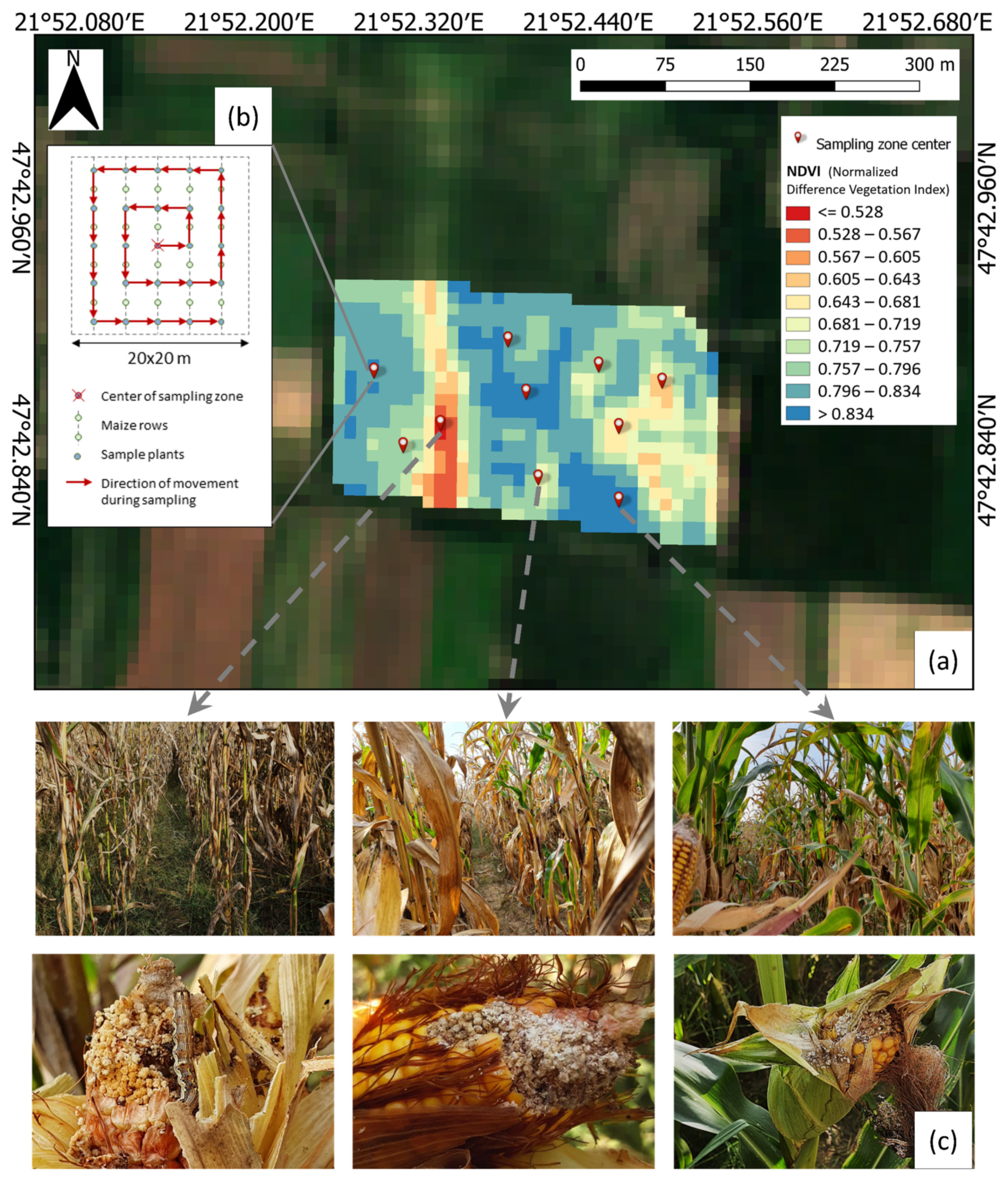
- Sampling zone selection: Georeferencing of field boundaries was performed manually. For each field, the NDVI was calculated based on Sentinel-2 imagery with a 20 m spatial resolution, and a grid of 20 × 20 m zones was applied to the fields. Ten sampling zones were selected in each field by the following method: The field’s NDVI values range was divided into ten equal sub-ranges. From each sub-range, one sampling zone was selected.
- Deploying sampling zones: The center point of the selected sampling zones was retrieved in QGIS and deployed on the fields based on GPS coordinates using a Trimble Juno 3B GPS device.
- Sample plant selection: In each sampling zone, 36 sample plants were selected following a spiral line from the sampling zone’s center with an equal distribution on the zone’s grid points.
- Damage observation: The ears of sample plants were visually inspected. The presence of apparent CBW larvae damage was observed by removing the ears’ husk and checking for chowed kernels and the typical excrement of the CBW (Figure 2). The extent of the damage to the ears was assumed to be negligible information and not estimated. The percentage of damaged ears was considered as characteristic of the sampling zone.
- maize plant density dropped below 60% due to waterlogging;
- maize plant density dropped below 60% due to agronomic failure;
- a large object was found in the sampling zone (e.g., light pole)
2.5. Cotton Bollworm Adult Flight Monitoring
2.6. Additional Field Observations
- BBCH 05–BBCH 17 Emergence, establishment, and mid-early development
- BBCH 18–BBCH 52 Canopy closure, organ, and stem elongation
- BBCH 53–BBCH 64 Tasseling, silking, pollination, and fertilization
- BBCH 65–BBCH 84 Grain filling
- BBCH 85–BBCH 89 Physiological maturation
- BBCH 99– After harvest
- FAO 300 maize hybrids: consists of mid–early maturing grain maize hybrids from FAO 290 to FAO 389;
- FAO 400 maize hybrids: consists of mid–late maturing grain maize hybrids from FAO 390 to FAO 489.
2.7. Statistical Analysis and Visualization
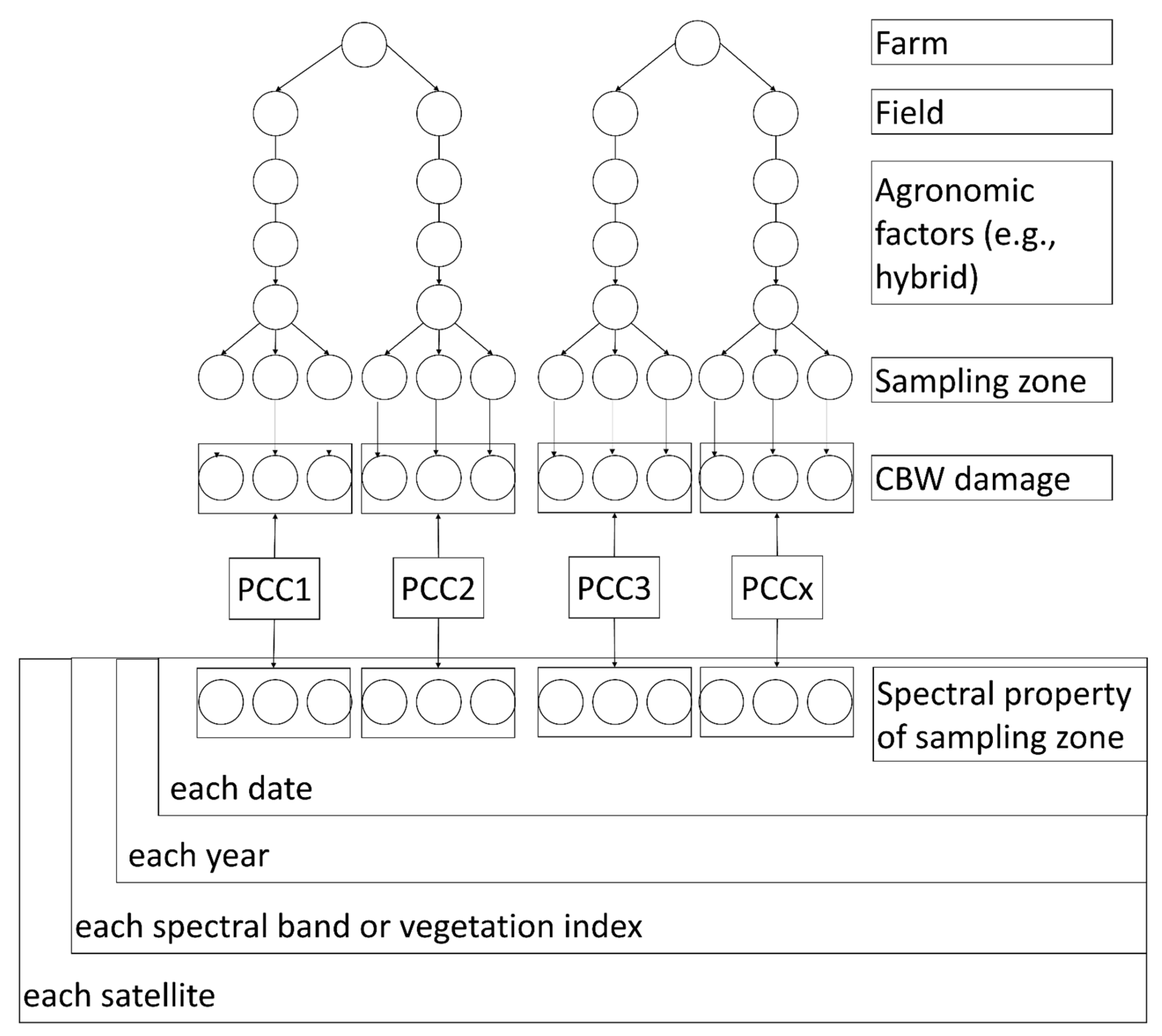
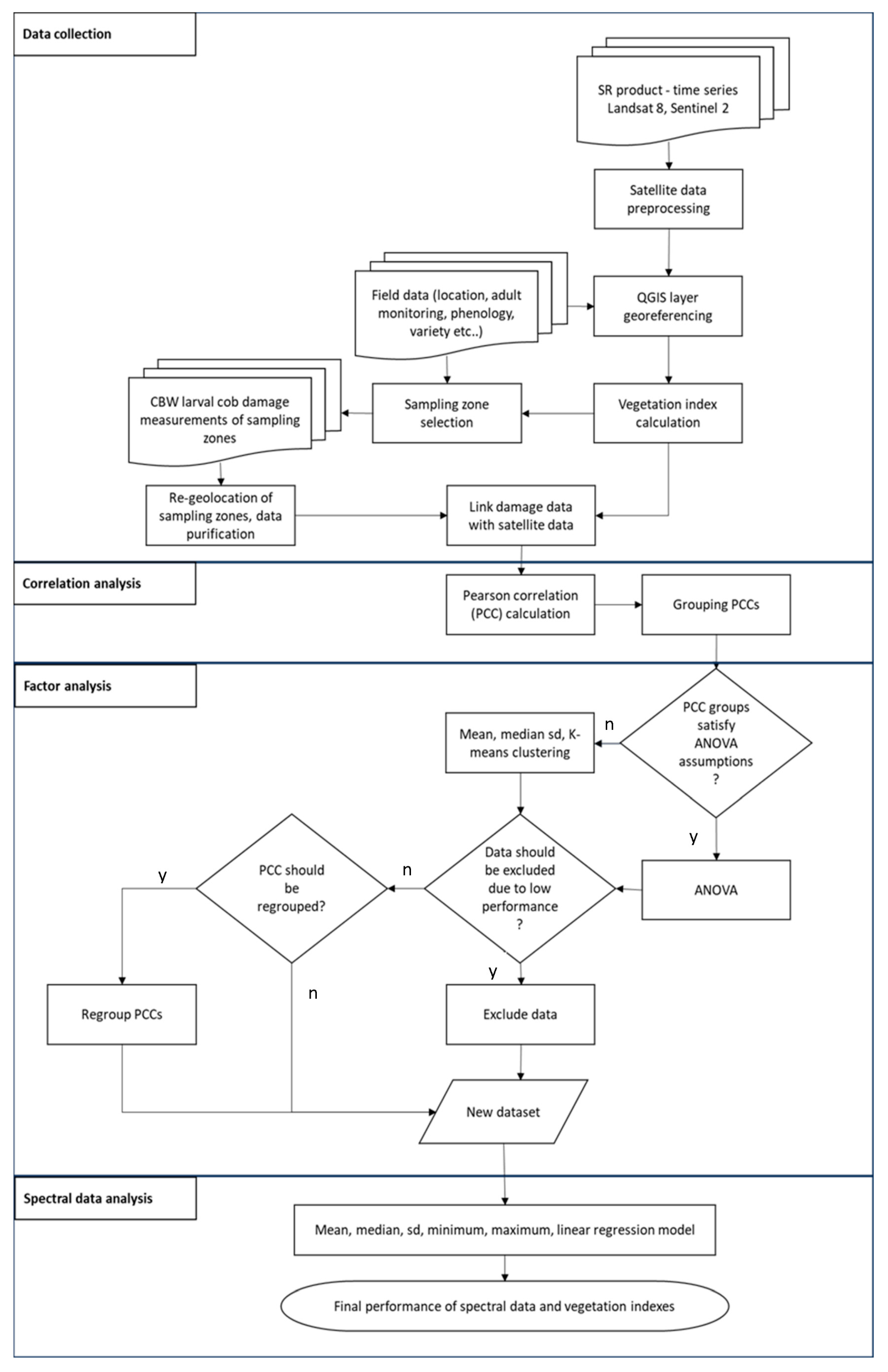
2.8. Summary of Methodology
3. Results
3.1. Cotton Bollworm Larval Damage to Maize Ears on Fields, Farms, and Years
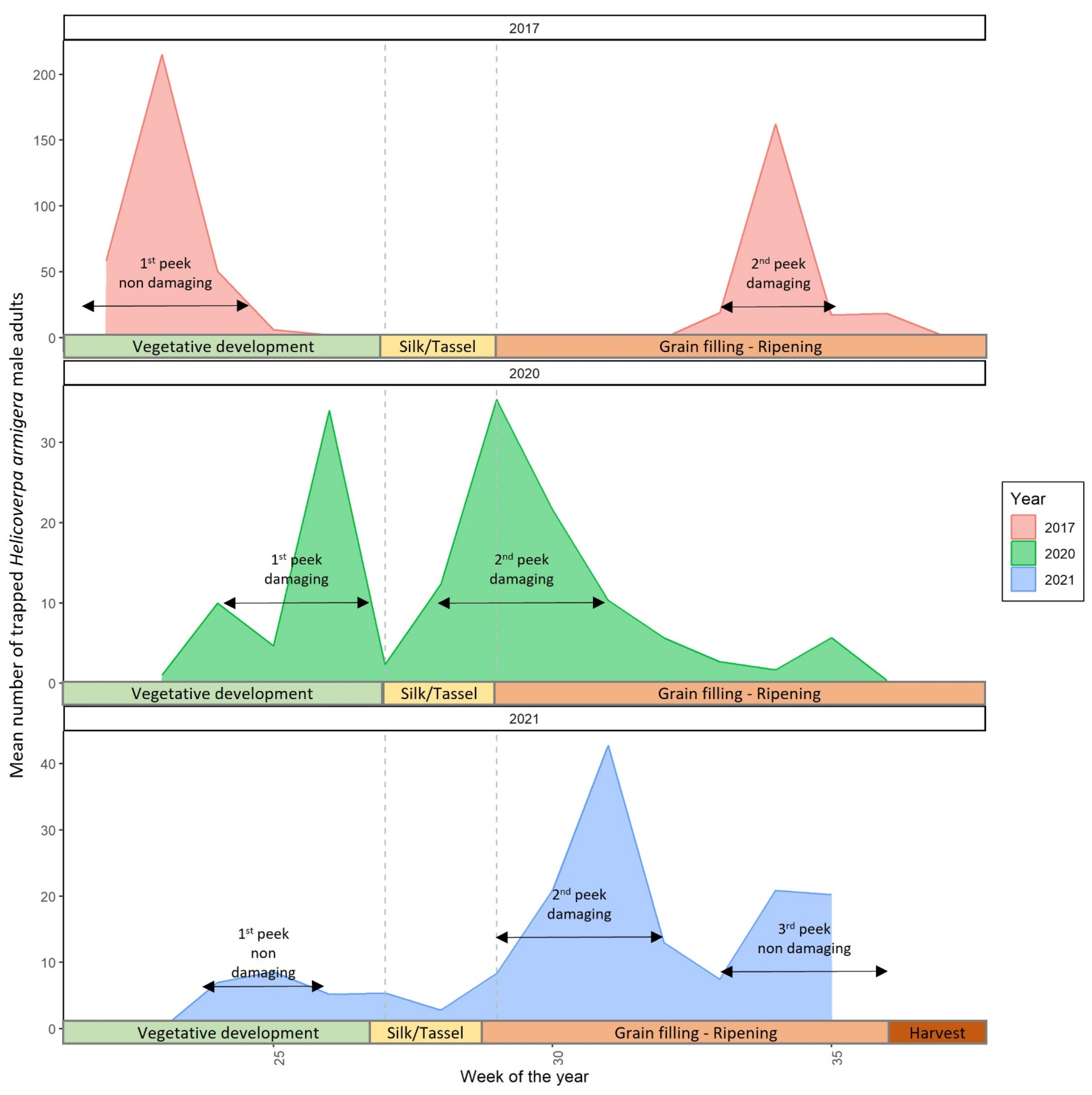
3.2. Cotton Bollworm Adult Monitoring and Annual Peaks of Their Appearance
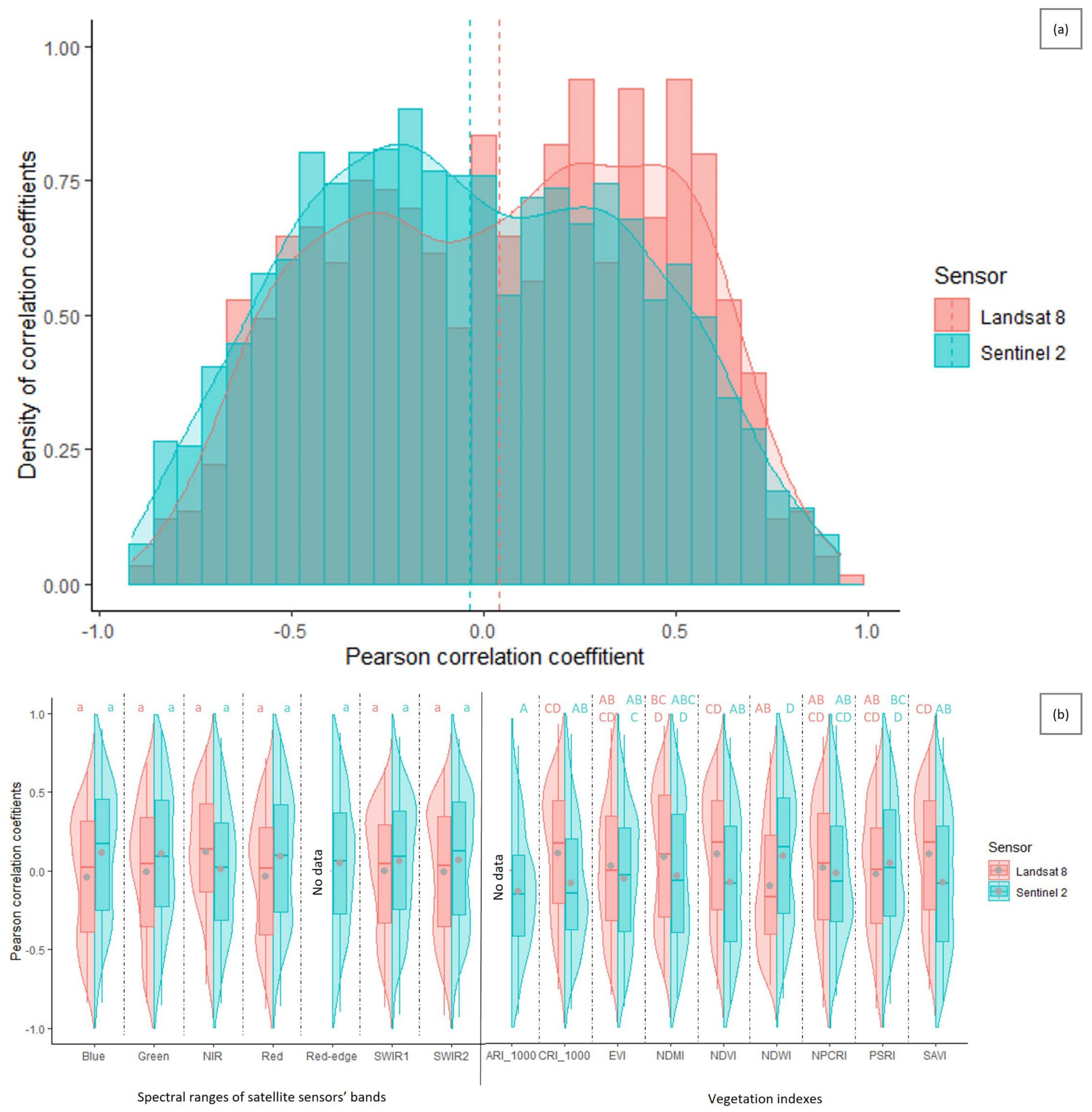
3.3. Suitability of Landsat 8 versus Sentinel-2 Satellites for Cotton Bollworm Damage Surveillance in Maize
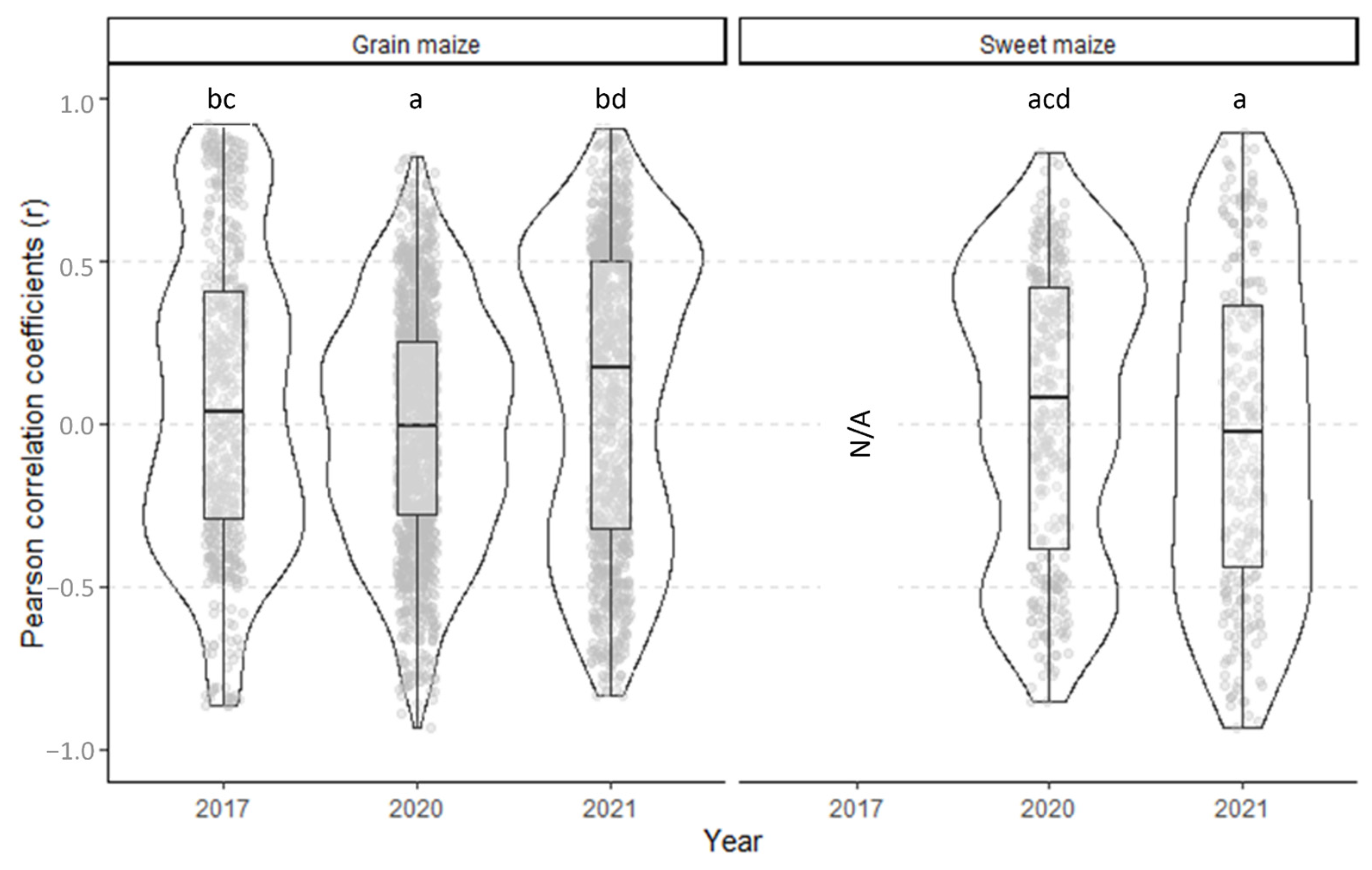
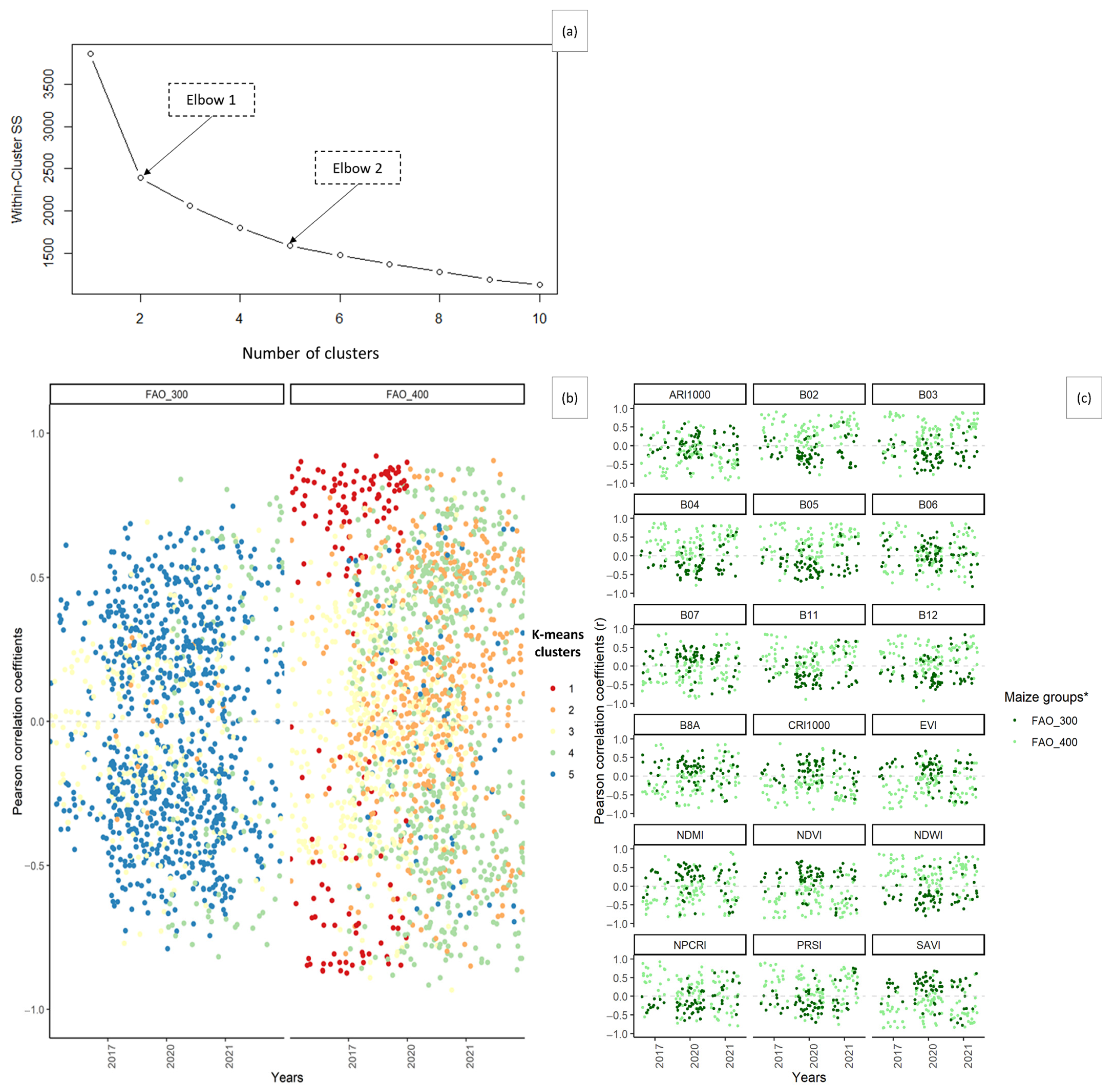
3.4. Cotton Bollworm Surveillance via Remote Sensing, Depending on Year, Maize Cultivation Purpose, and Maturity Group
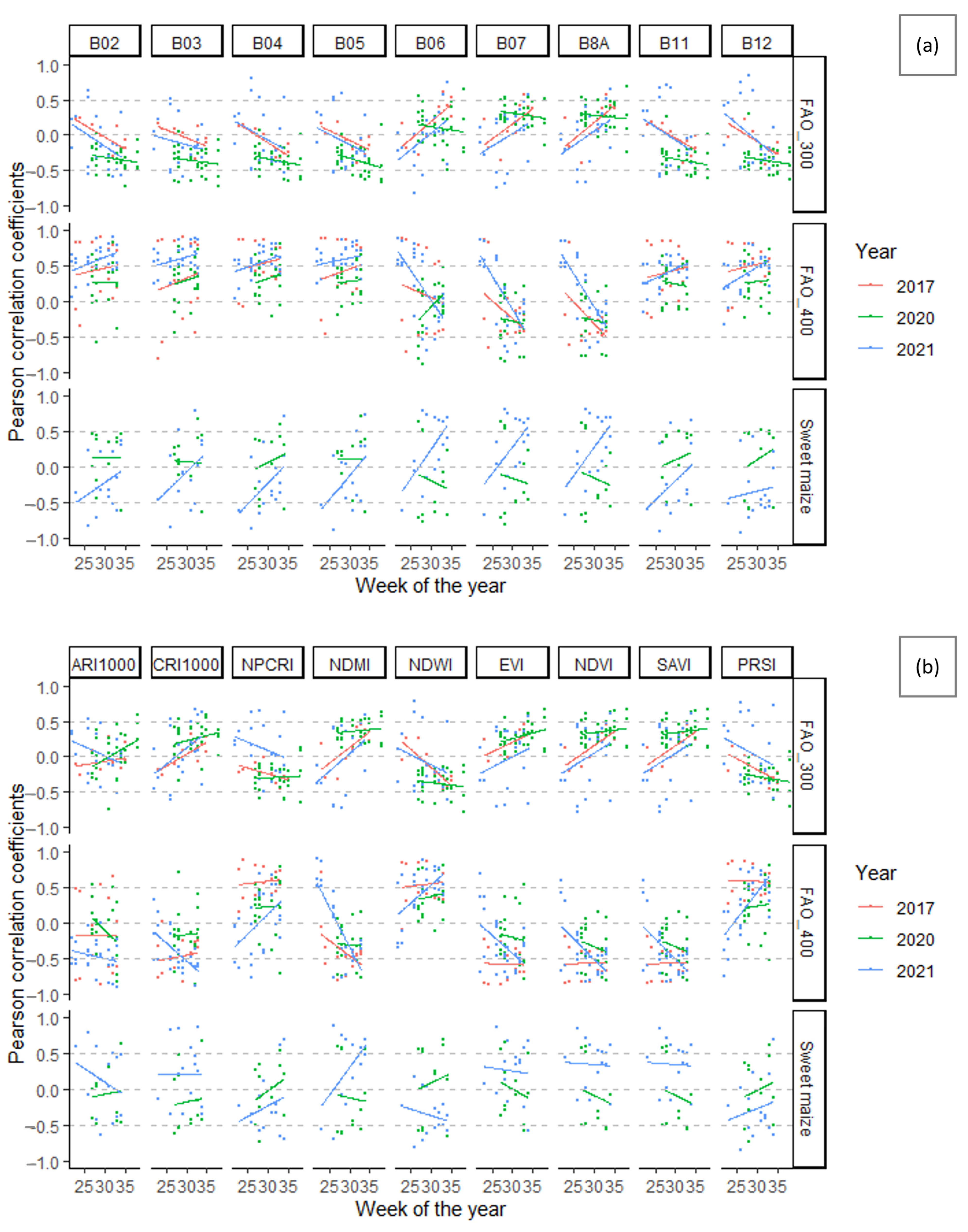
3.5. The Optimal Maize Phenology for Cotton Bollworm Surveillance
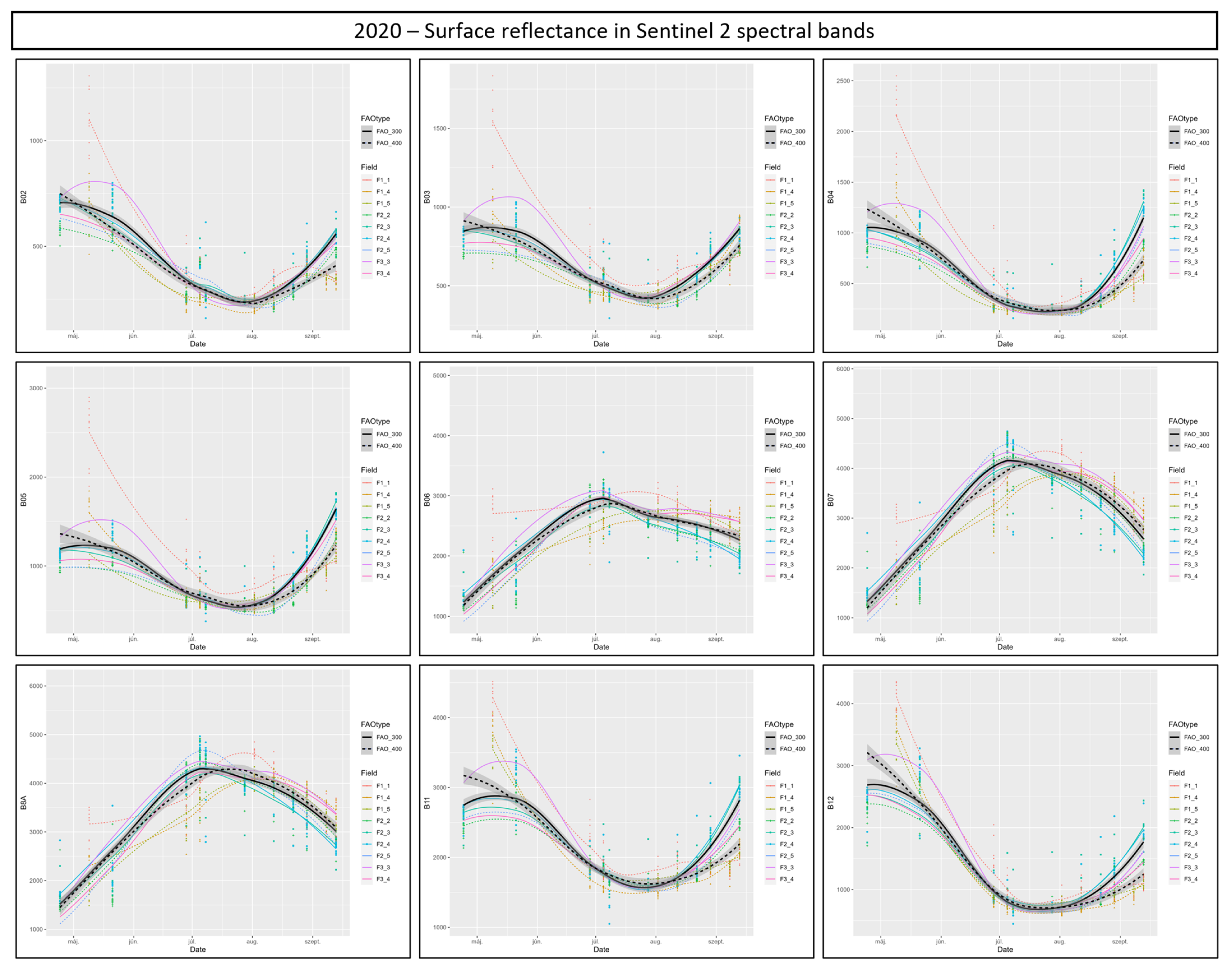
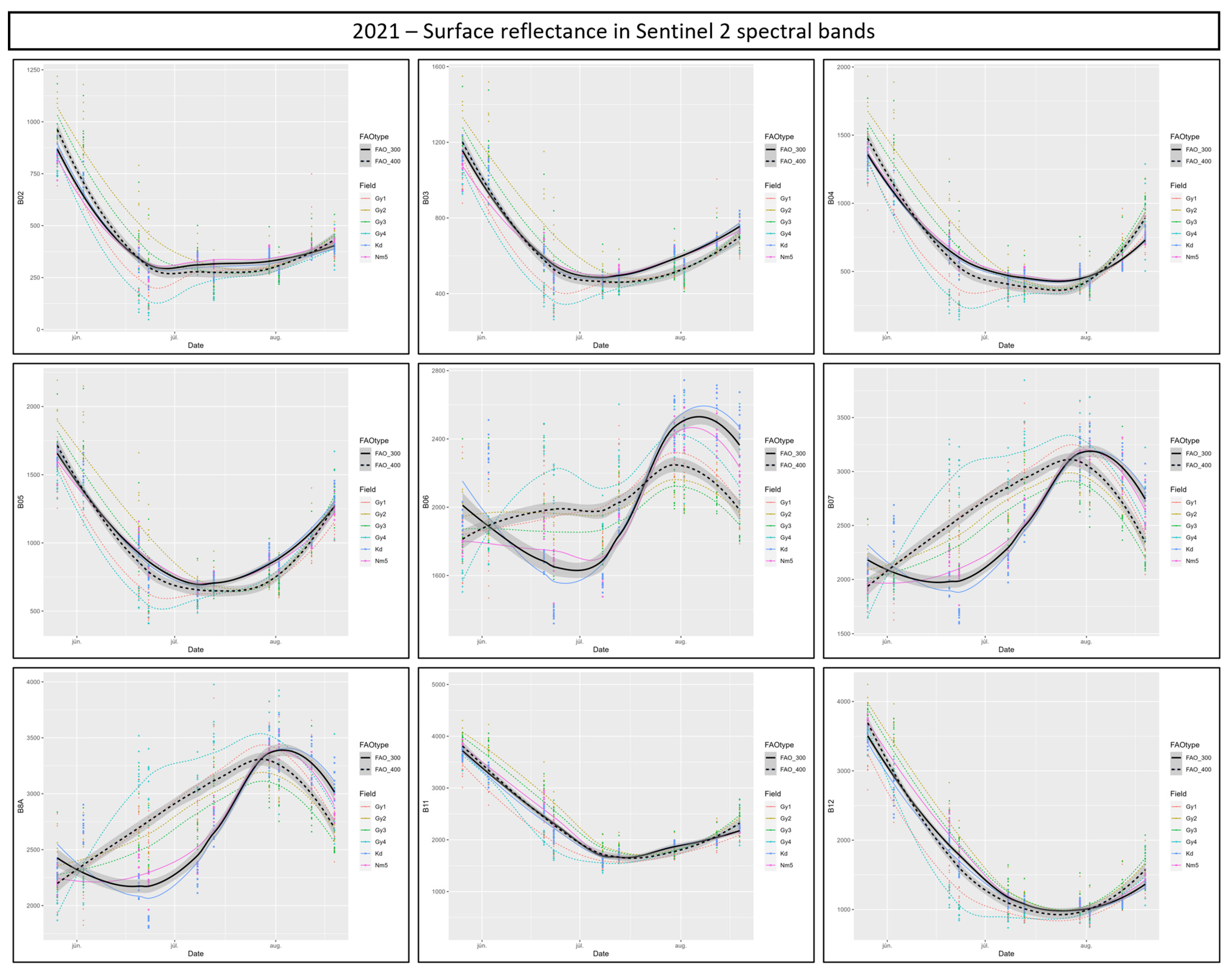
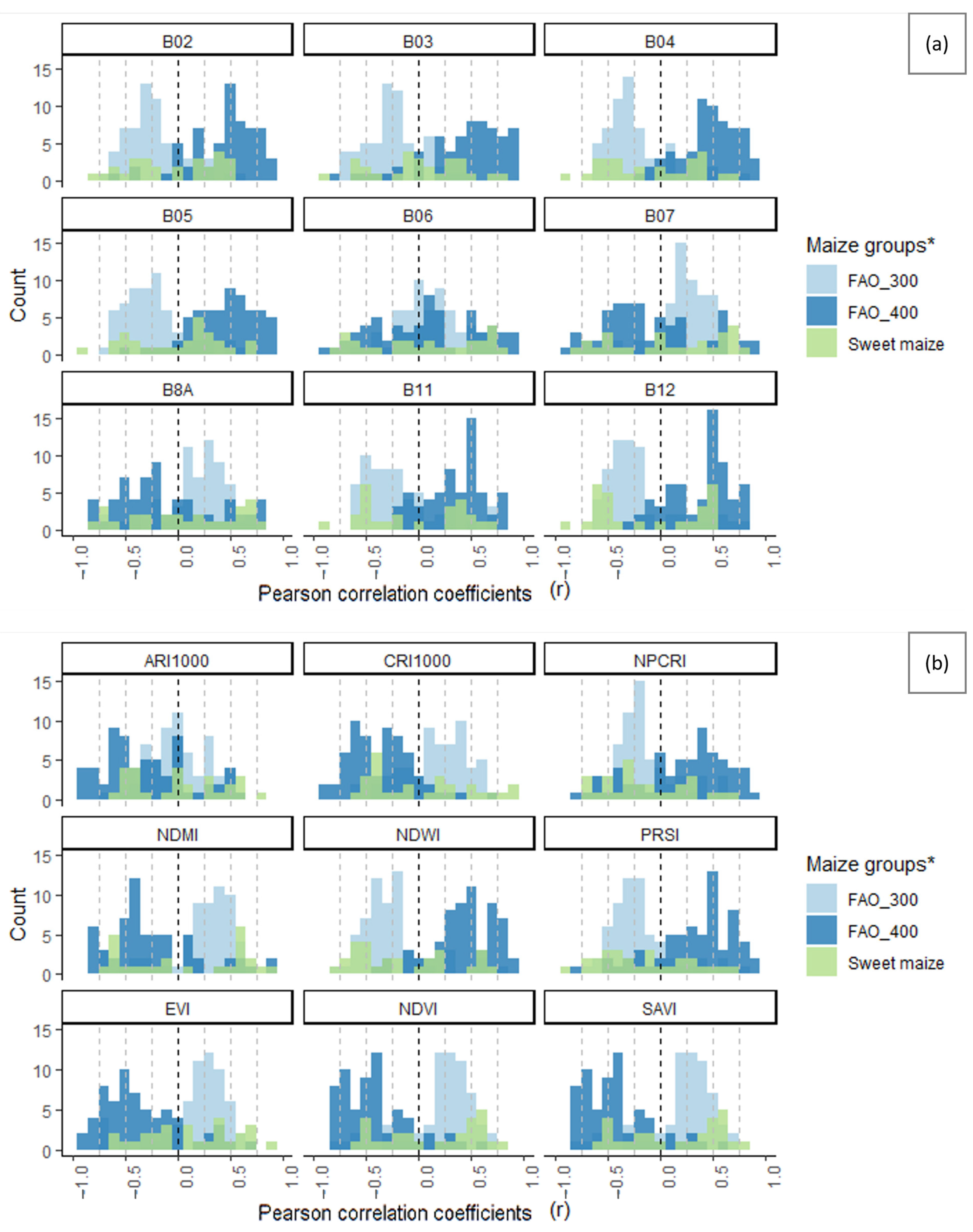
3.6. Suitability of Different Spectral Bands and Vegetation Indices for Cotton Bollworm Surveillance
4. Discussion
4.1. Limitations and Uncertainties
4.2. Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Farm | Field | X | Y | Cultivation Purpose | Hybrid | Maturity Group 1 | Adult Monitoring | Available Satellite Images | |
|---|---|---|---|---|---|---|---|---|---|---|
| Landsat-8 | Sentinel-2 | |||||||||
| 2017 | Farm 1 | F1_1 | 21.872793 | 47.714271 | Grain maize | Amandha | FAO_400 | Pheromone trap | 4 | 11 |
| 2017 | Farm 1 | F1_2 | 21.921102 | 47.721464 | Grain maize | Kinemas | FAO_300 | Pheromone trap | 4 | 11 |
| 2017 | Farm 1 | F1_3 | 21.807788 | 47.63241 | Grain maize | KWS 2482 | FAO_400 | Pheromone trap | 4 | 11 |
| 2020 | Farm 1 | F1_1 | 21.872793 | 47.714271 | Grain maize | Kathedralis | FAO_400 | Pheromone trap | 3 | 7 |
| 2020 | Farm 1 | F1_4 | 21.928877 | 47.725272 | Grain maize | Kathedralis | FAO_400 | Pheromone trap | 3 | 7 |
| 2020 | Farm 1 | F1_5 | 21.806356 | 47.629874 | Grain maize | Durango | FAO_400 | Pheromone trap | 3 | 7 |
| 2020 | Farm 2 | F2_2 | 20.799724 | 46.872995 | Grain maize | Fonard | FAO_400 | None | 6 | 10 |
| 2020 | Farm 2 | F2_3 | 20.886168 | 46.887758 | Grain maize | P9486 | FAO_300 | None | 6 | 10 |
| 2020 | Farm 2 | F2_4 | 20.86895 | 46.895381 | Grain maize | DKC4943 | FAO_300 | None | 6 | 10 |
| 2020 | Farm 2 | F2_5 | 20.817095 | 46.87975 | Grain maize | Fonard | FAO_400 | None | 6 | 10 |
| 2020 | Farm 3 | Nm1 | 20.474071 | 46.608599 | Sweet maize | SF1379 | na | None | 4 | 10 |
| 2020 | Farm 3 | Nm2 | 20.467449 | 46.610698 | Sweet maize | Kiara | na | None | 4 | 10 |
| 2020 | Farm 3 | F3_3 | 20.478401 | 46.600584 | Grain maize | PR37N01 | FAO_300 | None | 4 | 10 |
| 2020 | Farm 3 | F3_3 | 20.4809 | 46.62392 | Grain maize | PR37N01 | FAO_300 | None | 4 | 10 |
| 2021 | Farm 2 | Gy1 | 20.84142 | 46.881759 | Grain maize | DKC4897 | FAO_400 | None | 8 | 10 |
| 2021 | Farm 2 | Gy2 | 20.85435 | 46.859473 | Grain maize | DKC4897 | FAO_400 | None | 8 | 10 |
| 2021 | Farm 2 | Gy3 | 20.849997 | 46.858392 | Grain maize | DKC4897 | FAO_400 | None | 8 | 10 |
