A Review of Ocean Color Algorithms to Detect Trichodesmium Oceanic Blooms and Quantify Chlorophyll Concentration in Shallow Coral Lagoons of South Pacific Archipelagos
Abstract
1. Introduction
2. Data and Methods
2.1. Data
2.1.1. In Situ Reflectance and IOPs Collected in the SWTP
| Cruise | Year | Situation | ap, adg (m−1) | bb-H6 (m−1) | Rrs (sr−1) | Chl-a (mg m−3) | DW (g m−3) | Reference/DOI |
|---|---|---|---|---|---|---|---|---|
| FLUPAC | 1994 | Equatorial Upwelling | x | x | x | [43] | ||
| ZONAL FLUX | 1996 | Equatorial Upwelling | x | x | x | [44,45] | ||
| EBENE | 1996 | Equatorial Upwelling | x | x | x | [46] | ||
| TRICHONESIA 1 | 1998 | NC Vanuatu Fiji Tonga | x | x | x | x | x | [9,16] |
| TRICHONESIA 2 | 1999 | North Australia (Arafura Sea) | x | x | x | x | x | Dupouy, unpub. Res. |
| DIAPALIS (1 to 9) | 2001–2004 | NC Loyalty Isl. | x | x | x | x | x | [19,36]; 10.18142/85 |
| BISSECOTE | 2006 | NC West. lagoon | x | x | x | x | x | [38]; 10.17600/6100010 |
| ECHOLAG | 2007 | NC West. lagoon | x | x | x | x | x | [47]; 10.17600/7100010 |
| VALHYBIO | 2008 | NC West. lagoon | x | x | x | x | x | [42]; 10.17600/8100020 |
| Lagoon surveys | 2001–2013 | NC West. lagoon | x | x | x | x | x | [48]; 10.17600/3100080 |
| SPOT (1 to 14) | 2013–2018 | Vanuatu trench (6000 m) | x | x | x | x | x | 10.18142/237 |
| COMEVA 1,2 | 2015–2016 | Vanuatu | x | x | x | x | x | 10.17600/16003500; 10.17600/16004100 |
| OUTPACE | 2015 | NC Vanuatu Fiji Tonga Niue | x | x | x | x | x | [39]; 10.17600/15000900 |
| MOANAMATY 1,2 | 2018 | French Polynesia | x | x | x | 10.17600/18000580; 10.17600/18000887 | ||
| CALIOPE 1 | 2011 | NC East. lagoon | x | x | x | x | x | [42]; 10.17600/14000900 |
| CALIOPE 2 | 2014 | NC East. lagoon | x | x | x | x | x | [49]; 10.17600/14003900 |
| CALIOPE 3 | 2016 | NC East. lagoon | x | x | x | x | x | [50,51]; 10.17600/16003400 |
| MOISE station | 2012–2018 | NC West. lagoon | x | x | x | x | x | [52] |
| BULA (1 to 5) | 2000–2004 | Laucala Bay Fiji | x | x | x | x | [53]; 10.18142/71 | |
| Laucala Bay (1 to 4) | 2015–2017 | Laucala Bay Fiji | x | x | x | x | [54] | |
| SOKOWASA | 2022 | Viti Levu-Kadavu | x | x | x | x | x | 10.17600/18002025 |
2.1.2. Satellite Data Used for Our Studies
2.2. Algorithms
2.2.1. Trichodesmium Detection
2.2.2. Estimation of [Chl-a] and Turbidity
2.2.3. Extraction of Bathymetry and Seabed Color in Shallow Lagoon Waters
3. Results
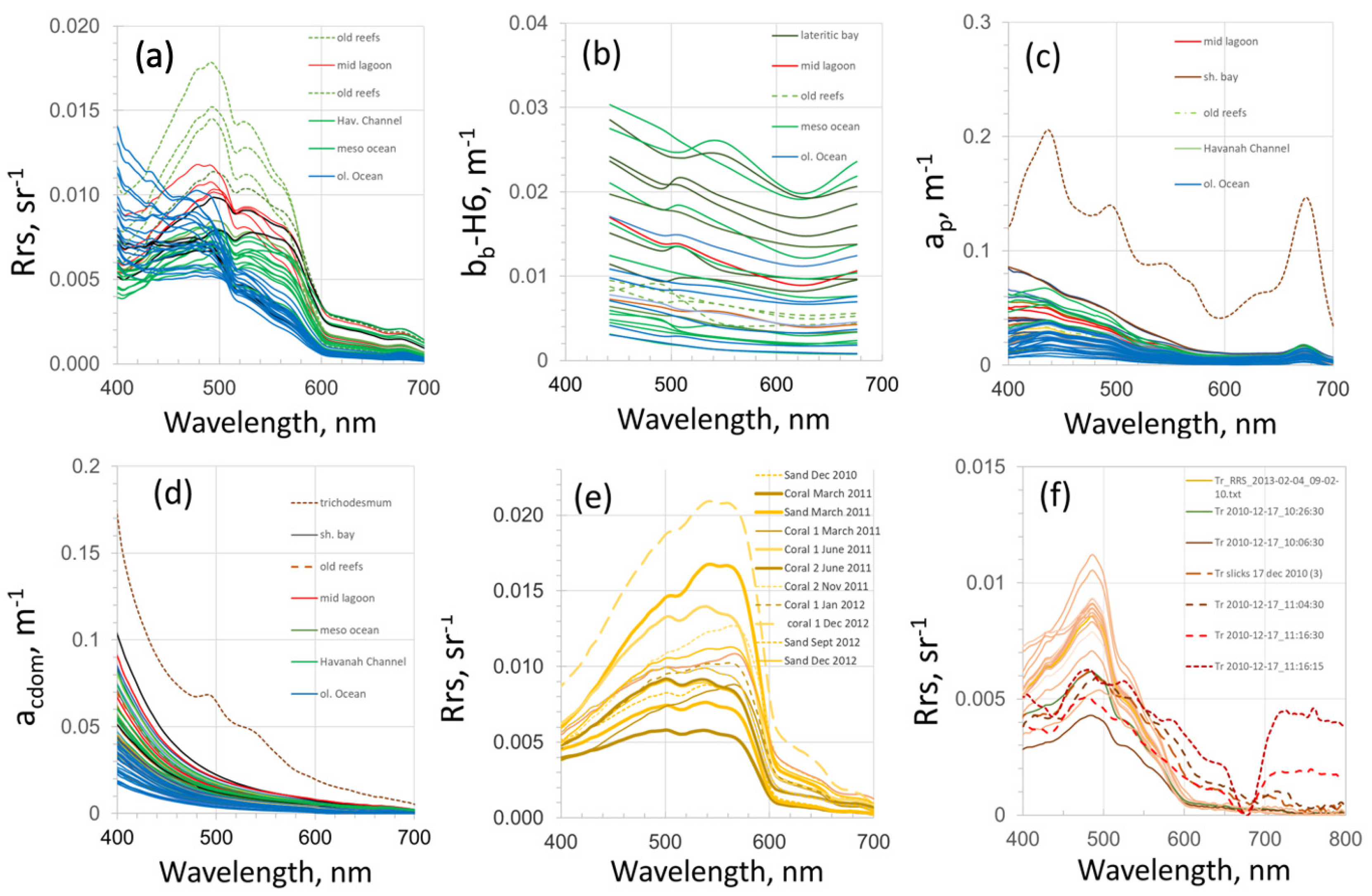
3.1. Variability of the Water Reflectance in the SWTP
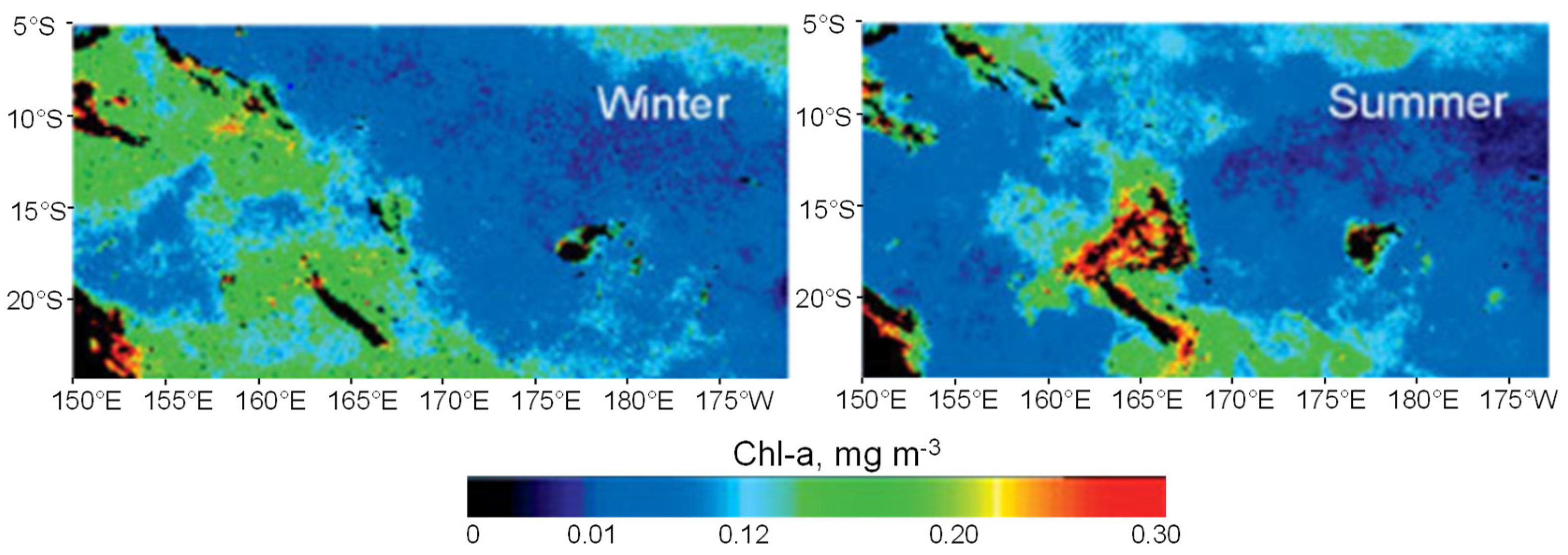
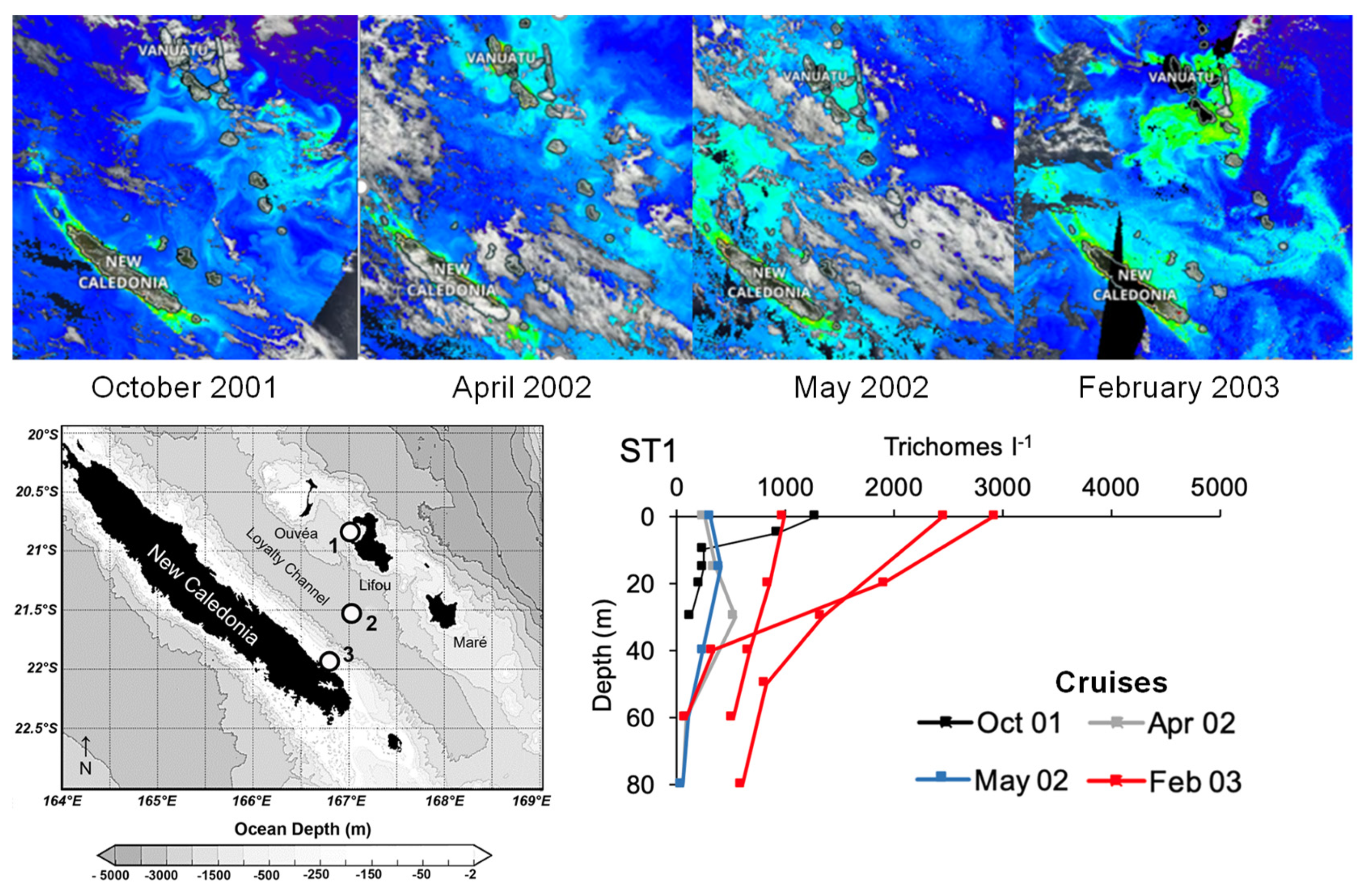
3.2. Trichodesmium Blooms in the SWTP
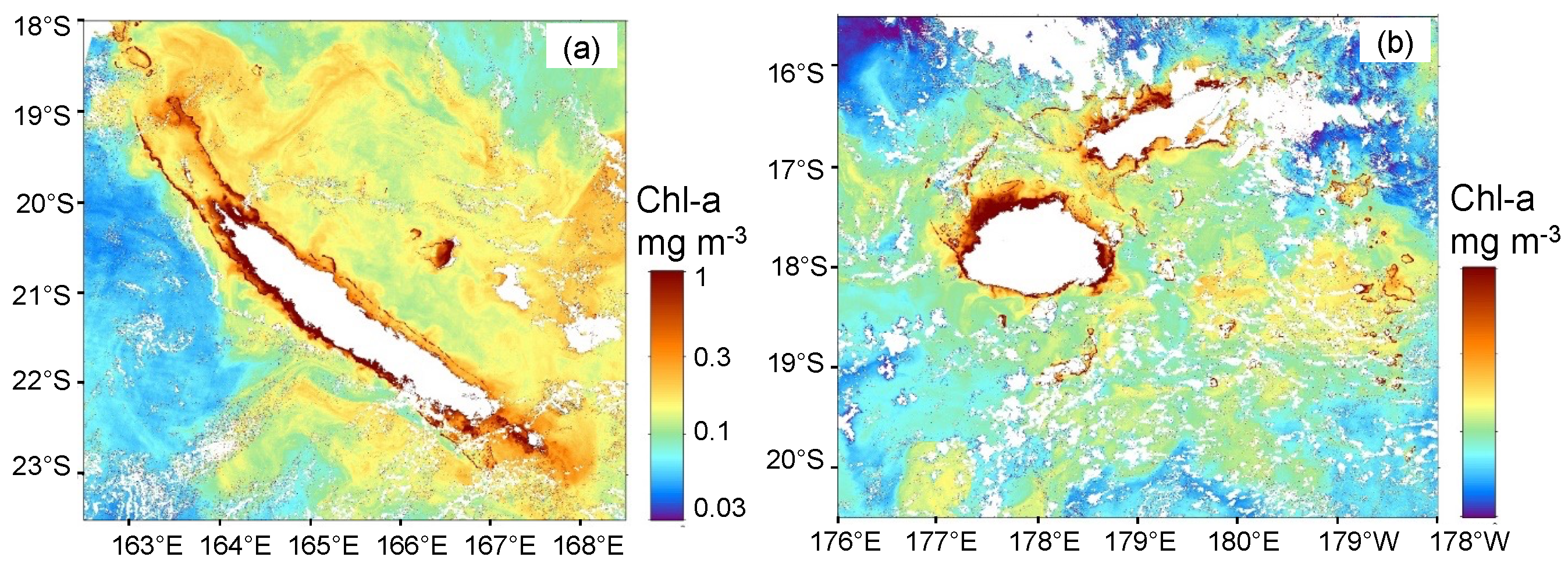
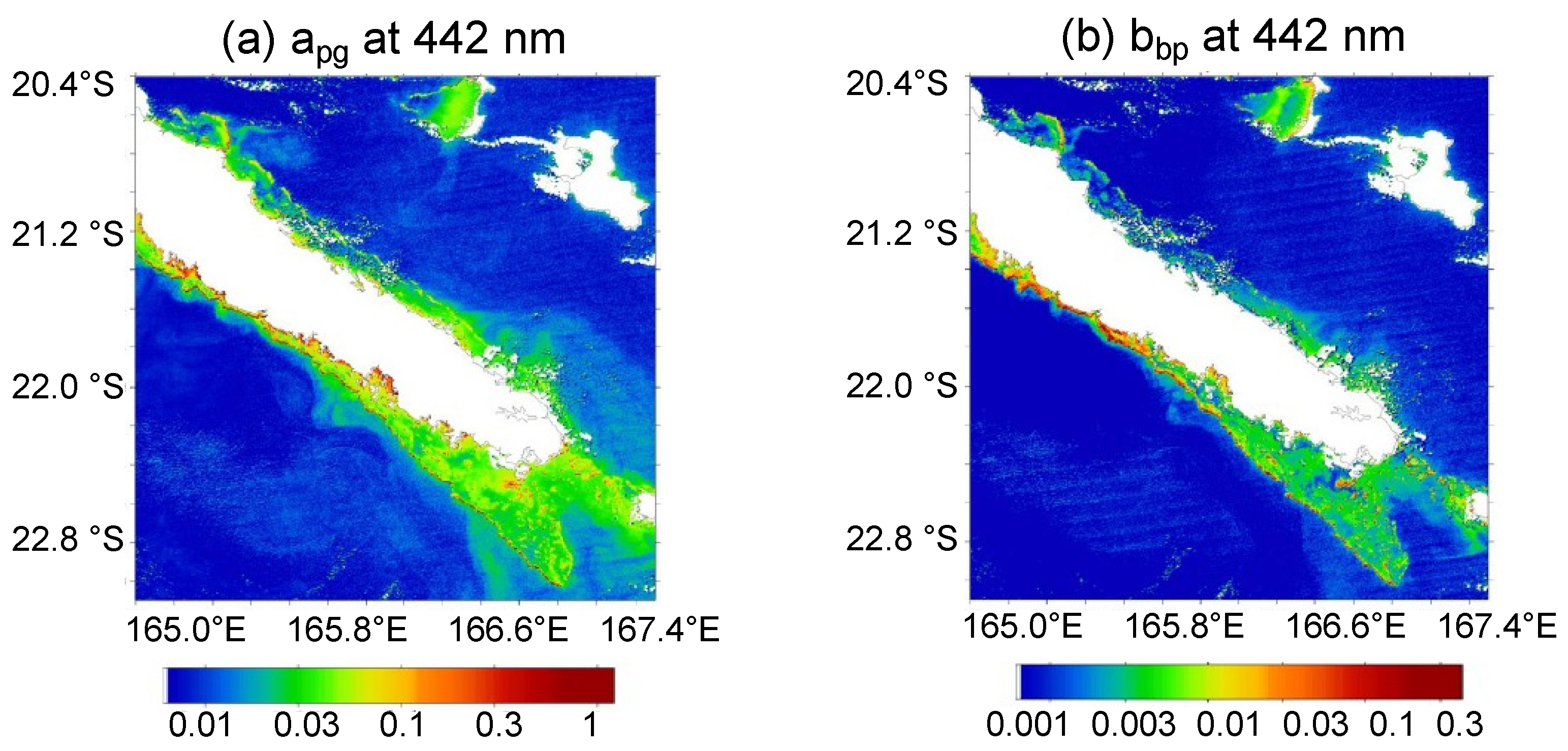
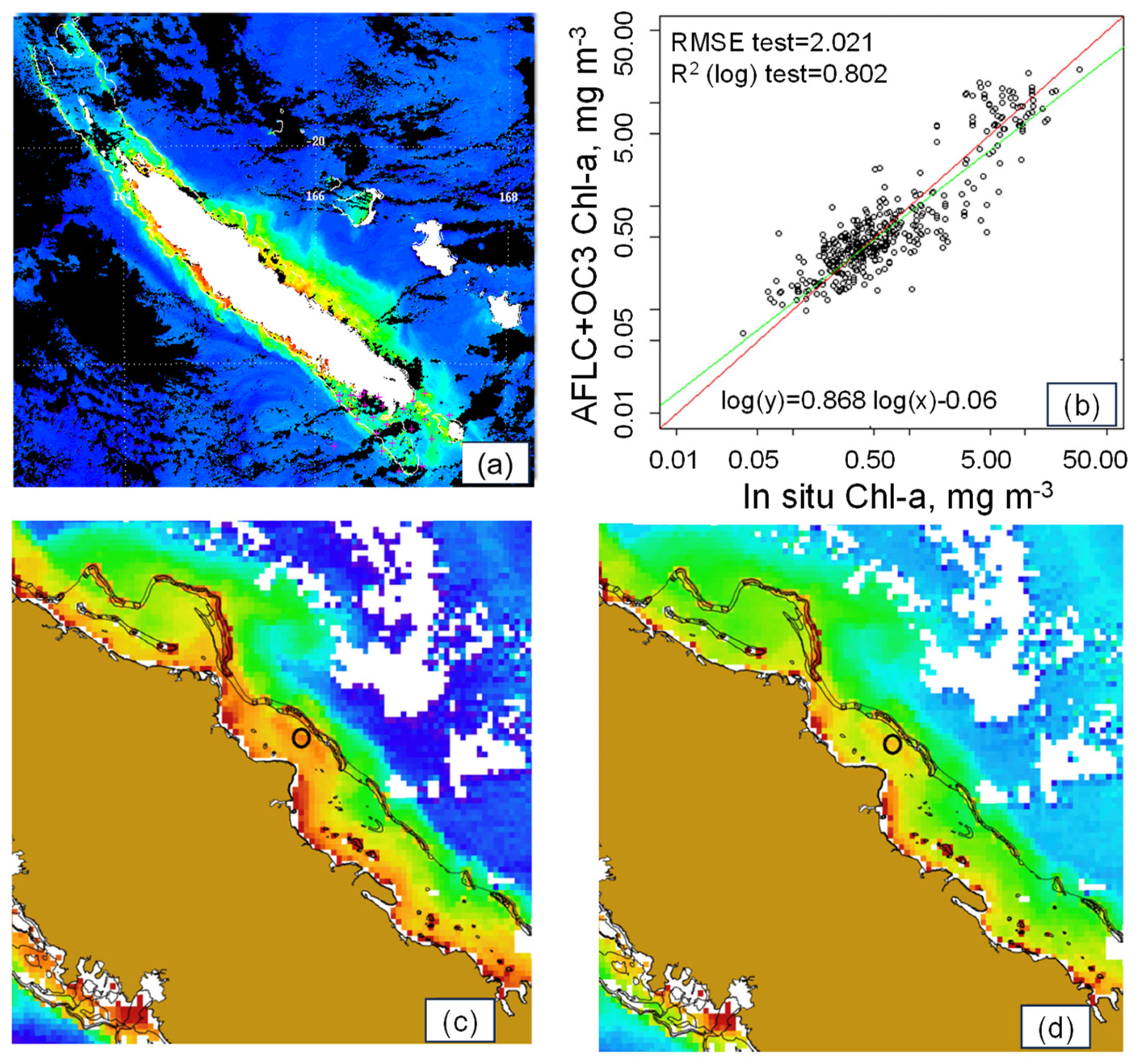
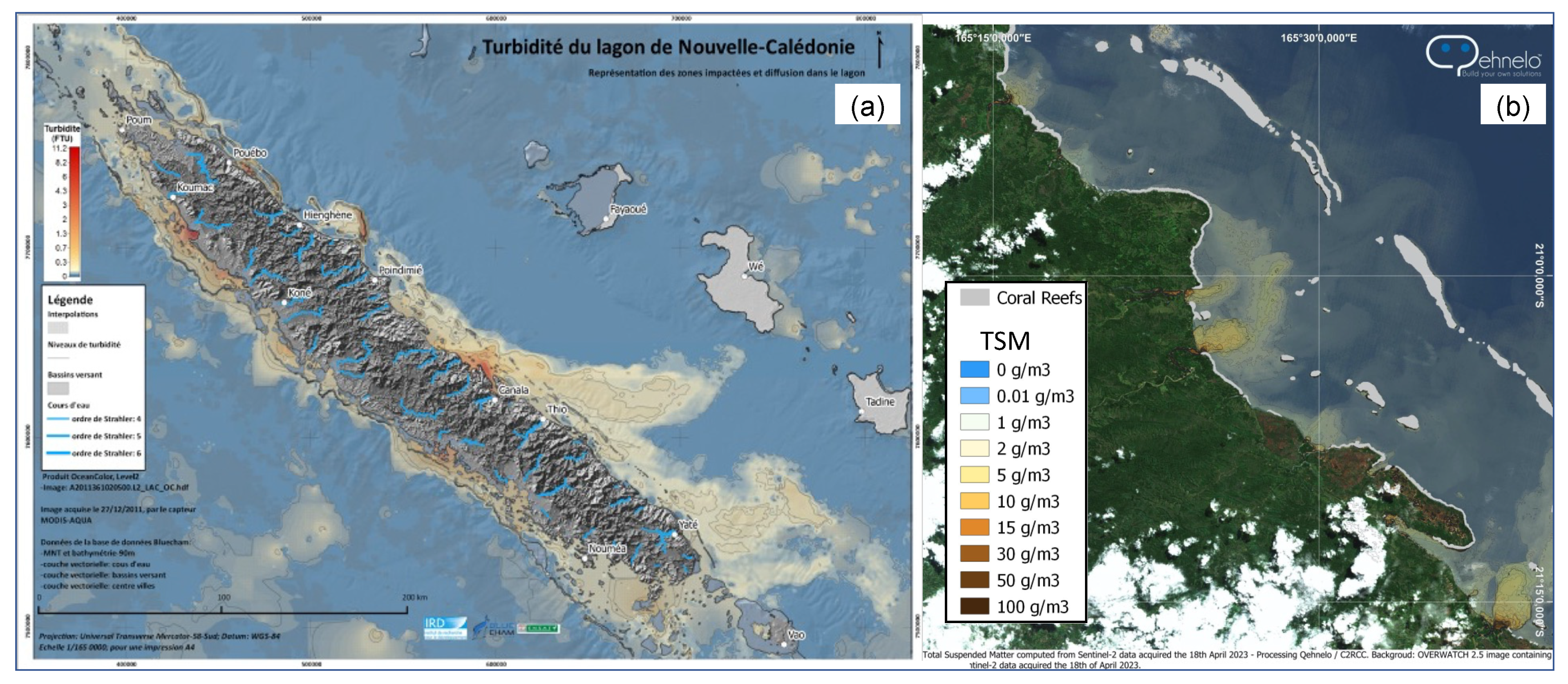
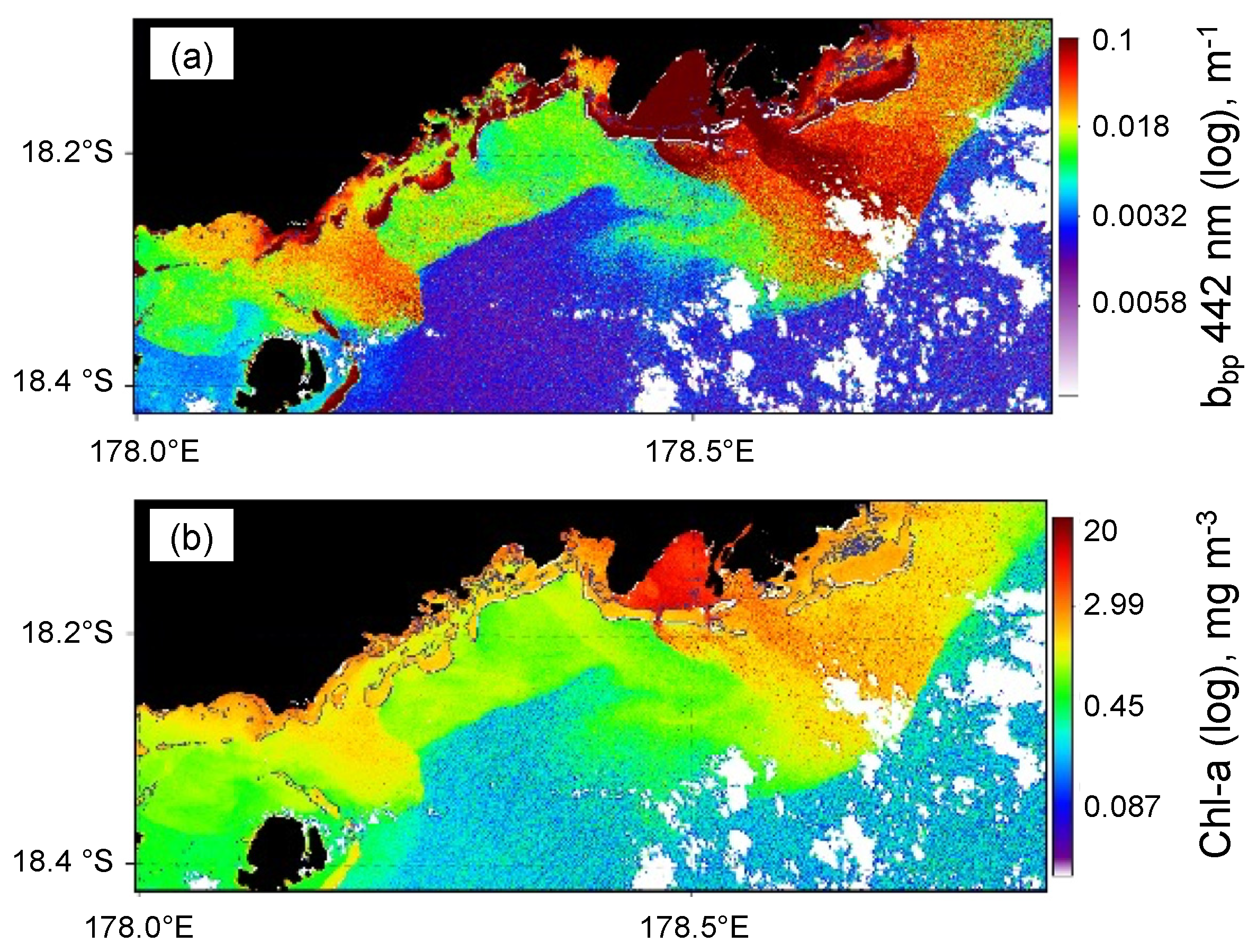
3.3. [Chl-a], IOPs, and Turbidity in New Caledonia Waters
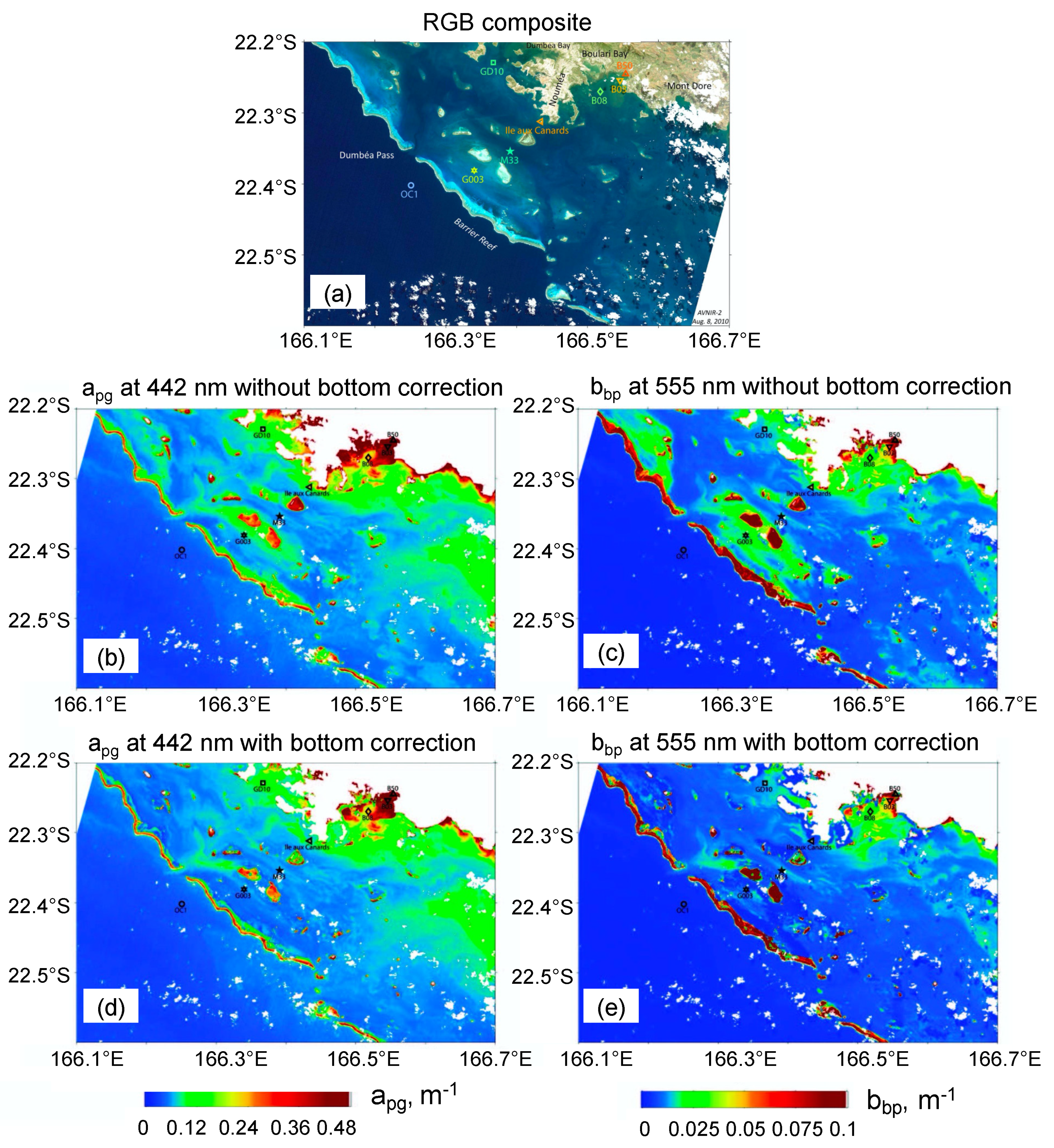
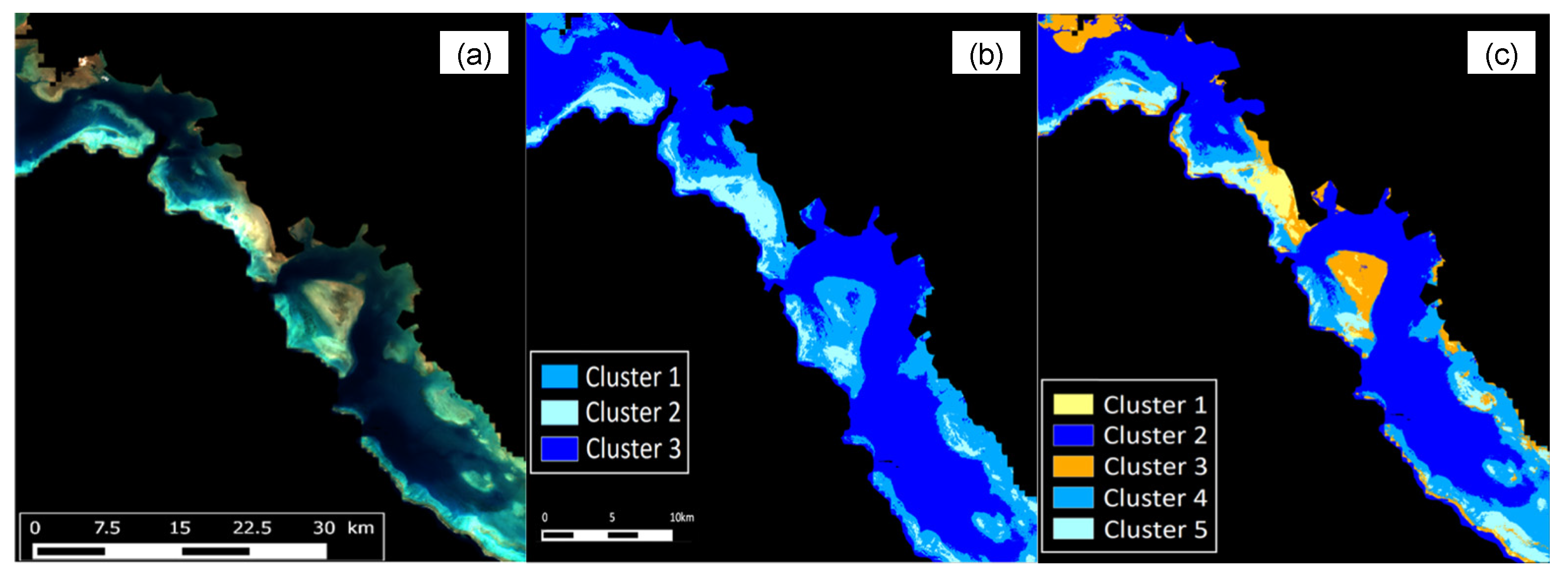
3.4. Bathymetry and Bottom Classification of the New Caledonia Lagoon
4. Discussion
4.1. Performance and Limitations of Our Algorithms for the SWTP
4.1.1. Trichodesmium Algorithms
4.1.2. Coastal Algorithms
4.2. Scientific Accomplishments and Perspectives from Ocean Color Remote Sensing in the SWTP
4.2.1. Novel Collection and Utilization of In Situ Optical Signatures
4.2.2. Algal Blooms and the Detection of Trichodesmium
4.2.3. Following Biogeochemistry of Pacific Island Lagoons
4.2.4. Long-Term Changes in the Ocean with Climate Change
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holland, E.; von Schuckmann, K.; Monier, M.; Legeais, J.-F.; Prado, S.; Sathyendranath, S.; Dupouy, C. Copernicus Marine Service Ocean State Report, Issue 3. J. Oper. Oceanogr. 2019, 12 (Suppl. S1), s43–s48. [Google Scholar] [CrossRef]
