Core of Sustainability Education: Bridging Theory and Practice in Teaching Climate Science to Future Mathematics and Physics Teachers
Abstract
1. Introduction
2. Theoretical Framework
2.1. The Fundamental Principles Guiding the Design of a TLS
2.2. The Guiding Principles for the Design of the Experimental Activities
3. Climate Models and Key Physics Concepts
3.1. A Brief History of the Climate Models
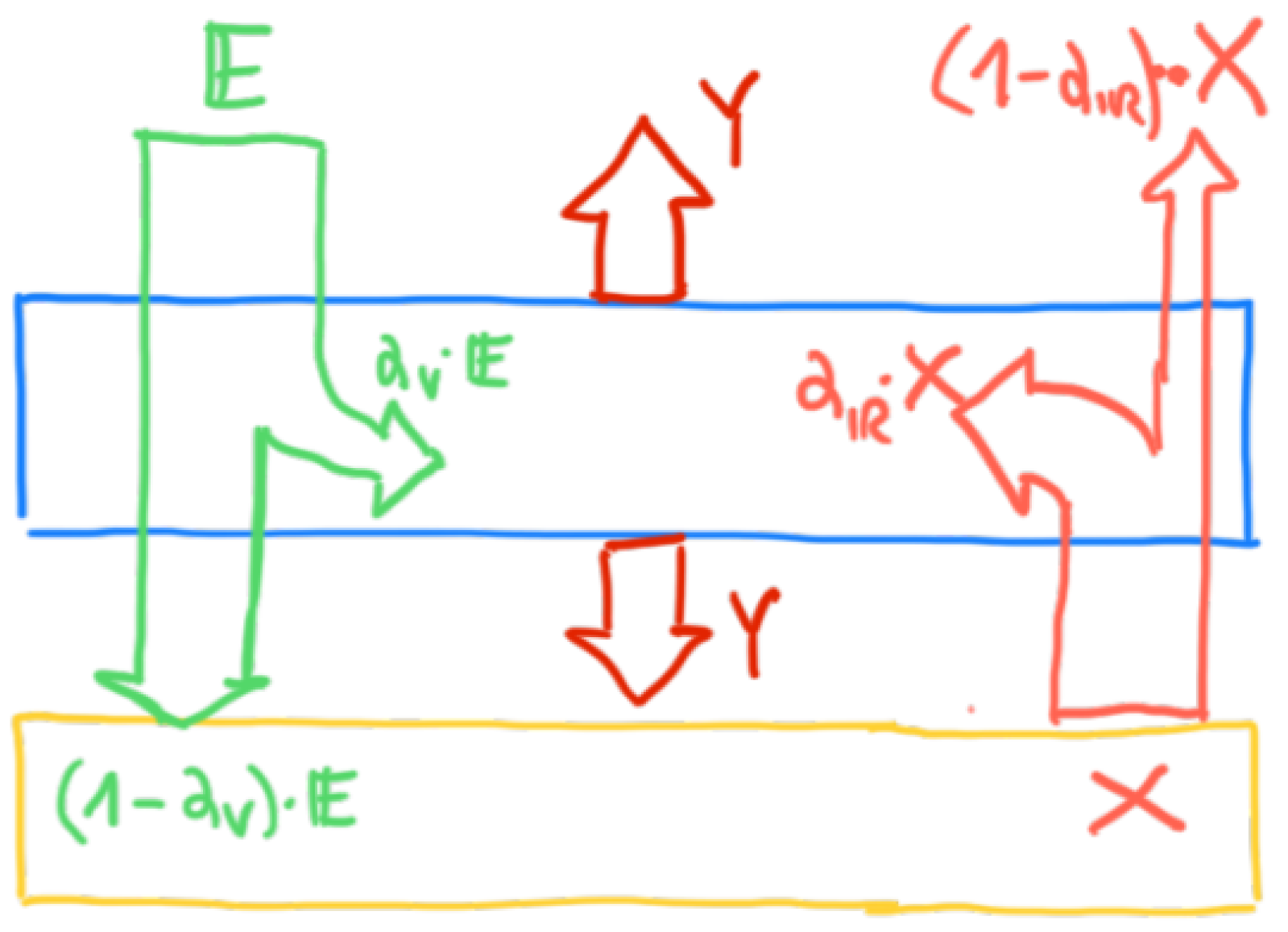
3.2. The 0-Dimensional Radiative Energy Budget Model
The Key Physics Concepts
- Electromagnetic (EM) spectrum: Knowing the range of the electromagnetic radiation spectrum beyond visible light and being able to sort different types of radiation by frequency and wavelength.
- Radiation–Matter interaction: Examining how electromagnetic radiation interacts with matter, including phenomena like reflection, refraction, absorption, scattering, and selective absorption.
- Thermal radiation: studying the emission of electromagnetic radiation from hot objects, such as the radiation from a filament bulb.