| 2021 | Farm 3 | Kd | 20.772472 | 46.727483 | Grain maize | PR37N01 | FAO_300 | Pheromone trap | 8 | 10 |
| 2021 | Farm 3 | Nm1 | 20.474071 | 46.608599 | Sweet maize | Kiara | na | None | 8 | 10 |
| 2021 | Farm 3 | Nm2 | 20.467449 | 46.610698 | Sweet maize | Kiara | na | None | 8 | 10 |
| 2021 | Farm 3 | Nm5 | 20.471139 | 46.598485 | Grain maize | PR37N01 | FAO_300 | Sex pheromone trap | 8 | 10 |
| FAO 300 Grain Maize Fields | FAO 400 Grain maize Fields | Sweet Maize Fields | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Band/ Index | Equation | R2 | p | Equation | R2 | p | Equation | R2 | p | |||
| 2017 | B02 | y = −0.03 x + 0.99 | 0.51 | 0.02 | * | y = 0.01 x + 0.07 | 0.01 | 0.69 | n/a | n/a | n/a | ||
| 2020 | B02 | y = −0.01 x − 0.05 | 0.04 | 0.25 | y = 0 x + 0.27 | 0.00 | 1.00 | y = 0 x + 0.16 | 0.00 | 0.99 | |||
| 2021 | B02 | y = −0.04 x + 0.99 | 0.15 | 0.10 | y = 0.02 x − 0.07 | 0.20 | 0.02 | * | y = 0.04 x − 1.4 | 0.11 | 0.23 | ||
| 2017 | B03 | y = −0.02 x + 0.66 | 0.32 | 0.09 | y = 0.02 x − 0.39 | 0.02 | 0.62 | n/a | n/a | n/a | |||
| 2020 | B03 | y = −0.01 x − 0.12 | 0.02 | 0.41 | y = 0.02 x − 0.3 | 0.03 | 0.48 | y = 0 x + 0.21 | 0.00 | 0.92 | |||
| 2021 | B03 | y = −0.02 x + 0.34 | 0.04 | 0.42 | y = 0.02 x + 0.15 | 0.10 | 0.10 | y = 0.06 x − 1.75 | 0.17 | 0.13 | |||
| 2017 | B04 | y = −0.04 x + 1 | 0.73 | 0.00 | * | y = 0.02 x + 0.1 | 0.03 | 0.55 | n/a | n/a | n/a | ||
| 2020 | B04 | y = −0.01 x + 0 | 0.06 | 0.19 | y = 0.02 x − 0.31 | 0.04 | 0.39 | y = 0.03 x − 0.8 | 0.03 | 0.56 | |||
| 2021 | B04 | y = −0.03 x + 0.89 | 0.08 | 0.23 | y = 0.02 x − 0.01 | 0.16 | 0.04 | * | y = 0.06 x − 2 | 0.22 | 0.08 | ||
| 2017 | B05 | y = −0.03 x + 0.75 | 0.43 | 0.04 | * | y = 0.02 x − 0.16 | 0.03 | 0.57 | n/a | n/a | n/a | ||
| 2020 | B05 | y = −0.02 x + 0.18 | 0.11 | 0.07 | y = 0.01 x + 0.08 | 0.00 | 0.76 | y = 0 x + 0.14 | 0.00 | 0.98 | |||
| 2021 | B05 | y = −0.03 x + 0.74 | 0.09 | 0.22 | y = 0.01 x + 0.25 | 0.07 | 0.18 | y = 0.07 x − 2.18 | 0.24 | 0.06 | |||
| 2017 | B06 | y = 0.05 x − 1.27 | 0.53 | 0.02 | * | y = −0.03 x + 0.81 | 0.02 | 0.60 | n/a | n/a | n/a | ||
| 2020 | B06 | y = −0.01 x + 0.4 | 0.02 | 0.47 | y = 0.06 x − 1.87 | 0.16 | 0.06 | y = −0.03 x + 0.63 | 0.02 | 0.66 | |||
| 2021 | B06 | y = 0.05 x − 1.37 | 0.24 | 0.03 | * | y = −0.08 x + 2.52 | 0.43 | 0.00 | * | y = 0.08 x − 2.24 | 0.37 | 0.02 | * |
| 2017 | B07 | y = 0.04 x − 1.1 | 0.41 | 0.04 | * | y = −0.05 x + 1.26 | 0.16 | 0.15 | n/a | n/a | n/a | ||
| 2020 | B07 | y = −0.01 x + 0.56 | 0.04 | 0.26 | y = −0.01 x + 0.12 | 0.01 | 0.67 | y = −0.02 x + 0.42 | 0.01 | 0.76 | |||
| 2021 | B07 | y = 0.04 x − 1.05 | 0.13 | 0.13 | y = −0.1 x + 2.73 | 0.55 | 0.00 | * | y = 0.07 x − 1.95 | 0.31 | 0.03 | * | |
| 2017 | B8A | y = 0.05 x − 1.23 | 0.47 | 0.03 | * | y = −0.06 x + 1.51 | 0.24 | 0.07 | n/a | n/a | n/a | ||
| 2020 | B8A | y = −0.01 x + 0.47 | 0.02 | 0.48 | y = −0.01 x + 0.13 | 0.01 | 0.66 | y = −0.03 x + 0.62 | 0.02 | 0.67 | |||
| 2021 | B8A | y = 0.04 x − 1.14 | 0.18 | 0.07 | y = −0.09 x + 2.69 | 0.55 | 0.00 | * | y = 0.08 x − 2.09 | 0.33 | 0.02 | * | |
| 2017 | B11 | y = −0.03 x + 0.96 | 0.53 | 0.02 | * | y = 0.01 x + 0 | 0.02 | 0.63 | n/a | n/a | n/a | ||
| 2020 | B11 | y = −0.01 x − 0.03 | 0.04 | 0.27 | y = −0.01 x + 0.61 | 0.01 | 0.59 | y = 0.03 x − 0.67 | 0.03 | 0.60 | |||
| 2021 | B11 | y = −0.04 x + 1.05 | 0.10 | 0.21 | y = 0.03 x − 0.36 | 0.22 | 0.02 | * | y = 0.06 x − 1.86 | 0.14 | 0.21 | ||
| 2017 | B12 | y = −0.04 x + 0.99 | 0.53 | 0.02 | * | y = 0.01 x + 0.1 | 0.03 | 0.57 | n/a | n/a | n/a | ||
| 2020 | B12 | y = −0.01 x − 0.07 | 0.04 | 0.30 | y = 0.01 x + 0.1 | 0.00 | 0.82 | y = 0.04 x − 1.03 | 0.04 | 0.51 | |||
| 2021 | B12 | y = −0.05 x + 1.36 | 0.16 | 0.09 | y = 0.03 x −0.56 | 0.25 | 0.01 | * | y = 0.01 x − 0.77 | 0.01 | 0.68 | ||
| 2017 | ARI | y = 0.01 x − 0.35 | 0.04 | 0.56 | y = 0 x −0.2 | 0.00 | 0.99 | n/a | n/a | n/a | |||
| 2020 | ARI | y = 0.03 x − 1.04 | 0.19 | 0.06 | y = −0.06 x + 1.57 | 0.14 | 0.08 | y = 0.01 x − 0.43 | 0.01 | 0.78 | |||
| 2021 | ARI | y = −0.03 x + 0.78 | 0.11 | 0.17 | y = −0.01 x − 0.1 | 0.05 | 0.27 | y = −0.04 x + 1.23 | 0.07 | 0.34 | |||
| 2017 | CRI | y = 0.03 x − 0.98 | 0.56 | 0.01 | * | y = 0.01 x − 0.79 | 0.03 | 0.57 | n/a | n/a | n/a | ||
| 2020 | CRI | y = 0.01 x − 0.2 | 0.06 | 0.19 | y = 0.01 x − 0.37 | 0.00 | 0.82 | y = 0.01 x − 0.56 | 0.01 | 0.80 | |||
| 2021 | CRI | y = 0.05 x −1.24 | 0.18 | 0.07 | y = −0.05 x + 0.98 | 0.41 | 0.00 | * | y = 0 x + 0.18 | 0.00 | 0.98 | ||
| 2017 | NPCRI | y = −0.02 x + 0.26 | 0.32 | 0.09 | y = 0.01 x + 0.38 | 0.01 | 0.68 | n/a | n/a | n/a | |||
| 2020 | NPCRI | y = 0 x −0.38 | 0.00 | 0.75 | y = 0.01 x + 0.07 | 0.00 | 0.88 | y = 0.04 x − 1.26 | 0.09 | 0.35 | |||
| 2021 | NPCRI | y = −0.02 x + 0.8 | 0.07 | 0.26 | y = 0.06 x − 1.58 | 0.23 | 0.01 | * | y = 0.03 x − 1.14 | 0.06 | 0.39 | ||
| 2017 | NDMI | y = 0.04 x − 1.2 | 0.48 | 0.03 | * | y = −0.04 x + 0.84 | 0.20 | 0.11 | n/a | n/a | n/a | ||
| 2020 | NDMI | y = 0.01 x + 0.19 | 0.02 | 0.46 | y = −0.01 x − 0.16 | 0.00 | 0.84 | y = −0.01 x + 0.28 | 0.00 | 0.83 | |||