- Ganachaud, A.; von Schuckmann, K.; Whiteside, A.; Dupouy, C.; Le Meur, P.-Y.; Monier, M.; van Wynsberge, S.; De Ramon N’Yeurt, A.; Costa, M.; Aucan, J.; et al. CMEMS SST and Chl-a Indicators for Two Pacific Islands: A Co-Construction Monitoring Framework for an Integrated, Transdisciplinary, Multi-Scale Approach. J. Oper. Oceanogr. 2022, 16, 1–15. [Google Scholar]
- Dandonneau, Y.; Gohin, F. Meridional and Seasonal Variations of the Sea Surface Chlorophyll Concentration in the Southwestern Tropical Pacific (14 to 32° S, 160 to 175° E). Deep Sea Res. 1984, 31, 1377–1393. [Google Scholar] [CrossRef]
- Dandonneau, Y.; Lemasson, L. Water-Column Chlorophyll in an Oligotrophic Environment: Correction for the Sampling Depths and Variations of the Vertical Structure of Density, and Observation of a Growth Period. J. Plankton Res. 1987, 9, 215–234. [Google Scholar] [CrossRef][Green Version]
- Blanchot, J.; Rodier, M. Picophytoplankton Abundance and Biomass in the Western Tropical Pacific Ocean during the 1992 El Niño Year: Results from Flow Cytometry. Deep Sea Res. 1996, 43, 877–895. [Google Scholar] [CrossRef]
- Le Bouteiller, A.; Blanchot, J.; Rodier, M. Size Distribution Patterns of Phytoplankton in the Western Pacific: Towards a Generalization for the Tropical Open Ocean. Deep Sea Res. 1992, 39, 805–823. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; McKinnon, A.D.; Andréfouët, S.; Allain, V.; Young, J.; Gledhill, D.C.; Flynn, A.; Bax, N.J.; Beaman, R.; Borsa, P.; et al. Chapter Four—The Coral Sea: Physical Environment, Ecosystem Status and Biodiversity Assets. In Advances in Marine Biology; Lesser, M., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 66, pp. 213–290. [Google Scholar]
- Wilson, C.; Qiu, X. Global Distribution of Summer Chlorophyll Blooms in the Oligotrophic Gyres. Prog. Oceanogr. 2008, 78, 107–134. [Google Scholar] [CrossRef]
- Dupouy, C.; Petit, M.; Dandonneau, Y. Satellite Detected Cyanobacteria Bloom in the Southwestern Tropical Pacific Implication for Oceanic Nitrogen Fixation. Int. J. Remote Sens. 1988, 9, 389–396. [Google Scholar] [CrossRef]
- Dupouy, C. La chlorophylle de surface observée par le satellite NIMBUS-7 CZCS autour de la Nouvelle Calédonie et de ses dépendances. Une première analyse. Bull. L’institut Océanographique Monaco 1990, 6, 125–148. [Google Scholar]
- Dupouy, C. Discoloured Waters in the Melanesian Archipelago (New Caledonia and Vanuatu). The Value of the Nimbus-7 Coastal Zone Colour Scanner Observations. In Marine Pelagic Cyanobacteria: Trichodesmium and other Diazotrophs; Carpenter, E.J., Capone, D.G., Rueter, J.G., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1992; pp. 177–191. ISBN 978-94-015-7977-3. [Google Scholar]
- Dupouy, C.; Neveux, J.; Subramaniam, A.; Mulholland, M.R.; Montoya, J.P.; Campbell, L.; Carpenter, E.J.; Capone, D.G. Satellite Captures Trichodesmium Blooms in the Southwestern Tropical Pacific. Eos Trans. Am. Geophys. Union 2000, 81, 13–16. [Google Scholar] [CrossRef]
- Dupouy, C.; Neveux, J.; Le Bouteiller, A. Spatial and temporal analysis of SeaWIFS sea surface chlorophyll, temperature, winds and sea level anomalies in the South Tropical Pacific Ocean (10° S–25° S, 150° E–180° E). In Proceedings of the 6ème Conférence PORSEC, Pan Ocean Remote Sensing Conference, Gayana, Conception, Chili, 29 November–3 December 2004; Gayana, Conception (Chili). Gayana 68(2) Suppl. I. Proc.. pp. 161–166. [Google Scholar] [CrossRef]
- Dupouy, C.; Dirgerg, G.; Tenório, M.M.B.; Neveux, J.; Le Bouteiller, A. Surveillance des Trichodesmium autour de la Nouvelle-Calédonie, du Vanuatu, de Fidji et de Tonga 1998–2004. Arch. Sci. Mer. 2004, 7, 51. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers14-03/010049853.pdf (accessed on 19 October 2023).
- Campbell, L.; Carpenter, E.; Montoya, J.P.; Kutska, A.B.; Capone, D. Picoplankton community structure within and outside a Trichodesmium bloom in the Southwestern Pacific Ocean. Vie Milieu 2005, 55, 185–195. [Google Scholar]