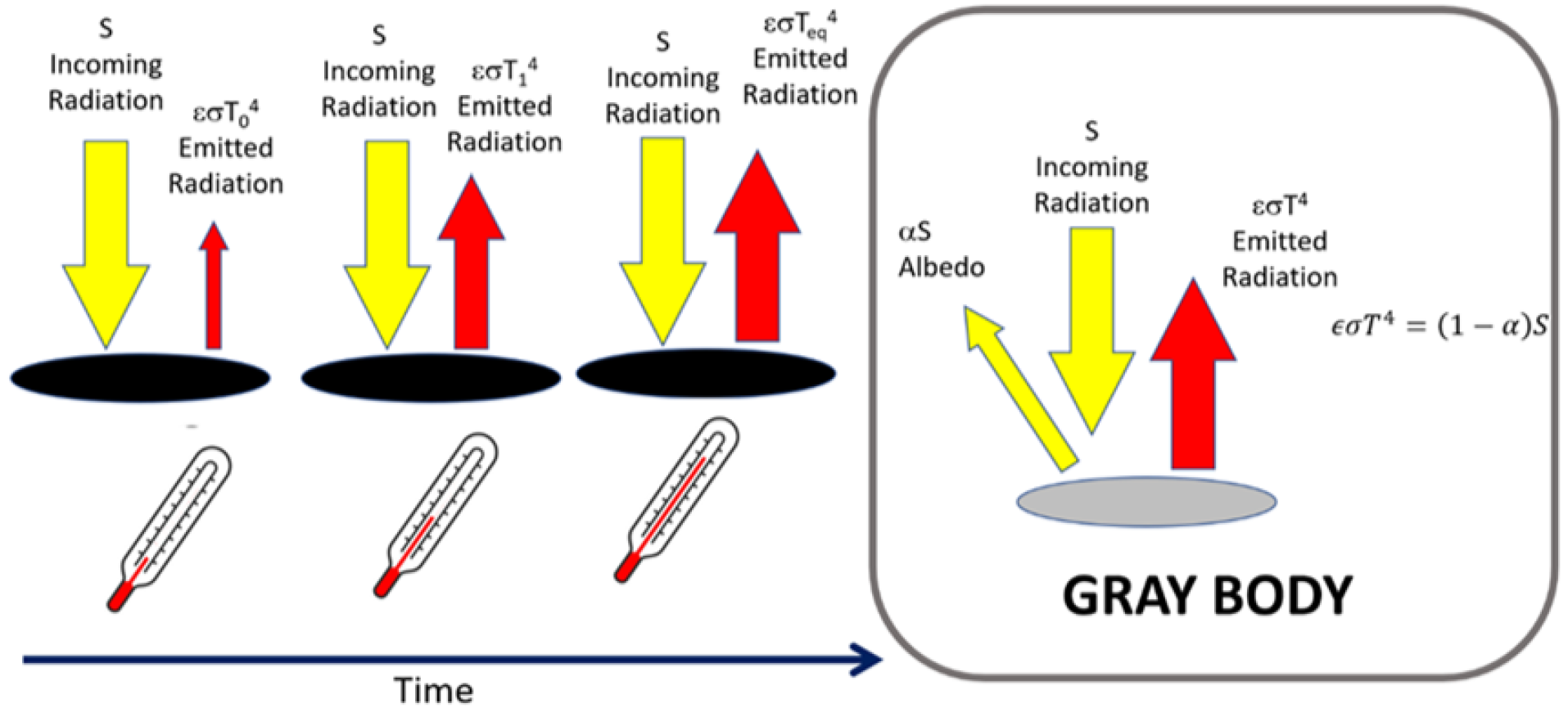
- Energy balance: understanding the balance between the radiation incident on a body and the radiation emitted, and then applying the concept to the Earth system.
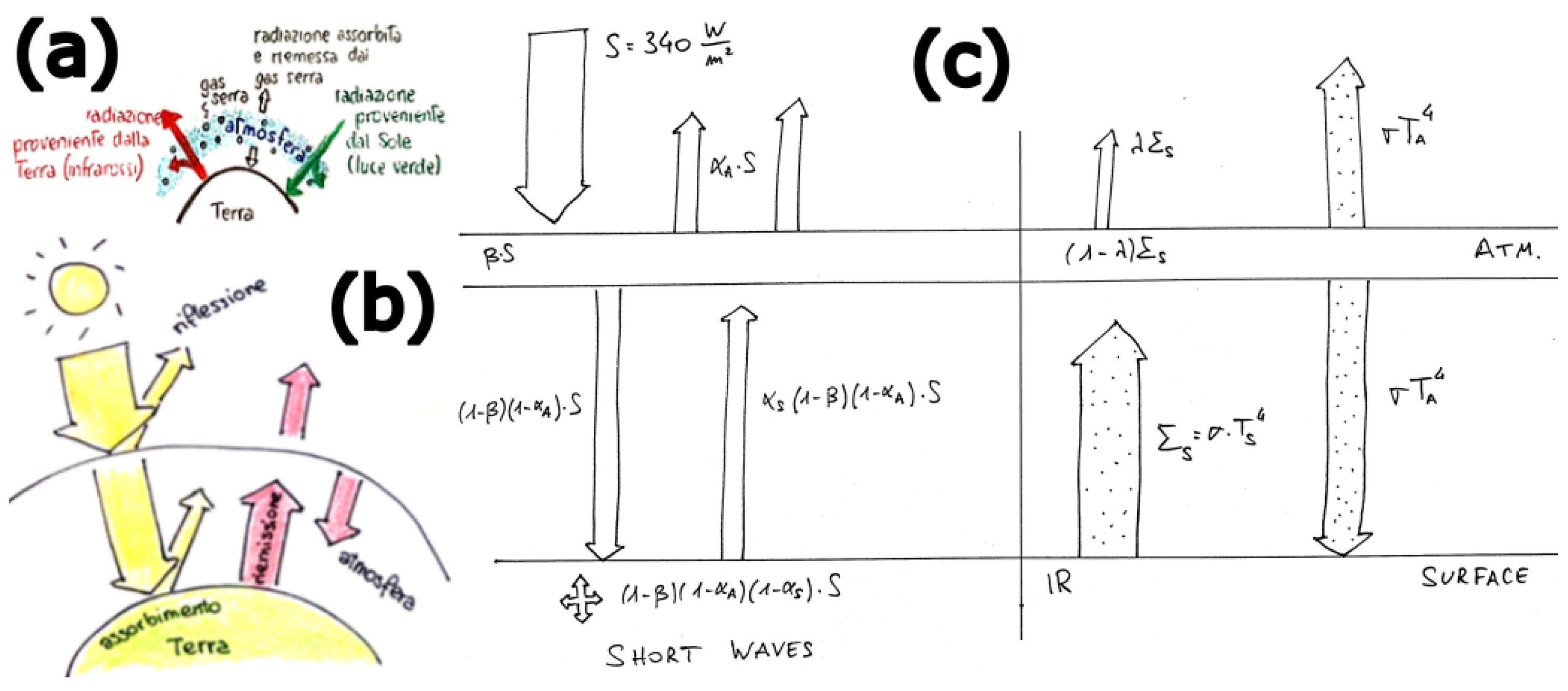
- Earth’s energy balance and GHE: exploring the role of greenhouse gases in trapping heat and influencing Earth’s temperature.