| 2021 | NDMI | y = 0.05 x − 1.45 | 0.18 | 0.09 | y = −0.11 x + 3.05 | 0.60 | 0.00 | * | y = 0.08 x − 2.02 | 0.26 | 0.07 | ||
| 2017 | NDWI | y = −0.05 x + 1.38 | 0.66 | 0.00 | * | y = 0.01 x + 0.35 | 0.01 | 0.76 | n/a | n/a | n/a | ||
| 2020 | NDWI | y = −0.01 x − 0.15 | 0.02 | 0.42 | y = 0.01 x − 0.02 | 0.02 | 0.58 | y = 0.03 x − 0.73 | 0.02 | 0.66 | |||
| 2021 | NDWI | y = −0.03 x + 0.74 | 0.07 | 0.28 | y = 0.05 x − 1 | 0.32 | 0.00 | * | y = −0.02 x + 0.17 | 0.04 | 0.47 | ||
| 2017 | EVI | y = 0.03 x − 0.61 | 0.44 | 0.04 | * | y = 0 x − 0.52 | 0.00 | 0.90 | n/a | n/a | n/a | ||
| 2020 | EVI | y = 0.02 x − 0.22 | 0.12 | 0.06 | y = −0.02 x + 0.25 | 0.01 | 0.63 | y = −0.03 x + 0.97 | 0.05 | 0.50 | |||
| 2021 | EVI | y = 0.03 x − 0.87 | 0.07 | 0.29 | y = −0.05 x + 1.09 | 0.24 | 0.01 | * | y = −0.01 x + 0.52 | 0.01 | 0.80 | ||
| 2017 | NDVI | y = 0.04 x −1.02 | 0.60 | 0.01 | * | y = 0 x − 0.61 | 0.00 | 0.94 | n/a | n/a | n/a | ||
| 2020 | NDVI | y = 0.01 x + 0.13 | 0.03 | 0.37 | y = −0.02 x + 0.38 | 0.04 | 0.36 | y = −0.03 x + 0.74 | 0.02 | 0.64 | |||
| 2021 | NDVI | y = 0.04 x − 1.02 | 0.10 | 0.20 | y = −0.06 x + 1.21 | 0.31 | 0.00 | * | y = 0 x + 0.48 | 0.00 | 0.88 | ||
| 2017 | SAVI | y = 0.04 x − 1.02 | 0.60 | 0.01 | * | y = 0 x − 0.61 | 0.00 | 0.94 | n/a | n/a | n/a | ||
| 2020 | SAVI | y = 0.01 x + 0.13 | 0.03 | 0.37 | y = −0.02 x + 0.38 | 0.04 | 0.36 | y = −0.03 x + 0.74 | 0.02 | 0.64 | |||
| 2021 | SAVI | y = 0.04 x − 1.02 | 0.10 | 0.20 | y = −0.06 x + 1.21 | 0.31 | 0.00 | * | y = 0 x + 0.48 | 0.00 | 0.88 | ||
| 2017 | PRSI | y = −0.03 x + 0.75 | 0.49 | 0.02 | * | y = 0 x + 0.64 | 0.00 | 0.92 | n/a | n/a | n/a | ||
| 2020 | PRSI | y = −0.01 x + 0.01 | 0.04 | 0.27 | y = 0.01 x − 0.05 | 0.00 | 0.76 | y = 0.03 x − 0.83 | 0.03 | 0.59 | |||
| 2021 | PRSI | y = −0.03 x + 0.96 | 0.08 | 0.24 | y = 0.07 x −1.8 | 0.44 | 0.00 | * | y = 0.02 x − 0.94 | 0.03 | 0.52 | ||

References
- Kriticos, D.J.; Ota, N.; Hutchison, W.D.; Beddow, J.; Walsh, T.; Tay, W.T.; Borchert, D.M.; Paula-Moreas, S.V.; Czepak, C.; Zalucki, M.P. The Potential Distribution of Invading Helicoverpa armigera in North America: Is It Just a Matter of Time? PLoS ONE 2015, 10, e0119618. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/ (accessed on 8 September 2023).
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A Review on Biological Interactions and Management of the Cotton Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Szőcs, G.; Tóth, M.; Újváry, I.; Szöcs, G.; Tóth, M.; Ujváry, I.; Szarukán, I. Development of a Pheromone Trap for Monitoring the Cotton Bollworm (Helicoverpa armigera Hbn.) an Upcoming Pest in Hungary. Növényvédelem 1995, 31, 261–266. [Google Scholar]
- Yadav, S.P.S.; Lahutiya, V.; Paudel, P. A Review on the Biology, Ecology, and Management Tactics of Helicoverpa armigera (Lepidoptera: Noctuidae). Turk. J. Agric.-Food Sci. Technol. 2022, 10, 2467–2476. [Google Scholar] [CrossRef]
- Lopes, H.M.; Bastos, C.S.; Boiteux, L.S.; Foresti, J.; Suinaga, F.A. A RAPD-PCR-Based Genetic Diversity Analysis of Helicoverpa armigera and H. zea Populations in Brazil. Genet. Mol. Res. GMR 2017, 16, gmr16038757. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Nowinszky, L.; Puskás, J. The Growing Abundance of Helicoverpa armigera in Hungary and Its Areal Shift Estimation. Cent. Eur. J. Biol. 2013, 8, 756–764. [Google Scholar] [CrossRef]
- Huang, J. Effects of Climate Change on Different Geographical Populations of the Cotton Bollworm Helicoverpa armigera (Lepidoptera, Noctuidae). Ecol. Evol. 2021, 11, 18357–18368. [Google Scholar] [CrossRef]
- Virić Gašparić, H.; Vučemilović Jurić, D.; Bažok, R. Assessment of a possible increase in the harmfulness of the cotton bollworm (Helicoverpa armigera Hubner) in Croatia. Entomol. Croat. 2022, 21, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Liu, J.; Zeng, J.; Li, Y. Long-Term Expansion of Cereal Crops Promotes Regional Population Increase of Polyphagous Helicoverpa armigera. Available online: https://www.researchsquare.com (accessed on 17 November 2023).
- Sagar, D.; Nebapure, S.M.; Chander, S. Development and Validation of Weather Based Prediction Model for Helicoverpa armigera in Chickpea. J. Agrometeorol. 2017, 19, 328–333. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Pál-Fám, F.; Kerepesi, I. Effect of Cotton Bollworm (Helicoverpa armigera Hübner) Caused Injury on Maize Grain Content, Especially Regarding to the Protein Alteration. Acta Biol. Hung. 2011, 62, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dömötör, I.; Kiss, J.; Szőcs, G. Coincidence of Silking Time of Corn, Zea mays and Flight Period of Cotton Bollworm, Helicoverpa armigera Hbn.: How Does It Affect Follow-up Abundancy of Larvae on Cobs and Grain Damage in Various Corn Hybrids? Acta Phytopathol. Entomol. Hung. 2009, 44, 315–326. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Puskás, J.; Nowinszky, L. Light-Trap Catch of Cotton Bollworm, Helicoverpa armigera in Connection with the Moon Phases and Geomagnetic H-Index. Biologia 2019, 74, 661–666. [Google Scholar] [CrossRef]