- Neveux, J.; Tenório, M.M.B.; Dupouy, C.; Villareal, T.A. Spectral diversity of phycoerythrins and diazotrophs abundance in tropical South Pacific. Limnol. Oceanogr. 2006, 51, 1689–1698. [Google Scholar] [CrossRef]
- Moisander, P.H.; Beinart, R.A.; Hewson, I.; White, A.E.; Johnson, K.S.; Carlson, C.A.; Montoya, J.P.; Zehr, J.P. Unicellular Cyanobacterial Distributions Broaden the Oceanic N2 Fixation Domain. Science 2010, 327, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, T.; Kodama, T.; Furuya, K. Large-Scale Impact of the Island Mass Effect through Nitrogen Fixation in the Western South Pacific Ocean. Geophy. Res. Lett. 2014, 41, 2907–2913. [Google Scholar] [CrossRef]
- Tenório, M.; Dupouy, C.; Rodier, M.; Neveux, J. Trichodesmium and other Filamentous Cyanobacteria in New Caledonian waters (South West Tropical Pacific) during an El Niño Episode. Aquat. Microb. Ecol. 2018, 81, 219–241. [Google Scholar] [CrossRef]
- Subramaniam, A.; Carpenter, E.J.; Karentz, D.; Falkowski, P.G. Bio-Optical Properties of the Marine Diazotrophic Cyanobacteria Trichodesmium spp. I. Absorption and Photosynthetic Action Spectra. Limnol. Oceanogr. 1999, 44, 608–617. [Google Scholar] [CrossRef]
- Subramaniam, A.; Brown, C.W.; Hood, R.R.; Carpenter, E.J.; Capone, D.G. Detecting Trichodesmium Blooms in SeaWiFS Imagery. Deep Sea Res. 2001, 49, 107–121. [Google Scholar] [CrossRef]
- Hu, C.; Cannizzaro, J.; Carder, K.L.; Muller-Karger, F.E.; Hardy, R. Remote Detection of Trichodesmium Blooms in Optically Complex Coastal Waters: Examples with MODIS Full-Spectral Data. Remote Sens. Environ. 2010, 114, 2048–2058. [Google Scholar] [CrossRef]
- Westberry, T.K.; Siegel, D.A.; Subramaniam, A. An Improved Bio-Optical Model for the Remote Sensing of Trichodesmium spp. Blooms. J. Geophys. Res. Oceans 2005, 110, C06012. [Google Scholar] [CrossRef]
- Gower, J.; King, S.; Young, E. Global remote sensing of Trichodesmium. Int. J. Remote Sens. 2014, 35, 5459–5466. [Google Scholar] [CrossRef]
- Dupouy, C.; Benielli-Gary, D.; Neveux, J.; Dandonneau, Y.; Westberry, T.K. An Algorithm for Detecting Trichodesmium Surface Blooms in the South Western Tropical Pacific. Biogeosciences 2011, 8, 3631–3647. [Google Scholar] [CrossRef]
- McKinna, L.I.W. Three Decades of Ocean-Color Remote-Sensing Trichodesmium spp. in the World’s Oceans: A Review. Prog. Oceanogr. 2015, 131, 177–199. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Brando, V.E.; Lønborg, C.; Leahy, S.M.; Dekker, A.G. Phenology of Trichodesmium spp. Blooms in the Great Barrier Reef Lagoon, Australia, from the ESA-MERIS 10-Year Mission. PLoS ONE 2018, 13, e0208010. [Google Scholar] [CrossRef]
- IOCCG. Remote Sensing of Ocean Color in Coastal, and Other Optically-Complex Waters; International Ocean-Colour Coordinating Group (IOCCG): Dartmouth, NS, Canada, 2000. [Google Scholar]
- IOCCG. Remote Sensing of Inherent Optical Properties: Fundamentals, Tests of Algorithms, and Applications; International Ocean-Colour Coordinating Group (IOCCG): Dartmouth, NS, Canada, 2006. [Google Scholar]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Bissett, P.; Brando, V.E.; Casey, B.; Fearns, P.; Hedley, J.; Klonowski, W.; Lee, Z.P.; et al. Intercomparison of Shallow Water Bathymetry, Hydro-Optics, and Benthos Mapping Techniques in Australian and Caribbean Coastal Environments. Limnol. Oceanogr. Methods 2011, 9, 396–425. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Gower, J.F.R.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A Review of Ocean Color Remote Sensing Methods and Statistical Techniques for the Detection, Mapping and Analysis of Phytoplankton Blooms in Coastal and Open Oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Blondeau-Patissier, D.; Schroeder, T.; Brando, V.E.; Maier, S.W.; Dekker, A.G.; Phinn, S. ESA-MERIS 10-Year Mission Reveals Contrasting Phytoplankton Bloom Dynamics in Two Tropical Regions of Northern Australia. Remote Sens. 2014, 6, 2963–2988. [Google Scholar] [CrossRef]