- Visualization of continuous and discrete spectra using a low-cost diffraction grating and smartphone camera to guide students to an understanding of the different spectral bands and reflections regarding different radiation emission mechanisms.
- Radiation–Matter interaction experiments (employing collimated beams and color filters): Both qualitative and quantitative experiments (Snell’s law) investigate Radiation–Matter interaction phenomena like reflection, refraction, absorption, scattering, and selective absorption.
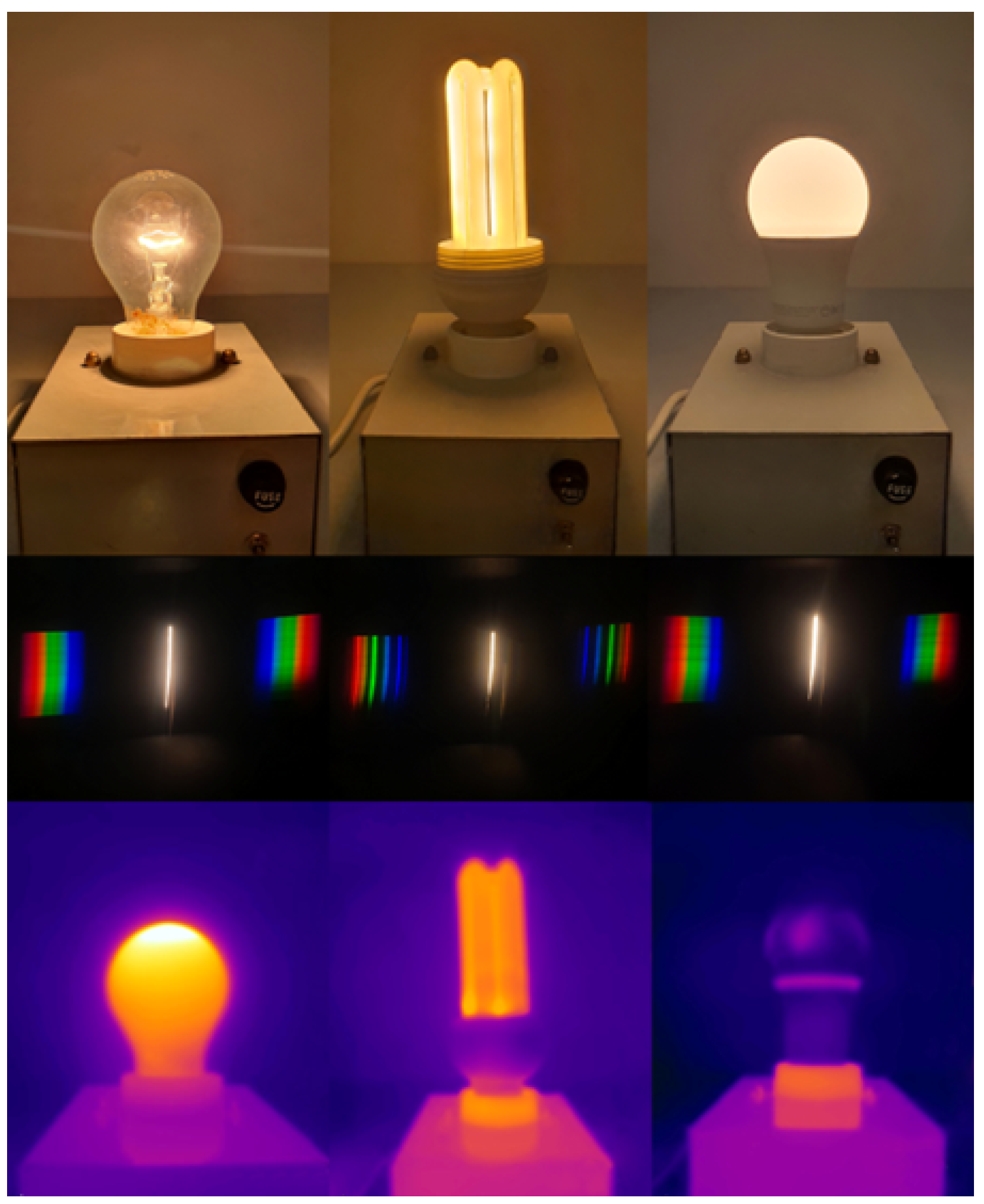
- The Stefan–Boltzmann law in thermal radiation: a quantitative experiment measuring the temperature of a bulb to study thermal radiation.
- Observation of the emission spectrum of a filament lamp using the diffraction grating and activity on the “Blackbody Spectrum” [46]. This activity is useful both for visualizing Wien’s law and for investigating blackbody radiation as a whole.