- Nowinszky, L.; Puskás, J. Light Trapping of Helicoverpa armigera in India and Hungary in Relation with the Moon Phases. Indian J. Agric. Sci. 2011, 81, 154–157. [Google Scholar]
- Pan, H.; Xu, Y.; Liang, G.; Wyckhuys, K.A.G.; Yang, Y.; Lu, Y. Field Evaluation of Light-Emitting Diodes to Trap the Cotton Bollworm, Helicoverpa armigera. Crop Prot. 2020, 137, 105267. [Google Scholar] [CrossRef]
- Nesbitt, B.F.; Beevor, P.S.; Hall, D.R.; Lester, R. Female Sex Pheromone Components of the Cotton Bollworm, Heliothis armigera. J. Insect Physiol. 1979, 25, 535–541. [Google Scholar] [CrossRef]
- Flórián, N.; Jósvai, J.K.; Tóth, Z.; Gergócs, V.; Sipőcz, L.; Tóth, M.; Dombos, M. Automatic Detection of Moths (Lepidoptera) with a Funnel Trap Prototype. Insects 2023, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Duffield, S.J.; Steer, A.P. The Ecology of Helicoverpa Spp. (Lepidoptera: Noctuidae) in the Riverina Region of South-Eastern Australia and the Implications for Tactical and Strategic Management. Bull. Entomol. Res. 2006, 96, 583–596. [Google Scholar] [CrossRef]
- Narava, R.; Kumar D V, S.R.; Jaba, J.; Kumar P, A.; Rao G V, R.; Rao V, S.; Mishra, S.P.; Kukanur, V. Development of Temporal Model for Forecasting of Helicoverpa armigera (Noctuidae: Lepidopetra) Using Arima and Artificial Neural Networks. J. Insect Sci. 2022, 22, 2. [Google Scholar] [CrossRef]
- Jallow, M.; Matsumura, M. Influence of Temperature on the Rate of Development of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2001, 36, 427–430. [Google Scholar] [CrossRef][Green Version]
- Mironidis, G.K. Development, Survivorship and Reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under Fluctuating Temperatures. Bull. Entomol. Res. 2014, 104, 751–764. [Google Scholar] [CrossRef]
- Mathukumalli, S.R.; Dammu, M.; Sengottaiyan, V.; Ongolu, S.; Biradar, A.K.; Kondru, V.R.; Karlapudi, S.; Bellapukonda, M.K.R.; Chitiprolu, R.R.A.; Cherukumalli, S.R. Prediction of Helicoverpa armigera Hubner on Pigeonpea during Future Climate Change Periods Using MarkSim Multimodel Data. Agric. For. Meteorol. 2016, 228–229, 130–138. [Google Scholar] [CrossRef]
- Kaneko, S.; Shirotsuka, K.; Shibao, M. Forecast of Peak Dates of Adult Emergence of Helicoverpa armigera in Osaka Prefecture by Using a Simulation Program Based on the Total Effective Temperature Provided by JPP-NET. Annu. Rep. Kansai Plant Prot. Soc. 2017, 59, 105–108. [Google Scholar] [CrossRef]
- Damos, P.; Papathanasiou, F.; Tsikos, E.; Kyriakidis, T.; Louta, M. Bayesian Non-Parametric Thermal Thresholds for Helicoverpa armigera and Their Integration into a Digital Plant Protection System. Agronomy 2022, 12, 2474. [Google Scholar] [CrossRef]
- Blum, M.; Nestel, D.; Cohen, Y.; Goldshtein, E.; Helman, D.; Lensky, I.M. Predicting Heliothis (Helicoverpa armigera) Pest Population Dynamics with an Age-Structured Insect Population Model Driven by Satellite Data. Ecol. Model. 2018, 369, 1–12. [Google Scholar] [CrossRef]
- Jokar, M. A Thermal Forecasting Model for the Overwintering Generation of Cotton Bollworm by Remote Sensing in the Southeast of Caspian Sea. Span. J. Agric. Res. 2022, 20, 1001. [Google Scholar] [CrossRef]
- Jokar, M.; López-Bernal, Á.; Kamkar, B. The Effect of Spring Flooding on Management and Distribution of Cotton Bollworm (Helicoverpa armigera) by Flood Mapping Using SAR Sentinel-1 and Optical Imagery Landsat-8; a Case Study in Golestan Province, Iran. Int. J. Pest Manag. 2022, 1–11. [Google Scholar] [CrossRef]
- Kumari, P.; Mishra, G.C.; Srivastava, C.P. Forecasting of Productivity and Pod Damage by Helicoverpa armigera Using Artificial Neural Network Model in Pigeonpea (Cajanus cajan). Int. J. Agric. Environ. Biotechnol. 2013, 6, 335–340. [Google Scholar]
- Perkins, L.E.; Cribb, B.W.; Hanan, J.; Zalucki, M.P. The Movement and Distribution of Helicoverpa armigera (Hübner) Larvae on Pea Plants Is Affected by Egg Placement and Flowering. Bull. Entomol. Res. 2010, 100, 591–598. [Google Scholar] [CrossRef]
- Gurung, B.; Dutta, S.; Singh, K.N.; Lama, A.; Vennila, S.; Gurung, B. The Development of a Statistical Model for Forewarning Helicoverpa armigera Infestation Using Beta Regression. J. Crop Weed 2023, 19, 210–215. [Google Scholar] [CrossRef]
- Sári-Barnácz, F.E.; Szalai, M.; Kun, M.; Iványi, D.; Chaddadi, M.; Barnácz, F.M.; Kiss, J. Satellite-Based Spectral Indices for Monitoring Helicoverpa armigera Damage in Maize. In Precision Agriculture? 21; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 209–216. ISBN 978-90-8686-363-1. [Google Scholar]
- Wei, G.; Zhang, Q.; Zhou, M.; Wu, W. Characteristic response of the compound eyes of Helicoverpa armigera to light. Acta Entomol. Sin. 2002, 45, 323–328. [Google Scholar]
- Gu, G.; Chen, X.; Han, J.; Ge, H.; Chen, H. Study on Phototaxis Action of Moth of Cotton Bollwor. J. Tianjin Agric. Coll. 2004, 11, 32–36. [Google Scholar]
- Jing, X.; Luo, F.; Zhu, F.; Huang, Q.; Lei, C. Effects of different light source and dark-adapted time on phototactic behavior of cotton bollworms (Helicoverpa armigera). Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2005, 16, 586–588. [Google Scholar]