- Hedley, J.D.; Roelfsema, C.; Brando, V.; Giardino, C.; Kutser, T.; Phinn, S.; Mumby, P.J.; Barrilero, O.; Laporte, J.; Koetz, B. Coral Reef Applications of Sentinel-2: Coverage, Characteristics, Bathymetry and Benthic Mapping with Comparison to Landsat 8. Remote Sens. Environ. 2018, 216, 598–614. [Google Scholar] [CrossRef]
- Zoffoli, M.L.; Frouin, R.; Kampel, M. Water Column Correction for Coral Reef Studies by Remote Sensing. Sensors 2014, 14, 16881–16931. [Google Scholar] [CrossRef]
- Dupouy, C.; Neveux, J.; Dirberg, G.; Röttgers, R.; Tenório, M.M.B.; Ouillon, S. Bio-optical properties of the marine cyanobacteria Trichodesmium spp. J. Appl. Remote Sens. 2008, 2, 023503. [Google Scholar] [CrossRef]
- Dupouy, C.; Neveux, J.; Ouillon, S.; Frouin, R.; Murakami, H.; Hochard, S.; Dirberg, G. Inherent Optical Properties and Satellite Retrieval of Chlorophyll Concentration in the Lagoon and Open Ocean Waters of New Caledonia. Mar. Pollut. Bull. 2010, 61, 503–518. [Google Scholar] [CrossRef]
- Froidefond, J.-M.; Ouillon, S. Introducing a Mini-Catamaran to Perform Reflectance Measurements above and below the Water Surface. Opt. Express 2005, 13, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Ouillon, S.; Douillet, P.; Petrenko, A.; Neveux, J.; Dupouy, C.; Froidefond, J.-M.; Andréfouët, S.; Muñoz-Caravaca, A. Optical Algorithms at Satellite Wavelengths for Total Suspended Matter in Tropical Coastal Waters. Sensors 2008, 8, 4165–4185. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, C.; Frouin, R.; Tedetti, M.; Maillard, M.; Rodier, M.; Lombard, F.; Guidi, L.; Picheral, M.; Neveux, J.; Duhamel, S.; et al. Diazotrophic Trichodesmium Impact on UV–Vis Radiance and Pigment Composition in the Western Tropical South Pacific. Biogeosciences 2018, 15, 5249–5269. [Google Scholar] [CrossRef]
- Ouillon, S.; Douillet, P.; Lefebvre, J.P.; Le Gendre, R.; Jouon, A.; Bonneton, P.; Fernandez, J.M.; Chevillon, C.; Magand, O.; Lefèvre, J.; et al. Circulation and Suspended Sediment Transport in a Coral Reef Lagoon: The South-West Lagoon of New Caledonia. Mar. Pollut. Bull. 2010, 61, 269–296. [Google Scholar] [CrossRef]
- Röttgers, R.; Dupouy, C.; Taylor, B.B.; Bracher, A.; Woźniak, S.B. Mass-Specific Light Absorption Coefficients of Natural Aquatic Particles in the near-Infrared Spectral Region. Limnol. Oceanogr. 2014, 59, 1449–1460. [Google Scholar] [CrossRef]
- Röttgers, R.; Doxaran, D.; Dupouy, C. Quantitative Filter Technique Measurements of Spectral Light Absorption by Aquatic Particles Using a Portable Integrating Cavity Absorption Meter (QFT-ICAM). Opt. Express 2016, 24, A1–A20. [Google Scholar] [CrossRef]
- Dupouy, C.; Neveux, J.; Andre’, J.M. Spectral Absorption Coefficient of Photosynthetically Active Pigments in the Equatorial Pacific Ocean (165°11–150° W). Deep. Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1881–1906. [Google Scholar] [CrossRef]
- Simeon, J.; Roesler, C.; Pegau, W.S.; Dupouy, C. Sources of Spatial Variability in Light Absorbing Components along an Equatorial Transect from 165° E to 150° W. J. Geophys. Res. Oceans 2003, 108, 3333. [Google Scholar] [CrossRef]
- Gross, L.; Frouin, R.; Dupouy, C.; André, J.M.; Thiria, S. Reducing Variability That Is Due to Secondary Pigments in the Retrieval of Chlorophyll a Concentration from Marine Reflectance: A Case Study in the Western Equatorial Pacific Ocean. Appl. Opt. 2004, 43, 4041–4054. [Google Scholar] [CrossRef]
- Dupouy, C.; Loisel, H.; Neveux, J.; Brown, S.L.; Moulin, C.; Blanchot, J.; Le Bouteiller, A.; Landry, M.R. Microbial Absorption and Backscattering Coefficients from in Situ and POLDER Satellite Data during an El Niño–Southern Oscillation Cold Phase in the Equatorial Pacific (180°). J. Geophys. Res. Oceans 2003, 108, 8138. [Google Scholar] [CrossRef]
- Neveux, J.; Lefebvre, J.-P.; Le Gendre, R.; Dupouy, C.; Gallois, F.; Courties, C.; Gérard, P.; Fernandez, J.-M.; Ouillon, S. Phytoplankton Dynamics in the Southern New Caledonian Lagoon during a Southeast Trade Winds Event. J. Mar. Syst. 2010, 82, 230–244. [Google Scholar] [CrossRef]