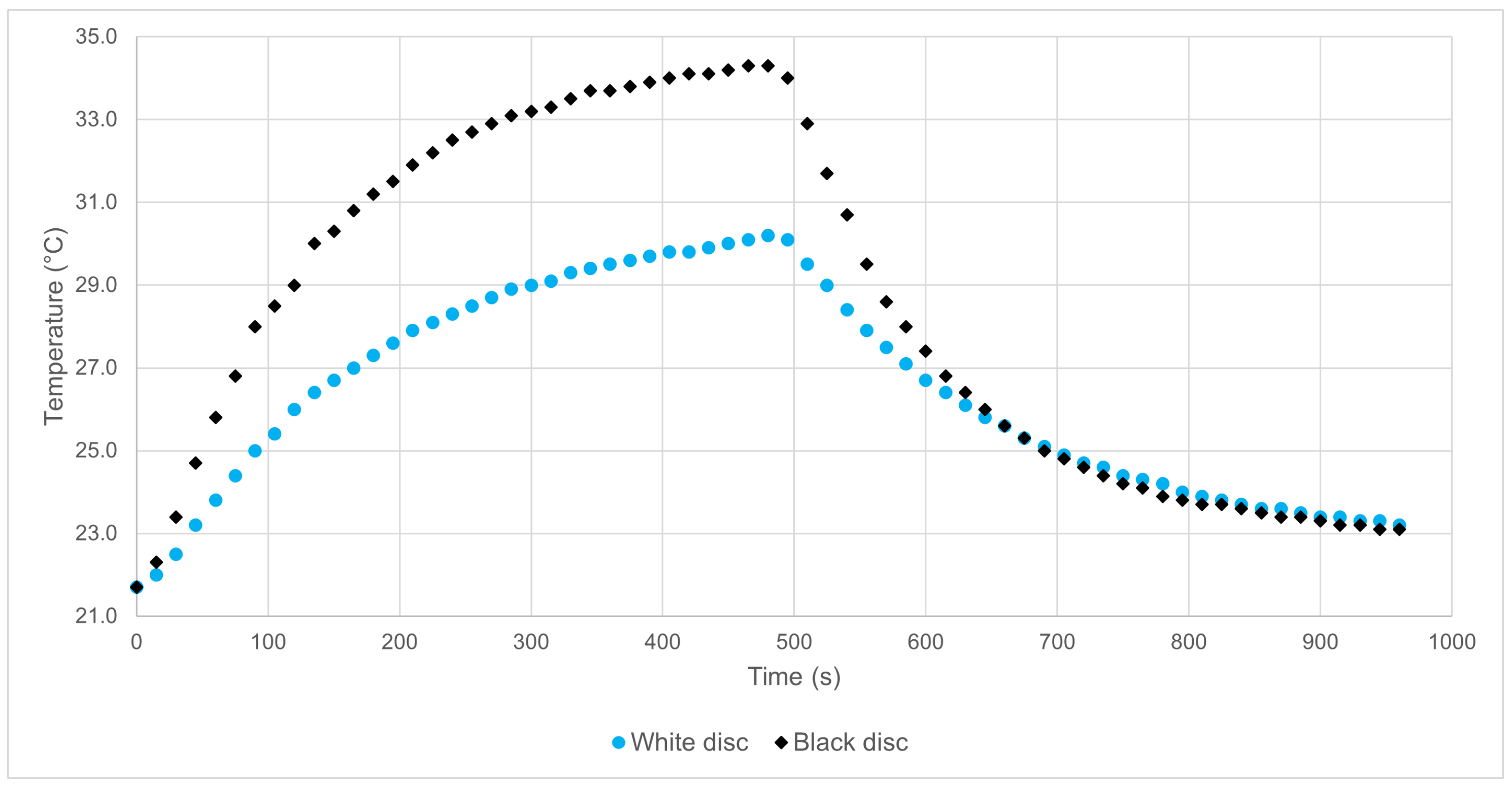
- Experiment with black and white discs: a quantitative experiment used to explain radiative equilibria, stationary states, introduce the concept of albedo, and discuss the mechanism of heat propagation.
- Measurement of the power emitted by a light bulb through a quantitative experiment introducing the concept of radiative equilibrium.
- Leaking bucket with one hole: a known demonstration to introduce the concept of radiative equilibrium and stationary states.
- Qualitative demonstration of Leslie cube with thermocouple and IR thermal camera: needed to explain the concept of emissivity characterizing the Stefan–Boltzmann law for gray bodies.
3.3. The 0-Dimensional Radiative Energy Budget Model Including Atmosphere
Key Physics Concepts
- Demonstration using an IR camera to distinguish between visible and IR radiation.
- Quantitative experiments and simulations concerning Beer’s law:
- (a)
- Conduct an experiment to measure the transmittance as a function of thickness by using many sheets of paper.
- (b)
- Perform an experiment to determine the transmittance as a function of the concentration of the absorber with a tray filled with dye.
- (c)
- Analyse the transmittance for the various spectral components of radiation incident through a liquid of different colours using light sources at certain wavelengths.
- (d)
- Use a PhET simulation [46] to explore Beer’s law by determining the transmittance as the substance and solute concentration change.
- Exploring selective transparency with an infrared digital camera for glass, plastic, and silicon wafers.
- Using PhET simulations [46] to study the photon-molecule interaction, particularly for certain gases relevant to the behavior of the atmosphere (GHG) at varying photon energy, thus exploring the microscopic aspects of selective transparency.