- JungBeom, Y.; Nomura, M.; Ishikura, S. Analysis of the flight activity of the cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) under yellow LED lighting. Jpn. J. Appl. Entomol. Zool. 2012, 56, 103–110. [Google Scholar]
- Satoh, A.; Kinoshita, M.; Arikawa, K. Innate Preference and Learning of Colour in the Male Cotton Bollworm Moth, Helicoverpa armigera. J. Exp. Biol. 2016, 219, 3857–3860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.; Zhang, S.; Jiang, X.; Yang, B.; Wang, G. Comparison of Phototactic Behavior between Two Migratory Pests, Helicoverpa armigera and Spodoptera frugiperda. Insects 2022, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Karakantza, E.; Rumbos, C.I.; Cavalaris, C.; Athanassiou, C.G. Different Trap Types Depict Dissimilar Spatio-Temporal Distribution of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Cotton Fields. Agronomy 2023, 13, 1256. [Google Scholar] [CrossRef]
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote Sensing for Precision Agriculture: Sentinel-2 Improved Features and Applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A Review: Application of Remote Sensing as a Promising Strategy for Insect Pests and Diseases Management. Environ. Sci. Pollut. Res. Int. 2020, 27, 33503–33515. [Google Scholar] [CrossRef]
- Jindo, K.; Kozan, O.; Iseki, K.; Maestrini, B.; van Evert, F.K.; Wubengeda, Y.; Arai, E.; Shimabukuro, Y.E.; Sawada, Y.; Kempenaar, C. Potential Utilization of Satellite Remote Sensing for Field-Based Agricultural Studies. Chem. Biol. Technol. Agric. 2021, 8, 58. [Google Scholar] [CrossRef]
- Rhodes, M.W.; Bennie, J.J.; Spalding, A.; ffrench-Constant, R.H.; Maclean, I.M.D. Recent Advances in the Remote Sensing of Insects. Biol. Rev. 2022, 97, 343–360. [Google Scholar] [CrossRef]
- Ashraf, A.; Ahmad, L.; Ferooz, K.; Ramzan, S.; Ashraf, I.; Khan, J.N.; Shehnaz, E.; Ul-Shafiq, M.; Akhter, S.; Nabi, A.; et al. Remote Sensing as a Management and Monitoring Tool for Agriculture: Potential Applications. Int. J. Environ. Clim. Chang. 2023, 13, 324–343. [Google Scholar] [CrossRef]
- Prabhakar, M.; Gopinath, K.A.; Kumar, N.R.; Thirupathi, M.; Sravan, U.S.; Kumar, G.S.; Siva, G.S.; Meghalakshmi, G.; Vennila, S. Detecting the Invasive Fall Armyworm Pest Incidence in Farm Fields of Southern India Using Sentinel-2A Satellite Data. Geocarto Int. 2022, 37, 3801–3816. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, H.; Roques, A. Early Monitoring of Forest Wood-Boring Pests with Remote Sensing. Annu. Rev. Entomol. 2023, 68, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Viña, A.; Gitelson, A.A.; Rundquist, D.C.; Keydan, G.; Leavitt, B.; Schepers, J. Monitoring Maize (Zea mays L.) Phenology with Remote Sensing. Agron. J. 2004, 96, 1139–1147. [Google Scholar] [CrossRef]
- Blickensdörfer, L.; Schwieder, M.; Pflugmacher, D.; Nendel, C.; Erasmi, S.; Hostert, P. Mapping of Crop Types and Crop Sequences with Combined Time Series of Sentinel-1, Sentinel-2 and Landsat 8 Data for Germany. Remote Sens. Environ. 2022, 269, 112831. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Revealing Annual Crop Type Distribution and Spatiotemporal Changes in Northeast China Based on Google Earth Engine. Remote Sens. 2022, 14, 4056. [Google Scholar] [CrossRef]
- Guo, Z.; Qi, W.; Huang, Y.; Zhao, J.; Yang, H.; Koo, V.-C.; Li, N. Identification of Crop Type Based on C-AENN Using Time Series Sentinel-1A SAR Data. Remote Sens. 2022, 14, 1379. [Google Scholar] [CrossRef]
- Abubakar, G.A.; Wang, K.; Koko, A.F.; Husseini, M.I.; Shuka, K.A.M.; Deng, J.; Gan, M. Mapping Maize Cropland and Land Cover in Semi-Arid Region in Northern Nigeria Using Machine Learning and Google Earth Engine. Remote Sens. 2023, 15, 2835. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Leung, H.; Zhang, G. Super-Resolution Mapping Based on Spatial–Spectral Correlation for Spectral Imagery. IEEE Trans. Geosci. Remote Sens. 2021, 59, 2256–2268. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Ren, T.; Liu, D.; Ma, Z.; Tong, L.; Zhang, C.; Zhou, T.; Zhang, X.; Li, S. Identification of Seed Maize Fields With High Spatial Resolution and Multiple Spectral Remote Sensing Using Random Forest Classifier. Remote Sens. 2020, 12, 362. [Google Scholar] [CrossRef]
- Chivasa, W.; Mutanga, O.; Biradar, C. Phenology-Based Discrimination of Maize (Zea mays L.) Varieties Using Multitemporal Hyperspectral Data. J. Appl. Remote Sens. 2019, 13, 017504. [Google Scholar] [CrossRef]
- Adak, A.; Murray, S.C.; Božinović, S.; Lindsey, R.; Nakasagga, S.; Chatterjee, S.; Anderson, S.L.; Wilde, S. Temporal Vegetation Indices and Plant Height from Remotely Sensed Imagery Can Predict Grain Yield and Flowering Time Breeding Value in Maize via Machine Learning Regression. Remote Sens. 2021, 13, 2141. [Google Scholar] [CrossRef]
- Nieto, L.; Houborg, R.; Zajdband, A.; Jumpasut, A.; Prasad, P.V.V.; Olson, B.J.S.C.; Ciampitti, I.A. Impact of High-Cadence Earth Observation in Maize Crop Phenology Classification. Remote Sens. 2022, 14, 469. [Google Scholar] [CrossRef]