- Neveux, J.; Tenório, M.M.B.; Jacquet, S.; Torréton, J.-P.; Douillet, P.; Ouillon, S.; Dupouy, C. Chlorophylls and Phycoerythrins as Markers of Environmental Forcings Including Cyclone Erica Effect (March 2003) on Phytoplankton in the Southwest Lagoon of New Caledonia and Oceanic Adjacent Area. Int. J. Oceanogr. 2009, 2009, e232513. [Google Scholar] [CrossRef]
- Favareto, L.R.; Rudorff, N.; Kampel, M.; Frouin, R.; Röttgers, R.; Doxaran, D.; Murakami, H.; Dupouy, C. Bio-Optical Characterization and Ocean Colour Inversion in the Eastern Lagoon of New Caledonia, South Tropical Pacific. Remote Sens. 2018, 10, 1043. [Google Scholar] [CrossRef]
- Martias, C.; Tedetti, M.; Lantoine, F.; Jamet, L.; Dupouy, C. Characterization and Sources of Colored Dissolved Organic Matter in a Coral Reef Ecosystem Subject to Ultramafic Erosion Pressure (New Caledonia, Southwest Pacific). Sci. Total Environ. 2018, 616–617, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, C.; Röttgers, R.; Tedetti, M.; Frouin, R.; Lantoine, F.; Rodier, M.; Martias, C.; Goutx, M. Impact of Contrasted Weather Conditions on CDOM Absorption/Fluorescence and Biogeochemistry in the Eastern Lagoon of New Caledonia. Front. Earth Sci. 2020, 8, 54. [Google Scholar] [CrossRef]
- Dupouy, C.; Rodier, M. MOISE Observing Station; OSU PYTHEAS Report; OSU PYTHEAS: Marseille, France, 2014. [Google Scholar]
- Fichez, R.D.P.; Chevillon, C.; Torreton, J.P.; Aung, T.H.; Chifflet, S.; Fernandez, J.M.; Gangaiya, P.; Garimella, S.; Gerard, P.; Ouillon, S.; et al. The Suva Lagoon Enviroment: An overview of a joint IRD Camelia Research Unit and USP Study. In At the Cross Roads: Science and Management of the Suva Lagoon; Morrison, R.J., Aalbersberg, W., Eds.; Institute of the Applied Sciences Press, University of the South Pacific: Suva, Fiji, 2006; Volume 1, pp. 93–105. [Google Scholar]
- Koliyavu, T.; Martias, C.; Singh, A.; Mounier, S.; Gérard, P.; Dupouy, C. In-Situ Variability of DOM in Relation with Biogeochemical and Physical Parameters in December 2017 in Laucala Bay (Fiji Islands) after a Strong Rain Event. J. Mar. Sci. Eng. 2021, 9, 241. [Google Scholar] [CrossRef]
- Lefèvre, J. The MODIS Data Processing Chain; Technical Document of the VALidation of Hyperspectral SATellite Data the Valhysat Project; IRD Nouméa: Nouméa, Nouvelle-Calédonie, 2010. [Google Scholar]
- Murakami, H.; Dupouy, C. Coastal ocean atmospheric correction for AVNIR-2 high resolution images. In Proceedings of the SPIE, Remote Sensing of the Coastal Ocean, Land, and Atmosphere Environment, Incheon, Republic of Korea, 11–14 October 2010; Volume 7858, p. 785802. [Google Scholar] [CrossRef]
- Murakami, H.; Dupouy, C.; Röttgers, R.; Frouin, R.J. Estimation of Inherent Optical Properties Using In Situ Hyperspectral Radiometer and MODIS Data along the East Coast of New Caledonia. In Proceedings of the SPIE, Remote Sensing of the Marine Environment II, Kyoto, Japan, 29 October–1 November 2012; Volume 8525, pp. 47–55. [Google Scholar]
- Wattelez, G.; Dupouy, C.; Mangeas, M.; Lefèvre, J.; Touraivane; Frouin, R. A Statistical Algorithm for Estimating Chlorophyll Concentration in the New Caledonian Lagoon. Remote Sens. 2016, 8, 45. [Google Scholar] [CrossRef]
- Wattelez, G.; Dupouy, C.; Lefèvre, J.; Ouillon, S.; Fernandez, J.-M.; Juillot, F. Application of the Support Vector Regression Method for Turbidity Assessment with MODIS on a Shallow Coral Reef Lagoon (Voh-Koné-Pouembout, New Caledonia). Water 2017, 9, 737. [Google Scholar] [CrossRef]
- Minghelli-Roman, A.; Dupouy, C. Influence of Water Column Chlorophyll Concentration on Bathymetric Estimations in the Lagoon of New Caledonia, Using Several MERIS Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 739–745. [Google Scholar] [CrossRef]
- Minghelli-Roman, A.; Dupouy, C. Correction of the Water Column Attenuation: Application to the Seabed Mapping of the Lagoon of New Caledonia Using MERIS Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2619–2629. [Google Scholar] [CrossRef]