- Implementing a “leaking bucket with two holes” experiment to discuss changes in the radiative equilibrium state.
4. The Experiments
4.1. Electromagnetic Spectrum and Infrared Radiation
4.1.1. Student Difficulties
4.1.2. Background Theory
4.1.3. Experimental Setup
4.1.4. Students’ Understanding and Learning Goals
4.2. The Stefan–Boltzmann Law
4.2.1. Student Difficulties
4.2.2. Background Theory
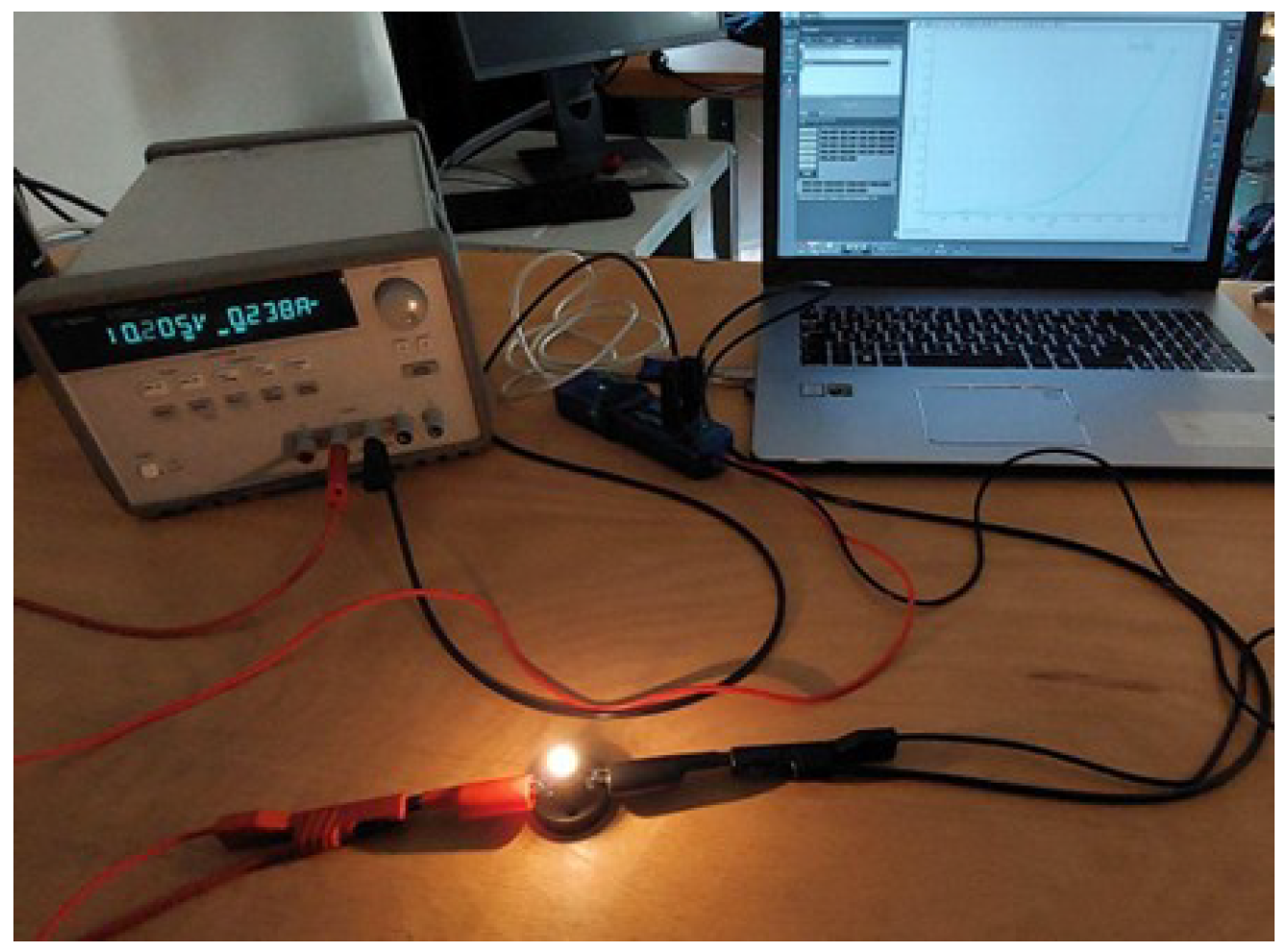
4.2.3. Experimental Setup
- Measurements are performed employing a lamp constructed to work at a voltage of 12 V, absorbing a power of 3 W. Included in the setup are a power supplier with adjustable output voltage and a voltage and current sensor manufactured by PASCO [62].
- By simply putting a diffraction grating in front of the smartphone camera, it is possible to capture the main features of the visible spectrum of the light bulb.
- Since the glass of the bulb is not transparent to IR radiation, to explicitly see what happens before a body becomes incandescent with the IR camera, we substituted the bulb with a graphite pencil lead (this is the same setup used above to demonstrate how thermal radiation is often invisible).
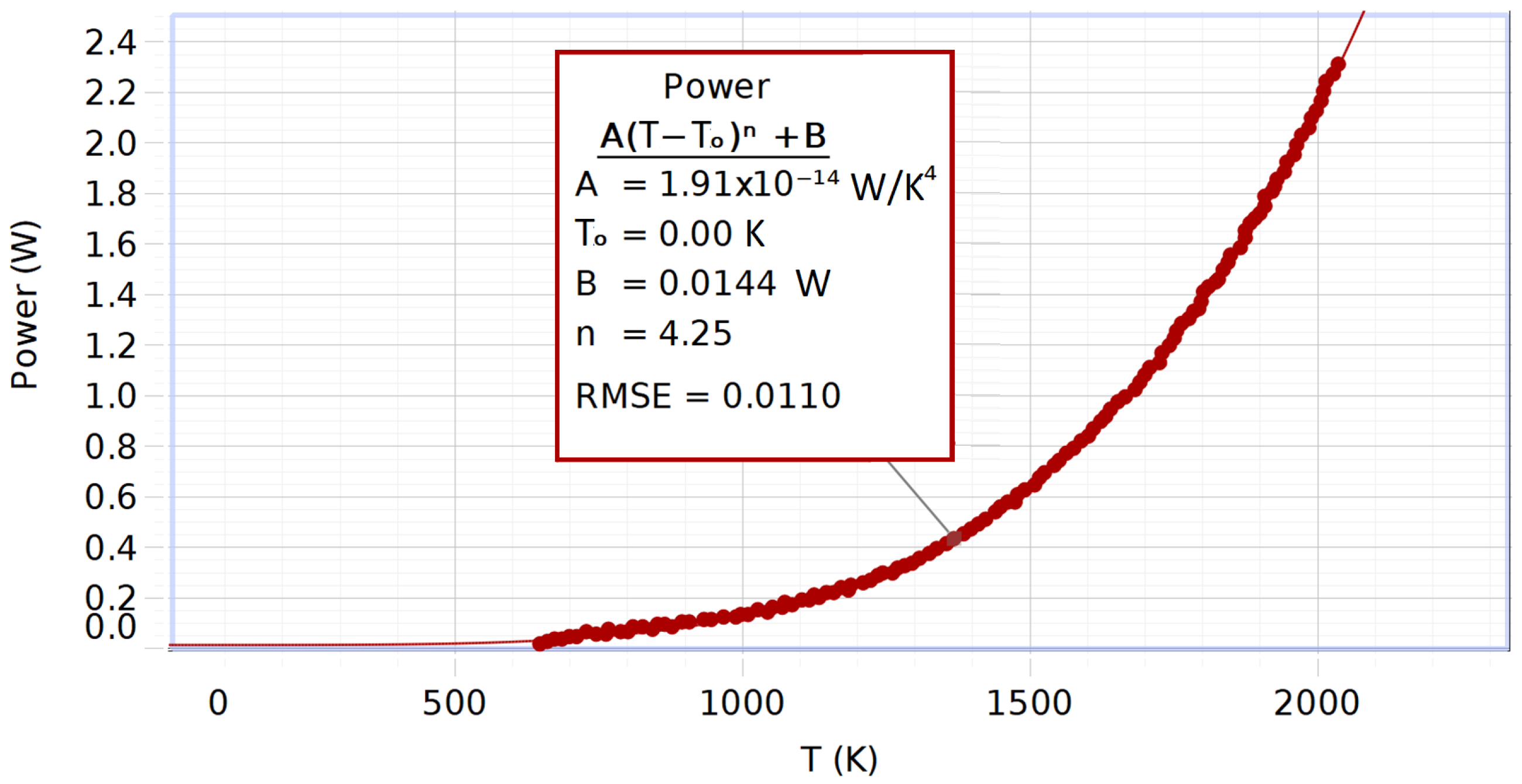
4.2.4. Results of the Measurements and Data Analysis
4.2.5. Students’ Understanding and Learning Goals
4.3. Radiation–Matter Interaction
4.3.1. Student Difficulties
4.3.2. Background Theory
4.3.3. Qualitative Experiments
4.3.4. Quantitative Experiments
- The first experiment uses a visible light source and a light sensor to measure how the intensity of transmitted light depends on the thickness of the material passed through. We used a table lamp as the source, a smartphone as the sensor, and several overlapping sheets of paper as the material. To control the ambient light sensor of the smartphone, we used the Physics Toolbox app [70] (Figure 8, left).