- Marshall, M.; Belgiu, M.; Boschetti, M.; Pepe, M.; Stein, A.; Nelson, A. Field-Level Crop Yield Estimation with PRISMA and Sentinel-2. ISPRS J. Photogramm. Remote Sens. 2022, 187, 191–210. [Google Scholar] [CrossRef]
- Pahlevan, N.; Mangin, A.; Balasubramanian, S.V.; Smith, B.; Alikas, K.; Arai, K.; Barbosa, C.; Bélanger, S.; Binding, C.; Bresciani, M.; et al. ACIX-Aqua: A Global Assessment of Atmospheric Correction Methods for Landsat-8 and Sentinel-2 over Lakes, Rivers, and Coastal Waters. Remote Sens. Environ. 2021, 258, 112366. [Google Scholar] [CrossRef]
- Lang, S.; Li, G.; Liu, Y.; Lu, W.; Zhang, Q.; Chao, K. A GAN-Based Augmentation Scheme for SAR Deceptive Jamming Templates with Shadows. Remote Sens. 2023, 15, 4756. [Google Scholar] [CrossRef]
- Doxani, G.; Vermote, E.F.; Roger, J.-C.; Skakun, S.; Gascon, F.; Collison, A.; De Keukelaere, L.; Desjardins, C.; Frantz, D.; Hagolle, O.; et al. Atmospheric Correction Inter-Comparison eXercise, ACIX-II Land: An Assessment of Atmospheric Correction Processors for Landsat 8 and Sentinel-2 over Land. Remote Sens. Environ. 2023, 285, 113412. [Google Scholar] [CrossRef]
- Bossung, C.; Schlerf, M.; Machwitz, M. Estimation of Canopy Nitrogen Content in Winter Wheat from Sentinel-2 Images for Operational Agricultural Monitoring. Precis. Agric. 2022, 23, 2229–2252. [Google Scholar] [CrossRef]
- Uribeetxebarria, A.; Castellón, A.; Aizpurua, A. A First Approach to Determine If It Is Possible to Delineate In-Season N Fertilization Maps for Wheat Using NDVI Derived from Sentinel-2. Remote Sens. 2022, 14, 2872. [Google Scholar] [CrossRef]
- Han, D.; Liu, S.; Du, Y.; Xie, X.; Fan, L.; Lei, L.; Li, Z.; Yang, H.; Yang, G. Crop Water Content of Winter Wheat Revealed with Sentinel-1 and Sentinel-2 Imagery. Sensors 2019, 19, 4013. [Google Scholar] [CrossRef]
- Avetisyan, D.; Nedkov, R.; Georgiev, N. Monitoring Maize (Zea mays L.) Phenology Response to Water Deficit Using Sentinel-2 Multispectral Data. In Proceedings of the Eighth International Conference on Remote Sensing and Geoinformation of the Environment (RSCy2020), Paphos, Cyprus, 16–18 March 2020; SPIE: Bellingham, WA, USA, 2020; Volume 11524, pp. 9–17. [Google Scholar]
- Ruan, C.; Dong, Y.; Huang, W.; Huang, L.; Ye, H.; Ma, H.; Guo, A.; Ren, Y. Prediction of Wheat Stripe Rust Occurrence with Time Series Sentinel-2 Images. Agriculture 2021, 11, 1079. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ren, B.; Huang, L.; Zheng, Q.; Ma, H. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Dhau, I.; Dube, T.; Mushore, T.D. Examining the Prospects of Sentinel-2 Multispectral Data in Detecting and Mapping Maize Streak Virus Severity in Smallholder Ofcolaco Farms, South Africa. Geocarto Int. 2021, 36, 1873–1883. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Dube, T.; Odindi, J.; Mafongoya, P.L. The Utility of the Upcoming HyspIRI’s Simulated Spectral Settings in Detecting Maize Gray Leafy Spot in Relation to Sentinel-2 MSI, VENµS, and Landsat 8 OLI Sensors. Agronomy 2019, 9, 846. [Google Scholar] [CrossRef]
- Ma, H.; Huang, W.; Jing, Y.; Yang, C.; Han, L.; Dong, Y.; Ye, H.; Shi, Y.; Zheng, Q.; Liu, L.; et al. Integrating Growth and Environmental Parameters to Discriminate Powdery Mildew and Aphid of Winter Wheat Using Bi-Temporal Landsat-8 Imagery. Remote Sens. 2019, 11, 846. [Google Scholar] [CrossRef]
- Shi, Y.; Han, L.; González-Moreno, P.; Dancey, D.; Huang, W.; Zhang, Z.; Liu, Y.; Huang, M.; Miao, H.; Dai, M. A Fast Fourier Convolutional Deep Neural Network for Accurate and Explainable Discrimination of Wheat Yellow Rust and Nitrogen Deficiency from Sentinel-2 Time Series Data. Front. Plant Sci. 2023, 14, 1250844. [Google Scholar] [CrossRef]
- Buchaillot, M.L.; Cairns, J.; Hamadziripi, E.; Wilson, K.; Hughes, D.; Chelal, J.; McCloskey, P.; Kehs, A.; Clinton, N.; Araus, J.L.; et al. Regional Monitoring of Fall Armyworm (FAW) Using Early Warning Systems. Remote Sens. 2022, 14, 5003. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, H.; Dong, Y.; Huang, W.; Hu, G.; Liu, Y.; Chen, H.; Geng, Y.; Bai, J.; Guo, P.; et al. Mapping the Spatio-Temporal Distribution of Fall Armyworm in China by Coupling Multi-Factors. Remote Sens. 2022, 14, 4415. [Google Scholar] [CrossRef]
- Obasekore, H.; Fanni, M.; Ahmed, S.M.; Parque, V.; Kang, B.-Y. Agricultural Robot-Centered Recognition of Early-Developmental Pest Stage Based on Deep Learning: A Case Study on Fall Armyworm (Spodoptera frugiperda). Sensors 2023, 23, 3147. [Google Scholar] [CrossRef]
- Adan, M.; Tonnang, H.E.Z.; Greve, K.; Borgemeister, C.; Goergen, G. Use of Time Series Normalized Difference Vegetation Index (NDVI) to Monitor Fall Armyworm (Spodoptera frugiperda) Damage on Maize Production Systems in Africa. Geocarto Int. 2023, 38, 2186492. [Google Scholar] [CrossRef]
- Rajapakse, C.N.K.; Walter, G.H. Polyphagy and Primary Host Plants: Oviposition Preference versus Larval Performance in the Lepidopteran Pest Helicoverpa armigera. Arthropod-Plant Interact 2007, 1, 17–26. [Google Scholar] [CrossRef]
- Open Access Hub. Available online: https://scihub.copernicus.eu/ (accessed on 16 October 2023).