- Wattelez, G.; Dupouy, C.; Juillot, F. Unsupervised Optical Classification of the Seabed Color in Shallow Oligotrophic Waters from Sentinel-2 Images: A Case Study in the Voh-Koné-Pouembout Lagoon (New Caledonia). Remote Sens. 2022, 14, 836. [Google Scholar] [CrossRef]
- Rousset, G.; De Boissieu, F.; Menkes, C.E.; Lefèvre, J.; Frouin, R.; Rodier, M.; Ridoux, V.; Laran, S.; Bonnet, S.; Dupouy, C. Remote Sensing of Trichodesmium spp. Mats in the Western Tropical South Pacific. Biogeosciences 2018, 15, 5203–5219. [Google Scholar] [CrossRef]
- Bazader, J.-F. Personal communication. 2023. [Google Scholar]
- Benavides, M.; Conradt, L.; Bonnet, S.; Berman-Frank, I.; Barrillon, S.; Petrenko, A.; Doglioli, A. Fine-Scale Sampling Unveils Diazotroph Patchiness in the South Pacific Ocean. ISME Commun. 2021, 1, 3. [Google Scholar] [CrossRef]
- Whiteside, A. Ocean Color Plume Monitoring around the Fiji Islands from Remote Sensing through Anthropogenic and Climatic Influences. Estimation of the Impact of the Cyanobacteria Trichodesmium, Thèse de 3eme Cycle; Aix-Marseille Université: Marseille, France, 7 November 2023. [Google Scholar]
- Bonnet, S.; Caffin, M.; Berthelot, H.; Moutin, T. Hot Spot of N2 Fixation in the Western Tropical South Pacific Pleads for a Spatial Decoupling between N2 Fixation and Denitrification. Proc. Natl. Acad. Sci. USA 2017, 114, E2800. [Google Scholar] [CrossRef]
- Messer, L.F.; Mahaffey, C.; M Robinson, C.; Jeffries, T.C.; Baker, K.G.; Bibiloni Isaksson, J.; Ostrowski, M.; Doblin, M.A.; Brown, M.V.; Seymour, J.R. High Levels of Heterogeneity in Diazotroph Diversity and Activity within a Putative Hotspot for Marine Nitrogen Fixation. ISME J. 2016, 10, 1499–1513. [Google Scholar] [CrossRef]
- Bonnet, S.; Caffin, M.; Berthelot, H.; Grosso, O.; Benavides, M.; Helias-Nunige, S.; Guieu, C.; Stenegren, M.; Foster, R.A. In-Depth Characterization of Diazotroph Activity across the Western Tropical South Pacific Hotspot of N2 Fixation (OUTPACE Cruise). Biogeosciences 2018, 15, 4215–4232. [Google Scholar] [CrossRef]
- Moutin, T.; Karl, D.M.; Duhamel, S.; Rimmelin, P.; Raimbault, P.; Van Mooy, B.A.S.; Claustre, H. Phosphate Availability and the Ultimate Control of New Nitrogen Input by Nitrogen Fixation in the Tropical Pacific Ocean. Biogeosciences 2008, 5, 95–109. [Google Scholar] [CrossRef]
- Bonnet, S.; Benavides, M.; Le Moigne, F.A.C.; Camps, M.; Torremocha, A.; Grosso, O.; Dimier, C.; Spungin, D.; Berman-Frank, I.; Garczarek, L.; et al. Diazotrophs Are Overlooked Contributors to Carbon and Nitrogen Export to the Deep Ocean. ISME J. 2023, 17, 47–58. [Google Scholar] [CrossRef]
- Yoon, J.-E.; King, D.; Longman, J.; Cronin, S.J. Differential Response of Chlorophyll-a Concentrations to Explosive Volcanism in the Western South Pacific. Front. Mar. Sci. 2023, 10, 1072610. [Google Scholar] [CrossRef]
- Whiteside, A.; Dupouy, C.; Singh, A.; Frouin, R.; Menkes, C.; Lefèvre, J. Automatic Detection of Optical Signatures within and around Floating Tonga-Fiji Pumice Rafts Using MODIS, VIIRS, and OLCI Satellite Sensors. Remote Sens. 2021, 13, 501. [Google Scholar] [CrossRef]
- Whiteside, A.; Dupouy, C.; Singh, A.; Bani, P.; Tan, J.; Frouin, R. Impact of Ashes from the 2022 Tonga Volcanic Eruption on Satellite Ocean Color Signatures. Front. Mar. Sci. 2023, 9, 1028022. [Google Scholar] [CrossRef]
- Martinez, E.; Antoine, D.; D’Ortenzio, F.; Gentili, B. Climate-Driven Basin-Scale Decadal Oscillations of Oceanic Phytoplankton. Science 2009, 326, 1253–1256. [Google Scholar] [CrossRef]
- Messié, M.; Petrenko, A.; Doglioli, A.M.; Aldebert, C.; Martinez, E.; Koenig, G.; Bonnet, S.; Moutin, T. The Delayed Island Mass Effect: How Islands Can Remotely Trigger Blooms in the Oligotrophic Ocean. Geophys. Res. Lett. 2020, 47, e2019GL085282. [Google Scholar] [CrossRef]