- The second activity reproduces the previous one but with an IR radiation source. In this case, the black face of a Leslie cube constitutes our source, a smartphone equipped with an IR camera, our intensity sensor, and some plastic sheets replace the sheets of paper. IR images are obtained by adding one plastic sheet at a time in front of the face of the cube and capturing images using the IR thermal camera.
- The third experiment aims to measure how the intensity of transmitted light depends on the nature of the material passed through, the color of the incident radiation, and the concentration of an absorber in solution. The source consists of an LED torch, and three colored filters are used to produce monochromatic light. A smartphone is used as a sensor. A plastic cube containing water is gradually infused with food coloring, drop by drop, as shown in Figure 8 (right). Initially, the light intensity is measured using the smartphone’s ambient light sensor without any filters. Subsequently, three colored filters are placed in front of the light source, one at a time. The dye concentration is then incrementally increased by adding one drop at a time, with measurements taken at each step. This entire procedure is repeated ten times.
4.3.5. Students’ Understanding and Learning Goals
- Students mentioning reflection: from 52% to 97%;
- Students mentioning refraction: from 82% to 89%;
- Students mentioning absorption: from 5% to 90%;
- Students mentioning scattering: from 4% to 68%;
- Students mentioning all four phenomena: from 0% to 61%.
4.4. Radiative Equilibrium
4.4.1. Student Difficulties
4.4.2. Background Theory
4.4.3. Experimental Setup
4.4.4. Experimental Results and Data Analysis
4.4.5. Students’ Understanding and Learning Goals
5. Results of the Tests
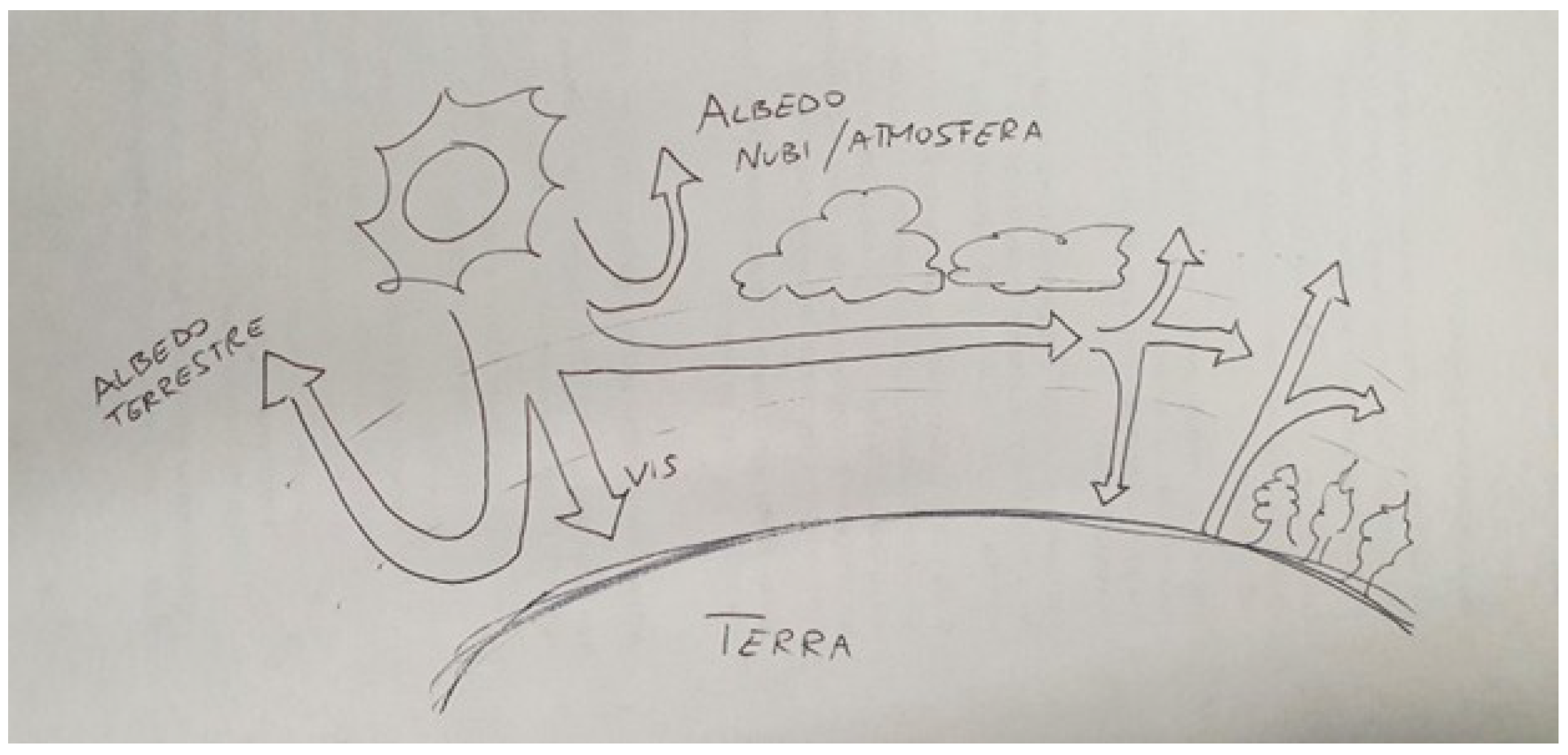
Students’ Drawings and Conceptual Understanding
- Trapping;
- Multiple reflections;
- The presence of a defined layer of greenhouse gases (or occasionally dust) acting as a physical barrier;
- Analogy with an agricultural greenhouse.