- Arlula—Access a Global Network of Satellite Imagery in Seconds. Available online: https://www.arlula.com/ (accessed on 16 October 2023).
- Sola, I.; García-Martín, A.; Sandonís-Pozo, L.; Álvarez-Mozos, J.; Pérez-Cabello, F.; González-Audícana, M.; Montorio Llovería, R. Assessment of Atmospheric Correction Methods for Sentinel-2 Images in Mediterranean Landscapes. Int. J. Appl. Earth Obs. Geoinform. 2018, 73, 63–76. [Google Scholar] [CrossRef]
- Main-Knorn, M.; Pflug, B.; Debaecker, V.; Louis, J. Calibration and validation plan for the l2a processor and products of the sentinel-2 mission. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, XL-7-W3, 1249–1255. [Google Scholar] [CrossRef]
- Congedo, L. Semi-Automatic Classification Plugin: A Python Tool for the Download and Processing of Remote Sensing Images in QGIS. J. Open Source Softw. 2021, 6, 3172. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and Physiological Basis for the Reflectance of Visible and Near-Infrared Radiation from Vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Ghamghami, M.; Ghahreman, N.; Irannejad, P.; Ghorbani, K. Comparison of Data Mining and GDD-Based Models in Discrimination of Maize Phenology. Int. J. Plant Prod. 2019, 13, 11–22. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the Radiometric and Biophysical Performance of the MODIS Vegetation Indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Ullah, M.I.; Arshad, M.; Khan, M.I.; Afzal, M.; Khan, A.A.; Zahid, S.M.A.; Saqib, M.; Abdullah, A.; Kousar, S.; Riaz, M. Plant Water Stress Affects the Feeding Performance of American Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) on Cotton Plants. Pak. J. Agric. Res. 2019, 34, 629–635. [Google Scholar] [CrossRef]
- Goodwin, N.R.; Coops, N.C.; Wulder, M.A.; Gillanders, S.; Schroeder, T.A.; Nelson, T. Estimation of Insect Infestation Dynamics Using a Temporal Sequence of Landsat Data. Remote Sens. Environ. 2008, 112, 3680–3689. [Google Scholar] [CrossRef]
- McFeeters, S.K. The Use of the Normalized Difference Water Index (NDWI) in the Delineation of Open Water Features. Int. J. Remote Sens. 1996, 17, 1425–1432. [Google Scholar] [CrossRef]
- Gao, B. NDWI—A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water from Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Weber, E.; Bleiholder, H. Erläuterungen Zu Den BBCH-Dezimal-Codes Für Die Entwicklungsstadien von Mais, Raps, Faba-Bohne, Sonnenblume Und Erbse-Mit Abbildungen. Gesunde Pflanz. 1990, 42, 308–321. [Google Scholar]
- Marton, L.C.; Szieberth, D.; Csuros, M. New Method to Determine FAO Number of Maize, Zea mays L. Genetika 2004, 36, 83–92. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S. Least Squares Quantization in PCM. IEEE Trans. Inf. Theory 1982, 28, 129–137. [Google Scholar] [CrossRef]
- Mengarelli, F.; Spoglianti, S.; Avenanti, A.; di Pellegrino, G. Cathodal tDCS Over the Left Prefrontal Cortex Diminishes Choice-Induced Preference Change. Cereb. Cortex 2015, 25, 1219–1227. [Google Scholar] [CrossRef][Green Version]
- Vallentin, C.; Harfenmeister, K.; Itzerott, S.; Kleinschmit, B.; Conrad, C.; Spengler, D. Suitability of Satellite Remote Sensing Data for Yield Estimation in Northeast Germany. Precis. Agric. 2022, 23, 52–82. [Google Scholar] [CrossRef]
- Alhassan, R.K.; Nketiah-Amponsah, E.; Afaya, A.; Salia, S.M.; Abuosi, A.A.; Nutor, J.J. Global Health Security Index Not a Proven Surrogate for Health Systems Capacity to Respond to Pandemics: The Case of COVID-19. J. Infect. Public Health 2023, 16, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Vuković, S.; Indić, D.; Grahovac, M.; Franeta, F. Protection of sweet corn from Ostrinia nubilalis hbn. and Helicoverpa armigera hbn. Commun. Agric. Appl. Biol. Sci. 2015, 80, 161–167. [Google Scholar] [PubMed]
- R Studio Team. R Studio: Integrated Development Environment for R; R Studio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. 2023. Available online: https://dplyr.tidyverse.org (accessed on 17 April 2023).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Package “Emmeans”. 2018. Available online: http://cran.r-project.org/package=emmeans (accessed on 17 April 2023).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Meyer, L.H.; Heurich, M.; Beudert, B.; Premier, J.; Pflugmacher, D. Comparison of Landsat-8 and Sentinel-2 Data for Estimation of Leaf Area Index in Temperate Forests. Remote Sens. 2019, 11, 1160. [Google Scholar] [CrossRef]
- Ghayour, L.; Neshat, A.; Paryani, S.; Shahabi, H.; Shirzadi, A.; Chen, W.; Al-Ansari, N.; Geertsema, M.; Pourmehdi Amiri, M.; Gholamnia, M.; et al. Performance Evaluation of Sentinel-2 and Landsat 8 OLI Data for Land Cover/Use Classification Using a Comparison between Machine Learning Algorithms. Remote Sens. 2021, 13, 1349. [Google Scholar] [CrossRef]
- Nasiri, V.; Deljouei, A.; Moradi, F.; Sadeghi, S.M.M.; Borz, S.A. Land Use and Land Cover Mapping Using Sentinel-2, Landsat-8 Satellite Images, and Google Earth Engine: A Comparison of Two Composition Methods. Remote Sens. 2022, 14, 1977. [Google Scholar] [CrossRef]
- Chakhar, A.; Ortega-Terol, D.; Hernández-López, D.; Ballesteros, R.; Ortega, J.F.; Moreno, M.A. Assessing the Accuracy of Multiple Classification Algorithms for Crop Classification Using Landsat-8 and Sentinel-2 Data. Remote Sens. 2020, 12, 1735. [Google Scholar] [CrossRef]
- Faqe Ibrahim, G.R.; Rasul, A.; Abdullah, H. Sentinel-2 Accurately Estimated Wheat Yield in a Semi-Arid Region Compared with Landsat 8. Int. J. Remote Sens. 2023, 44, 4115–4136. [Google Scholar] [CrossRef]
- Najafi, P.; Eftekhari, A.; Sharifi, A. Evaluation of Time-Series Sentinel-2 Images for Early Estimation of Rice Yields in South-West of Iran. Aircr. Eng. Aerosp. Technol. 2023, 95, 741–748. [Google Scholar] [CrossRef]
- Achour, H.; Toujani, A.; Trabelsi, H.; Jaouadi, W. Evaluation and Comparison of Sentinel-2 MSI, Landsat 8 OLI, and EFFIS Data for Forest Fires Mapping. Illustrations from the Summer 2017 Fires in Tunisia. Geocarto Int. 2022, 37, 7021–7040. [Google Scholar] [CrossRef]
- Abdullah, H.; Skidmore, A.K.; Darvishzadeh, R.; Heurich, M. Sentinel-2 Accurately Maps Green-Attack Stage of European Spruce Bark Beetle (Ips typographus, L.) Compared with Landsat-8. Remote Sens. Ecol. Conserv. 2019, 5, 87–106. [Google Scholar] [CrossRef]
- Chakhvashvili, E.; Siegmann, B.; Muller, O.; Verrelst, J.; Bendig, J.; Kraska, T.; Rascher, U. Retrieval of Crop Variables from Proximal Multispectral UAV Image Data Using PROSAIL in Maize Canopy. Remote Sens. 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Wen, T.; Xu, J.; Huang, B.; Yan, S.; Gao, G.; Zhao, Y.; Li, H.; Qiao, J.; et al. On Correlation between Canopy Vegetation and Growth Indexes of Maize Varieties with Different Nitrogen Efficiencies. Open Life Sci. 2023, 18, 20220566. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Vergara-Díaz, O.; Thierfelder, C.; Cairns, J.E.; Kefauver, S.C.; Araus, J.L. Phenotyping Conservation Agriculture Management Effects on Ground and Aerial Remote Sensing Assessments of Maize Hybrids Performance in Zimbabwe. Remote Sens. 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Loladze, A.; Rodrigues, F.A.; Toledo, F.; San Vicente, F.; Gérard, B.; Boddupalli, M.P. Application of Remote Sensing for Phenotyping Tar Spot Complex Resistance in Maize. Front. Plant Sci. 2019, 10, 552. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Yang, H.; Xu, B.; Li, Z.; Yang, X. Clustering Field-Based Maize Phenotyping of Plant-Height Growth and Canopy Spectral Dynamics Using a UAV Remote-Sensing Approach. Front. Plant Sci. 2018, 9, 1638. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, W.; Rezaei, E.E.; Li, J.; Sun, Z.; Zhang, J. The Optimal Phenological Phase of Maize for Yield Prediction with High-Frequency UAV Remote Sensing. Remote Sens. 2022, 14, 1559. [Google Scholar] [CrossRef]
- Johnson, D.M. An Assessment of Pre- and within-Season Remotely Sensed Variables for Forecasting Corn and Soybean Yields in the United States. Remote Sens. Environ. 2014, 141, 116–128. [Google Scholar] [CrossRef]
- Skakun, S.; Kalecinski, N.I.; Brown, M.G.L.; Johnson, D.M.; Vermote, E.F.; Roger, J.-C.; Franch, B. Assessing Within-Field Corn and Soybean Yield Variability from WorldView-3, Planet, Sentinel-2, and Landsat 8 Satellite Imagery. Remote Sens. 2021, 13, 872. [Google Scholar] [CrossRef]
- Skakun, S.; Vermote, E.; Franch, B.; Roger, J.-C.; Kussul, N.; Ju, J.; Masek, J. Winter Wheat Yield Assessment from Landsat 8 and Sentinel-2 Data: Incorporating Surface Reflectance, Through Phenological Fitting, into Regression Yield Models. Remote Sens. 2019, 11, 1768. [Google Scholar] [CrossRef]