- Messié, M.; Petrenko, A.; Doglioli, A.M.; Martinez, E.; Alvain, S. Basin-Scale Biogeochemical and Ecological Impacts of Islands in the Tropical Pacific Ocean. Nat. Geosci. 2022, 15, 469–474. [Google Scholar] [CrossRef]
- Bonnet, S.; Guieu, C.; Taillandier, V.; Boulart, C.; Bouruet-Aubertot, P.; Gazeau, F.; Scalabrin, C.; Bressac, M.; Knapp, A.N.; Cuypers, Y.; et al. Natural Iron Fertilization by Shallow Hydrothermal Sources Fuels Diazotroph Blooms in the Ocean. Science 2023, 380, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.; Horvat, C. Extreme South Pacific Phytoplankton Blooms Induced by Tropical Cyclones. Geophys. Res. Lett. 2023, 50, e2022GL100821. [Google Scholar] [CrossRef]
- Andrews, J.; Gentien, P. Upwelling as a Source of Nutrients for the Great Barrier Reef Ecosystems: A Solution to Darwin’s Question? Mar. Ecol. Prog. Ser. 1982, 8, 257–269. [Google Scholar] [CrossRef]
- Brodie, J.E.; Kroon, F.J.; Schaffelke, B.; Wolanski, E.C.; Lewis, S.E.; Devlin, M.J.; Bohnet, I.C.; Bainbridge, Z.T.; Waterhouse, J.; Davis, A.M. Terrestrial Pollutant Runoff to the Great Barrier Reef: An Update of Issues, Priorities and Management Responses. Mar. Pollut. Bull. 2012, 65, 81–100. [Google Scholar] [CrossRef]
- Oubelkheir, K.; Ford, P.W.; Cherukuru, N.; Clementson, L.A.; Petus, C.; Devlin, M.; Schroeder, T.; Steven, A.D.L. Impact of a Tropical Cyclone on Terrestrial Inputs and Bio-Optical Properties in Princess Charlotte Bay (Great Barrier Reef Lagoon). Remote Sens. 2023, 15, 652. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Logan, M.; Weeks, S.; Brodie, J. The Effects of River Run-off on Water Clarity across the Central Great Barrier Reef. Mar. Pollut. Bull. 2014, 84, 191–200. [Google Scholar] [CrossRef]
- Fuchs, R.; Dupouy, C.; Douillet, P.; Caillaud, M.; Mangin, A.; Pinazo, C. Modelling the Impact of a La Niña Event on a South West Pacific Lagoon. Mar. Pollut. Bull. 2012, 64, 1596–1613. [Google Scholar] [CrossRef]
- Fuchs, R.; Pinazo, C.; Douillet, P.; Fraysse, M.; Grenz, C.; Mangin, A.; Dupouy, C. Modelling Ocean–Lagoon Interaction during Upwelling Processes in the Southwest of New Caledonia. Estuar. Coast. Shelf Sci. 2013, 135, 5–17. [Google Scholar] [CrossRef]
- Chevalier, C.; Sous, D.; Devenon, J.-L.; Pagano, M.; Rougier, G.; Blanchot, J. Impact of Cross-Reef Water Fluxes on Lagoon Dynamics: A Simple Parameterization for Coral Lagoon Circulation Model, with Application to the Ouano Lagoon, New Caledonia. Ocean Dyn. 2015, 65, 1509–1534. [Google Scholar] [CrossRef]
- Roche, R.C.; Heenan, A.; Taylor, B.M.; Schwarz, J.N.; Fox, M.D.; Southworth, L.K.; Williams, G.J.; Turner, J.R. Linking Variation in Planktonic Primary Production to Coral Reef Fish Growth and Condition. R. Soc. Open Sci. 2022, 9, 201012. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in Tropical Scleractinian Corals. Biol. Rev. Camb. Philos. Soc. 2009, 84, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rodier, M.; Le Borgne, R. Population dynamics and environmental conditions affecting Trichodesmium spp. (filamentous cyanobacteria) blooms in the south-west lagoon of New Caledonia. J. Exp. Mar. Biol. Ecol. 2008, 358, 20–32. [Google Scholar] [CrossRef]
- Rodier, M.; Le Borgne, R. Population and Trophic Dynamics of Trichodesmium Thiebautii in the SE Lagoon of New Caledonia. Comparison with T. Erythraeum in the SW Lagoon. Mar. Pollut. Bull. 2010, 61, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Saulia, E.; Benavides, M.; Henke, B.; Turk-Kubo, K.; Cooperguard, H.; Grosso, O.; Desnues, A.; Rodier, M.; Dupouy, C.; Riemann, L.; et al. Seasonal Shifts in Diazotrophs Players: Patterns Observed Over a Two-Year Time Series in the New Caledonian Lagoon (Western Tropical South Pacific Ocean). Front. Mar. Sci. 2020, 7, 581755. [Google Scholar] [CrossRef]
- Boatman, T.G.; Upton, G.J.G.; Lawson, T.; Geider, R.J. Projected Expansion of Trichodesmium’s Geographical Distribution and Increase in Growth Potential in Response to Climate Change. Glob. Chang. Biol. 2020, 26, 6445–6456. [Google Scholar] [CrossRef]