6. Discussion
6.1. Disciplinary Aspects
6.1.1. Inquiry-Based Learning, Critical Thinking, and Metacognition
6.1.2. Difficulties for Students in Experimental Activities
6.2. Individual Internalization of Sustainability
6.2.1. Psychology in Climate Change Education
6.2.2. Activities Devoted to Overcome Psychological Obstacles
6.2.3. Some Results from Our Students
6.3. Denialism and Misinformation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Versteijlen, M.; Wals, A.E.J. Developing Design Principles for Sustainability-Oriented Blended Learning in Higher Education. Sustainability 2023, 15, 8150. [Google Scholar] [CrossRef]
- Tilbury, D. Education for Sustainable Development: An Expert Review of Processes and Learning. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000191442. (accessed on 28 February 2025).
- Sterling, S. Science, Society, and Sustainability; Number 27 in Routledge Research in Education; Routledge: New York, NY, USA, 2009; Chapter Sustainable Education. [Google Scholar]
- Ramakrishna, S. Incorporating sustainability into the university curriculum. Dry. Technol. 2021, 39, 985–988. [Google Scholar] [CrossRef]
- Gosselin, D.; Parnell, R.; Smith-Sebasto, N.J.; Vincent, S. Integration of sustainability in higher education: Three case studies of curricular implementation. J. Environ. Stud. Sci. 2013, 3, 316–330. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bryan, A. Climate Change Education in the Context of Education for Sustainable Development: Rationale and Principles. J. Educ. Sustain. Dev. 2015, 9, 4–26. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2023; pp. 3–32. [Google Scholar] [CrossRef]
- UNESCO. Climate Change Education. 2024. Available online: https://www.unesco.org/en/climate-change/education. (accessed on 28 February 2025).
- Schubatzky, T. Climate Change in Physics Education: Why, What and How? In Proceedings of the 4th World Conference on Physics Education 2024, Kraków, Poland, 26–30 August 2024. [Google Scholar]
- Chew Hung, C. Climate Change Education: Knowing, Doing and Being; Routledge: London, UK, 2022. [Google Scholar] [CrossRef]
- Kasperson, R.E.; Kasperson, J. Social Contours of Risk: Volume I Publics, Risk Communication and the Social; Taylor & Francis Group: Abingdon, UK, 2012; p. 376. [Google Scholar]
- Steffen, W.; Grinevald, J.; Crutzen, P.; McNeill, J. The Anthropocene: Conceptual and historical perspectives. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 842–867. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Ghil, M.; Lucarini, V. The physics of climate variability and climate change. Rev. Mod. Phys. 2020, 92, 035002. [Google Scholar] [CrossRef]
- AAPT. Recommendations for the Undergraduate Physics Laboratory Curriculum; American Association of Physics Teachers (AAPT): College Park, MD, USA, 2010. [Google Scholar]
- Steffen, W.; Persson, A.; Deutsch, L.; Zalasiewicz, J.; Williams, M.; Richardson, K.; Crumley, C.; Crutzen, P.; Folke, C.; Gordon, L.; et al. The Anthropocene: From Global Change to Planetary Stewardship. Ambio 2011, 40, 739–761. [Google Scholar] [CrossRef]
- Ding, D.; Maibach, E.W.; Zhao, X.; Roser-Renouf, C.; Leiserowitz, A. Support for climate policy and societal action are linked to perceptions about scientific agreement. Nat. Clim. Chang. 2011, 1, 462–466. [Google Scholar] [CrossRef]
- Besson, U.; De Ambrosis, A.; Mascheretti, P. Studying the physical basis of global warming: Thermal effects of the interaction between radiation and matter and greenhouse effect. Eur. J. Phys. 2010, 31, 375–388. [Google Scholar] [CrossRef]
- Moggio, L. Design and Experimentation of Communication and of a Teaching Sequence on Atmospheric Physics. Ph.D. Thesis, Università degli studi di Trento, Trento, Italy, 2017. [Google Scholar]
- Wallace, J.M. Atmospheric Science: An Introductory Survey, 2nd ed.; Number Volume 92 in International Geophysics; Academic Press: Cambridge, MA, USA; Elsevier: Burlington, MA, USA, 2006. [Google Scholar]
- Gautier, C.; Deutsch, K.; Rebich, S. Misconceptions About the Greenhouse Effect. J. Geosci. Educ. 2006, 54, 386–395. [Google Scholar] [CrossRef]
- Jarrett, L. Investigating Secondary School Students’ Understanding of Climate Change. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2013. [Google Scholar]
- Koulaidis, V.; Christidou, V. Models of students’ thinking concerning the greenhouse effect and teaching implications. Sci. Educ. 1999, 83, 559–576. [Google Scholar] [CrossRef]
- Shepardson, D.P.; Niyogi, D.; Choi, S.; Charusombat, U. Students’ conceptions about the greenhouse effect, global warming, and climate change. Clim. Change 2010, 104, 481–507. [Google Scholar] [CrossRef]
- Ke, L.; Sadler, T.D.; Zangori, L.; Friedrichsen, P.J. Developing and Using Multiple Models to Promote Scientific Literacy in the Context of Socio-Scientific Issues. Sci. Educ. 2021, 30, 589–607. [Google Scholar] [CrossRef]
- Kumar, V.; Choudhary, S.K.; Singh, R. Environmental socio-scientific issues as contexts in developing scientific literacy in science education: A systematic literature review. Soc. Sci. Humanit. Open 2024, 9, 100765. [Google Scholar] [CrossRef]
- Castiglione, A.; Brick, C.; Holden, S.; Miles-Urdan, E.; Aron, A.R. Discovering the psychological building blocks underlying climate action—A longitudinal study of real-world activism. R. Soc. Open Sci. 2021, 9, 210006. [Google Scholar] [CrossRef]