- Hartlieb, E.; Rembold, H. Behavioral Response of female Helicoverpa (Heliothis) armigera HB. (Lepidoptera: Noctuidae) Moths to Synthetic Pigeonpea (Cajanus cajan L.) Kairomone. J. Chem. Ecol. 1996, 22, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Røstelien, T.; Stranden, M.; Borg-Karlson, A.-K.; Mustaparta, H. Olfactory Receptor Neurons in Two Heliothine Moth Species Responding Selectively to Aliphatic Green Leaf Volatiles, Aromatic Compounds, Monoterpenes and Sesquiterpenes of Plant Origin. Chem. Senses 2005, 30, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Gregg, P.C.; Del Socorro, A.P.; Henderson, G.S. Development of a Synthetic Plant Volatile-Based Attracticide for Female Noctuid Moths. II. Bioassays of Synthetic Plant Volatiles as Attractants for the Adults of the Cotton Bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- Kuester, T.; Spengler, D. Structural and Spectral Analysis of Cereal Canopy Reflectance and Reflectance Anisotropy. Remote Sens. 2018, 10, 1767. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Cibils-Stewart, X.; Wuhrer, R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Leaf Silicification Provides Herbivore Defence Regardless of the Extensive Impacts of Water Stress. Funct. Ecol. 2021, 35, 1200–1211. [Google Scholar] [CrossRef]
- Srinivasa Rao, M.; Rama Rao, C.A.; Raju, B.M.K.; Subba Rao, A.V.M.; Gayatri, D.L.A.; Islam, A.; Prasad, T.V.; Navya, M.; Srinivas, K.; Pratibha, G.; et al. Pest Scenario of Helicoverpa armigera (Hub.) on Pigeonpea during Future Climate Change Periods under RCP Based Projections in India. Sci. Rep. 2023, 13, 6788. [Google Scholar] [CrossRef]



| Sentinel-2 | Landsat 8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spectral Band | Central Wavelength (nm) | Bandwidth (nm) | Resolution | Resolution of Use (m) | Spectral Band | Central Wavelength (nm) | Bandwidth (nm) | Resolution | Resolution of Use (m) | |
| Blue | B02 | 492.7 | 65 | 10 | 20 | B2 | 482 | 60 | 30 | 30 |
| Green | B03 | 559.8 | 35 | 10 | B3 | 561.5 | 57 | 30 | ||
| Red | B04 | 664.6 | 30 | 10 | B4 | 654.5 | 37 | 30 | ||
| Red-edge | B05 | 704.1 | 14 | 20 | ||||||
| Red-edge | B06 | 740.5 | 14 | 20 | ||||||
| Red-edge | B07 | 782.8 | 19 | 20 | ||||||
| Near Infrared (NIR) | B08 | 832.8 | 105 | 10 | B5 | 865 | 28 | 30 | ||
| Short-Wave Infrared (SWIR) | B11 | 1613.7 | 90 | 20 | B6 | 1608.5 | 85 | 30 | ||
| Short-Wave Infrared (SWIR) | B12 | 2202.4 | 174 | 20 | B7 | 2200.5 | 187 | 30 | ||
| Abbr. | Name | Sentinel-2 | Landsat 8 | |
|---|---|---|---|---|
| Moisture-related vegetation indices | ||||
| NDWI | normalized difference water index | [90] | ||
| NDMI | normalized difference moisture index | [91] | ||
| Pigment-related vegetation indices | ||||
| NPCRI | normalized pigment chlorophyll ratio index | [86] | ||
| ARI | anthocyanin reflectance index | - | [86] | |
| CRI | carotenoid reflectance index | [87] | ||
| General vegetation indices | ||||
| EVI | enhanced vegetation index | [84] | ||
| NDVI | normalized difference vegetation index | [81] | ||
| SAVI | soil adjusted vegetation index | [82] | ||
| Senescence- and ripening-related vegetation index | ||||
| PSRI | plant senescence reflectance index | [85] | ||
| Pearson Correlation Coefficient | Interpretation | |
|---|---|---|
| −1 | 1 | Perfect |
| −0.95–−0.99 | +0.95–+0.99 | Very Strong |
| −0.75–−0.95 | +0.75–+0.95 | Strong |
| −0.3–−0.75 | +0.3–+0.75 | Moderate |
| −0.1–−0.3 | +0.1–+0.3 | Low |
| 0–−0.1 | 0–+0.1 | No correlation |
| Year | Farm | Field | Cultivation Purpose | Mean ± SD 1 | CLD 2 | Median | Min | Max | CV 3 | Mean ± SD of Farms | CLD | Mean ± SD of the Year | CLD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Farm 1 | F1_1 | Grain | 48.8 | ± | 11.9 | abc | 47.0 | 28.0 | 68.8 | 0.24 | 35.6 | ± | 14.7 | AB | 35.6 | ± | 14.7 | a |
| F1_2 | Grain | 32.1 | ± | 6.7 | adefg | 33.3 | 19.4 | 38.9 | 0.21 | ||||||||||
| F1_3 | Grain | 23.0 | ± | 10.8 | ehi | 20.8 | 8.3 | 39.4 | 0.47 | ||||||||||
| 2020 | Farm 1 | F1_1 | Grain | 53.9 | ± | 18.6 | bc | 53.5 | 19.4 | 80.6 | 0.35 | 46.9 | ± | 14.3 | A | 34.5 | ± | 16.7 | a |
| F1_4 | Grain | 44.7 | ± | 10.8 | bf | 46.0 | 29.7 | 67.7 | 0.24 | ||||||||||
| F1_5 | Grain | 40.0 | ± | 6.5 | efg | 41.7 | 33.3 | 50.0 | 0.16 | ||||||||||
| Farm 2 | F2_2 | Grain | 43.2 | ± | 7.9 | cf | 44.6 | 29.7 | 59.5 | 0.18 | 34.5 | ± | 10.4 | BC | |||||
| F2_3 | Grain | 34.1 | ± | 9.5 | adefg | 31.1 | 24.3 | 51.3 | 0.28 | ||||||||||
| F2_4 | Grain | 30.4 | ± | 10.8 | defg | 25.7 | 16.2 | 48.7 | 0.35 | ||||||||||
| F2_5 | Grain | 31.1 | ± | 9.0 | defg | 29.7 | 18.9 | 48.7 | 0.29 | ||||||||||
| Farm 3 | Nm1 | Sweet | 21.0 | ± | 12.9 | dhi | 14.3 | 8.6 | 42.9 | 0.61 | 25.8 | ± | 18.5 | B | |||||
| Nm2 | Sweet | 1.7 | ± | 2.4 | j | 0.0 | 0.0 | 5.7 | 1.40 | ||||||||||
| F3_3 | Grain | 41.7 | ± | 5.4 | cfg | 41.4 | 31.4 | 51.4 | 0.13 | ||||||||||
| F3_4 | Grain | 38.3 | ± | 11.5 | cefg | 37.1 | 20.0 | 60.0 | 0.30 | ||||||||||
| 2021 | Farm 2 | Gy1 | Grain | 26.2 | ± | 12.1 | gi | 23.0 | 10.8 | 48.7 | 0.46 | 30.5 | ± | 12.6 | BC | 32.4 | ± | 20.0 | a |
| Gy2 | Grain | 38.6 | ± | 11.1 | cefg | 33.8 | 24.3 | 54.0 | 0.29 | ||||||||||
| Gy3 | Grain | 26.5 | ± | 11.4 | gi | 24.3 | 16.2 | 54.0 | 0.43 | ||||||||||
| Farm 3 | Kd | Grain | 60.0 | ± | 8.7 | b | 58.0 | 44.0 | 76.0 | 0.14 | 37.2 | ± | 25.6 | AC | |||||
| Nm1 | Sweet | 10.5 | ± | 4.8 | ij | 10.0 | 4.0 | 20.0 | 0.45 | ||||||||||
| Nm2 | Sweet | 6.3 | ± | 3.9 | hj | 4.0 | 4.0 | 12.0 | 0.62 | ||||||||||
| Nm5 | Grain | 54.7 | ± | 7.2 | bc | 52.0 | 44.0 | 68.0 | 0.13 | ||||||||||
| All | 33.9 | ± | 17.7 | 33.3 | 0.0 | 80.6 | 0.5 | ||||||||||||
| Sentinel-2 | Landsat 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | ||
| B02 | 0.11 | 0.42 | −0.85 | 0.90 | B2 | −0.04 | 0.40 | −0.84 | 0.65 |
| B03 | 0.11 | 0.44 | −0.86 | 0.90 | B3 | 0.00 | 0.42 | −0.84 | 0.69 |
| B04 | 0.09 | 0.42 | −0.86 | 0.87 | B4 | −0.03 | 0.40 | −0.88 | 0.72 |
| B05 | 0.10 | 0.43 | −0.89 | 0.88 | |||||
| B06 | 0.04 | 0.42 | −0.89 | 0.88 | |||||
| B07 | 0.01 | 0.42 | −0.86 | 0.86 | |||||
| B8A | 0.01 | 0.42 | −0.84 | 0.85 | B5 | 0.12 | 0.38 | −0.72 | 0.80 |
| B11 | 0.06 | 0.39 | −0.92 | 0.85 | B6 | 0.00 | 0.38 | −0.87 | 0.65 |
| B12 | 0.07 | 0.42 | −0.93 | 0.84 | B7 | −0.01 | 0.40 | −0.92 | 0.67 |
| ARI*1000 | −0.13 | 0.38 | −0.92 | 0.79 | CRI*1000 | ||||
| CRI*1000 | −0.08 | 0.40 | −0.89 | 0.87 | 0.11 | 0.41 | −0.76 | 0.93 | |
| EVI | −0.05 | 0.41 | −0.87 | 0.86 | EVI | 0.03 | 0.42 | −0.80 | 0.90 |
| NDMI | −0.04 | 0.45 | −0.82 | 0.90 | NDMI | 0.09 | 0.43 | −0.70 | 0.92 |
| NDVI | −0.08 | 0.43 | −0.85 | 0.84 | NDVI | 0.10 | 0.41 | −0.76 | 0.79 |
| NDWI | 0.09 | 0.43 | −0.82 | 0.88 | NDWI | −0.10 | 0.39 | −0.73 | 0.75 |
| NPCRI | −0.02 | 0.40 | −0.80 | 0.92 | NPCRI | 0.02 | 0.42 | −0.88 | 0.85 |
| PSRI | 0.04 | 0.41 | −0.85 | 0.89 | PRSI | −0.02 | 0.41 | −0.88 | 0.80 |
| SAVI | −0.08 | 0.43 | −0.85 | 0.84 | SAVI | 0.10 | 0.41 | −0.76 | 0.79 |
| All | 0.015 | 0.42 | −0.93 | 0.92 | All | 0.021 | 0.41 | −0.92 | 0.93 |
| Band/Index | Mid–Early Grain Maize (FAO 300 Group) | Mid–Late Grain Maize (FAO 400 Group) | Sweet Maize | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | Mean | Median | SD | ||
| Visible bands | B02 | −0.21 | −0.28 | 0.28 | 0.44 | 0.51 | 0.33 | −0.06 | 0.00 | 0.42 |
| B03 | −0.23 | −0.27 | 0.27 | 0.42 | 0.50 | 0.39 | −0.01 | 0.02 | 0.43 | |
| B04 | −0.21 | −0.30 | 0.32 | 0.46 | 0.49 | 0.27 | −0.09 | −0.22 | 0.44 | |
| Red-edge bands | B05 | −0.23 | −0.26 | 0.30 | 0.44 | 0.50 | 0.31 | −0.02 | 0.06 | 0.43 |
| B06 | 0.08 | 0.06 | 0.31 | 0.07 | 0.07 | 0.49 | 0.05 | 0.07 | 0.53 | |
| B07 | 0.18 | 0.23 | 0.30 | −0.10 | −0.18 | 0.46 | 0.08 | 0.05 | 0.53 | |
| NIR band | B8A | 0.18 | 0.23 | 0.29 | −0.09 | −0.20 | 0.46 | 0.08 | 0.07 | 0.52 |
| SWIR bands | B11 | −0.20 | −0.27 | 0.33 | 0.34 | 0.39 | 0.28 | −0.06 | −0.16 | 0.49 |
| B12 | −0.20 | −0.29 | 0.34 | 0.38 | 0.49 | 0.29 | −0.14 | −0.41 | 0.50 | |
| Pigment-based VIs | ARI | 0.02 | 0.02 | 0.28 | −0.28 | −0.33 | 0.41 | 0.03 | 0.03 | 0.43 |
| CRI | 0.16 | 0.17 | 0.31 | −0.35 | −0.35 | 0.32 | 0.04 | −0.06 | 0.48 | |
| NPCRI | −0.15 | −0.21 | 0.30 | 0.21 | 0.33 | 0.43 | −0.13 | −0.23 | 0.41 | |
| Water-based VIs | NDMI | 0.21 | 0.28 | 0.34 | −0.22 | −0.35 | 0.43 | 0.09 | 0.15 | 0.55 |
| NDWI | −0.25 | −0.28 | 0.32 | 0.43 | 0.46 | 0.30 | −0.16 | −0.27 | 0.47 | |
| General VIs | EVI | 0.17 | 0.25 | 0.32 | −0.34 | −0.44 | 0.37 | 0.14 | 0.13 | 0.42 |
| NDVI | 0.22 | 0.28 | 0.33 | −0.41 | −0.45 | 0.33 | 0.16 | 0.34 | 0.47 | |
| SAVI | 0.22 | 0.28 | 0.33 | −0.41 | −0.45 | 0.33 | 0.16 | 0.34 | 0.47 | |
| Senescence | PSRI | −0.17 | −0.25 | 0.32 | 0.33 | 0.42 | 0.38 | −0.15 | −0.24 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sári-Barnácz, F.E.; Zalai, M.; Toepfer, S.; Milics, G.; Iványi, D.; Tóthné Kun, M.; Mészáros, J.; Árvai, M.; Kiss, J. Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae. Remote Sens. 2023, 15, 5602. https://doi.org/10.3390/rs15235602
Sári-Barnácz FE, Zalai M, Toepfer S, Milics G, Iványi D, Tóthné Kun M, Mészáros J, Árvai M, Kiss J. Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae. Remote Sensing. 2023; 15(23):5602. https://doi.org/10.3390/rs15235602
Chicago/Turabian StyleSári-Barnácz, Fruzsina Enikő, Mihály Zalai, Stefan Toepfer, Gábor Milics, Dóra Iványi, Mariann Tóthné Kun, János Mészáros, Mátyás Árvai, and József Kiss. 2023. "Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae" Remote Sensing 15, no. 23: 5602. https://doi.org/10.3390/rs15235602
APA StyleSári-Barnácz, F. E., Zalai, M., Toepfer, S., Milics, G., Iványi, D., Tóthné Kun, M., Mészáros, J., Árvai, M., & Kiss, J. (2023). Suitability of Satellite Imagery for Surveillance of Maize Ear Damage by Cotton Bollworm (Helicoverpa armigera) Larvae. Remote Sensing, 15(23), 5602. https://doi.org/10.3390/rs15235602