- De Monte, S.; Soccodato, A.; Alvain, S.; d’Ovidio, F. Can We Detect Oceanic Biodiversity Hotspots from Space? ISME J. 2013, 7, 2054–2056. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Marinov, I.; Cabre, A.; Kostadinov, T.; Singh, A. Increasing Biomass in the Warm Oceans: Unexpected New Insights from SeaWiFS. Geophys. Res. Lett. 2019, 46, 3900–3910. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Bréon, F.M. Remote Sensing of Phytoplankton Groups in Case 1 Waters from Global SeaWiFS Imagery. Deep Sea Res. 2005, 52, 1989–2004. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Loisel, H. Seasonal Distribution and Succession of Dominant Phytoplankton Groups in the Global Ocean: A Satellite View. Glob. Biogeochem. Cycles 2008, 22, GB3001. [Google Scholar] [CrossRef]
- De Boissieu, F.; Menkes, C.; Dupouy, C.; Rodier, M.; Bonnet, S.; Mangeas, M.; Frouin, R.J. Phytoplankton Global Mapping from Space with a Support Vector Machine Algorithm. In Proceedings of the SPIE, Ocean Remote Sensing and Monitoring from Space, Beijing, China, 13–16 October 2014; Volume 9261, pp. 354–367. [Google Scholar]


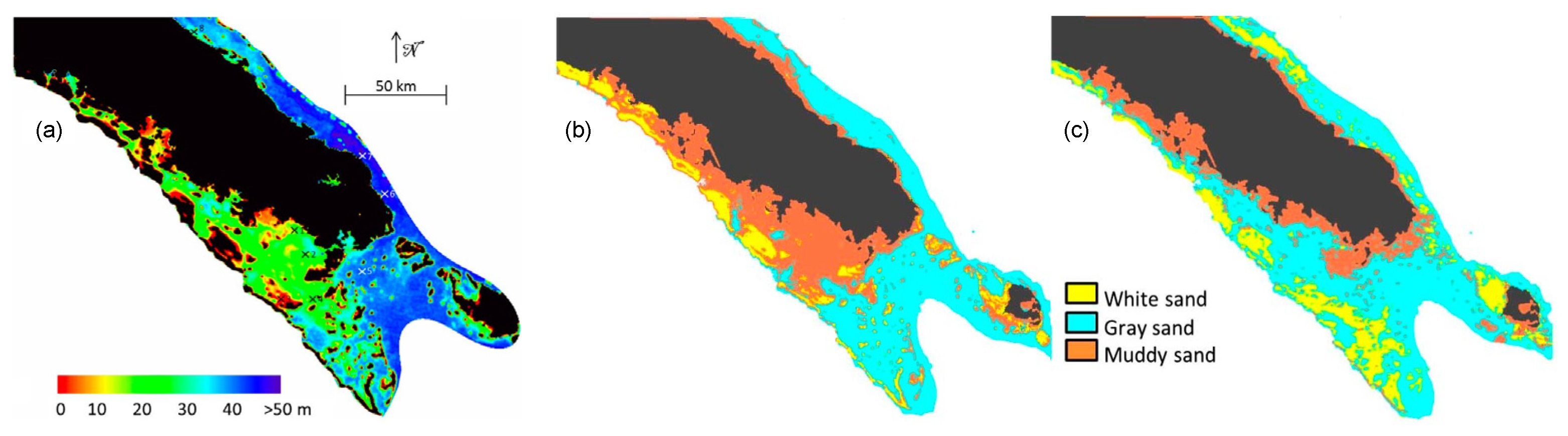
 : white bottom;
: white bottom;  : grey bottom;
: grey bottom;  : brown bottom. (c,d) Turbidity maps (in FTU) retrieved from the MODIS image with the [38] New Caledonia turbidity algorithm compared with one from the SVR algorithm (using bathymetry and reflectance combination (see text for details)). (Adapted from [59]).
: brown bottom. (c,d) Turbidity maps (in FTU) retrieved from the MODIS image with the [38] New Caledonia turbidity algorithm compared with one from the SVR algorithm (using bathymetry and reflectance combination (see text for details)). (Adapted from [59]).
 : white bottom;
: white bottom;  : grey bottom;
: grey bottom;  : brown bottom. (c,d) Turbidity maps (in FTU) retrieved from the MODIS image with the [38] New Caledonia turbidity algorithm compared with one from the SVR algorithm (using bathymetry and reflectance combination (see text for details)). (Adapted from [59]).
: brown bottom. (c,d) Turbidity maps (in FTU) retrieved from the MODIS image with the [38] New Caledonia turbidity algorithm compared with one from the SVR algorithm (using bathymetry and reflectance combination (see text for details)). (Adapted from [59]).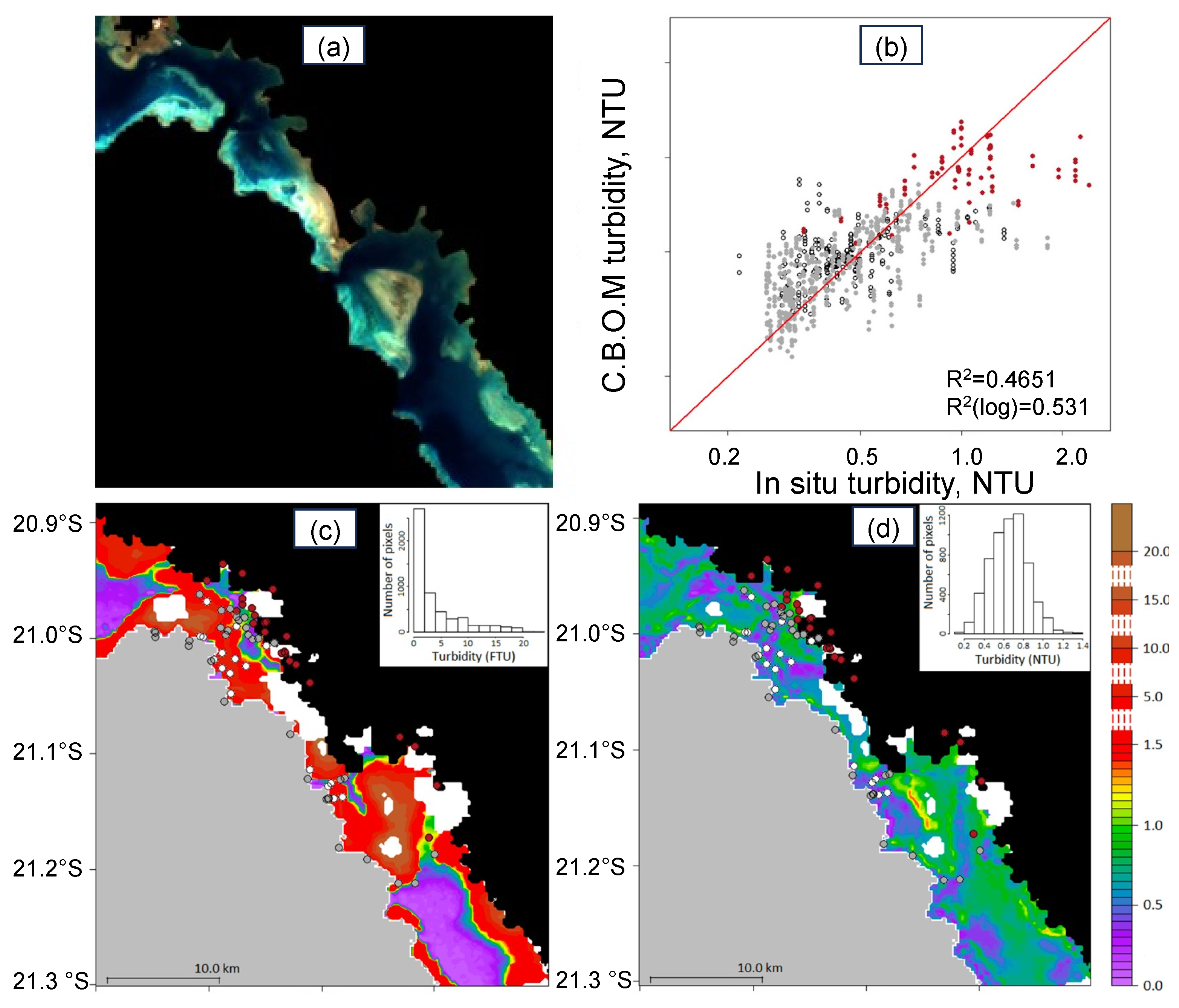
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupouy, C.; Whiteside, A.; Tan, J.; Wattelez, G.; Murakami, H.; Andréoli, R.; Lefèvre, J.; Röttgers, R.; Singh, A.; Frouin, R. A Review of Ocean Color Algorithms to Detect Trichodesmium Oceanic Blooms and Quantify Chlorophyll Concentration in Shallow Coral Lagoons of South Pacific Archipelagos. Remote Sens. 2023, 15, 5194. https://doi.org/10.3390/rs15215194
Dupouy C, Whiteside A, Tan J, Wattelez G, Murakami H, Andréoli R, Lefèvre J, Röttgers R, Singh A, Frouin R. A Review of Ocean Color Algorithms to Detect Trichodesmium Oceanic Blooms and Quantify Chlorophyll Concentration in Shallow Coral Lagoons of South Pacific Archipelagos. Remote Sensing. 2023; 15(21):5194. https://doi.org/10.3390/rs15215194
Chicago/Turabian StyleDupouy, Cécile, Andra Whiteside, Jing Tan, Guillaume Wattelez, Hiroshi Murakami, Rémi Andréoli, Jérôme Lefèvre, Rüdiger Röttgers, Awnesh Singh, and Robert Frouin. 2023. "A Review of Ocean Color Algorithms to Detect Trichodesmium Oceanic Blooms and Quantify Chlorophyll Concentration in Shallow Coral Lagoons of South Pacific Archipelagos" Remote Sensing 15, no. 21: 5194. https://doi.org/10.3390/rs15215194
APA StyleDupouy, C., Whiteside, A., Tan, J., Wattelez, G., Murakami, H., Andréoli, R., Lefèvre, J., Röttgers, R., Singh, A., & Frouin, R. (2023). A Review of Ocean Color Algorithms to Detect Trichodesmium Oceanic Blooms and Quantify Chlorophyll Concentration in Shallow Coral Lagoons of South Pacific Archipelagos. Remote Sensing, 15(21), 5194. https://doi.org/10.3390/rs15215194