- Niebert, K.; Gropengiesser, H. The model of educational reconstruction: A framework for the design of theory-based content specific interventions. The example of climate change. In Educational Design Research–Part B: Illustrative Cases; SLO—Netherlands Institute for Curriculum Development: Enschede, The Netherlands, 2013; pp. 511–531. [Google Scholar]
- Toffaletti, S.; Di Mauro, M.; Rosi, T.; Malgieri, M.; Onorato, P. Guiding Students towards an Understanding of Climate Change through a Teaching–Learning Sequence. Educ. Sci. 2022, 12, 759. [Google Scholar] [CrossRef]
- Marzari, A.; Di Mauro, M.; Rosi, T.; Onorato, P.; Malgieri, M. Investigating the Principle of Relativity and the Principle of Equivalence in Classical Mechanics: Design and Evaluation of a Teaching–Learning Sequence Based on Experiments and Simulations. Educ. Sci. 2023, 13, 712. [Google Scholar] [CrossRef]
- Ambrosis, A.D.; Malgieri, M.; Mascheretti, P.; Onorato, P. Investigating the role of sliding friction in rolling motion: A teaching sequence based on experiments and simulations. Eur. J. Phys. 2015, 36, 035020. [Google Scholar] [CrossRef]
- Besson, U.; Borghi, L.; De Ambrosis, A.; Mascheretti, P. A Three-Dimensional Approach and Open Source Structure for the Design and Experimentation of Teaching-Learning Sequences: The case of friction. Int. J. Sci. Educ. 2010, 32, 1289–1313. [Google Scholar] [CrossRef][Green Version]
- Pesman, H.; Ari, U.; Cirit, D.K.; Ayazgok, B. Effect of Amount of Guidance in Inquiry-Based Physics Laboratory on Conceptual Understanding and Metacognitive Awareness. Sci. Educ. 2024, 34, 1–21. [Google Scholar] [CrossRef]
- Holstermann, N.; Grube, D.; Bögeholz, S. Hands-on Activities and Their Influence on Students’ Interest. Res. Sci. Educ. 2010, 40, 743–757. [Google Scholar] [CrossRef]
- Kampourakis, K. On the Meaning of Concepts in Science Education. Sci. Educ. 2018, 27, 591–592. [Google Scholar] [CrossRef]
- Girwidz, R.; Theyßen, H.; Widenhorn, R. Experiments in Physics Teaching. In Physics Education; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 269–296. [Google Scholar] [CrossRef]
- Redish, E.F.; Burciaga, J.R. Teaching Physics with the Physics Suite. Am. J. Phys. 2004, 72, 414. [Google Scholar] [CrossRef]
- Kranz, J.; Baur, A.; Möller, A. Learners’ challenges in understanding and performing experiments: A systematic review of the literature. Stud. Sci. Educ. 2022, 59, 321–367. [Google Scholar] [CrossRef]
- Zimmerman, C. The development of scientific thinking skills in elementary and middle school. Dev. Rev. 2007, 27, 172–223. [Google Scholar] [CrossRef]
- Brewe, E. Modeling theory applied: Modeling Instruction in introductory physics. Am. J. Phys. 2008, 76, 1155–1160. [Google Scholar] [CrossRef]
- Hestenes, D. Toward a modeling theory of physics instruction. Am. J. Phys. 1987, 55, 440–454. [Google Scholar] [CrossRef]
- Etkina, E.; Warren, A.; Gentile, M. The Role of Models in Physics Instruction. Phys. Teach. 2005, 44, 34–39. [Google Scholar] [CrossRef]
- Zwickl, B.M.; Finkelstein, N.; Lewandowski, H.J. The process of transforming an advanced lab course: Goals, curriculum, and assessments. Am. J. Phys. 2012, 81, 63–70. [Google Scholar] [CrossRef]
- Wilcox, B.R.; Lewandowski, H.J. Open-ended versus guided laboratory activities:Impact on students’ beliefs about experimental physics. Phys. Rev. Phys. Educ. Res. 2016, 12, 020132. [Google Scholar] [CrossRef]
- Monteiro, M.; Martí, A.C. Resource Letter MDS-1: Mobile devices and sensors for physics teaching. Am. J. Phys. 2022, 90, 328–343. [Google Scholar] [CrossRef]
- PhET. 2025. Available online: https://phet.colorado.edu/ (accessed on 28 February 2025).
- Wieman, C.E.; Adams, W.K.; Loeblein, P.; Perkins, K.K. Teaching Physics Using PhET Simulations. Phys. Teach. 2010, 48, 225–227. [Google Scholar] [CrossRef]
- Houghton, J. Global Warming: The Complete Briefing, 5th ed.; Cambridge University Press: West Nyack, NY, USA, 2015. [Google Scholar]
- Edwards, P.N. History of climate modeling. WIREs Clim. Chang. 2010, 2, 128–139. [Google Scholar] [CrossRef]
- Krauss, L.M. The Physics of Climate Change; Head of Zeus: London, UK, 2024. [Google Scholar]
- Fourier, J. Mémoire sur les températures du globe terrestre et des espaces planétaires. Mémoires L’académie R. Sci. L’institut Fr. 1827, 7, 570–604. [Google Scholar]
- Tyndall, J. On the absorption and radiation of heat by gases and vapours, and on the physical connexion of radiation, absorption, and conduction. Philos. Trans. R. Soc. Lond. 1861, 151, 1–36. [Google Scholar]
- Arrhenius, S., XXXI. On the influence of carbonic acid in the air upon the temperature of the ground. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 41, 237–276. [Google Scholar] [CrossRef]
- Manabe, S.; Wetherald, R.T. Thermal Equilibrium of the Atmosphere with a Given Distribution of Relative Humidity. J. Atmos. Sci. 1967, 24, 241–259. [Google Scholar] [CrossRef]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Toffaletti, S.; Di Mauro, M.; Malgieri, M.; Rosi, T.; Tufino, E.; Onorato, P.; Oss, S. A teaching-learning sequence about climate change: From theory to practice. Am. J. Phys. 2023, 91, 676–684. [Google Scholar] [CrossRef]
- Onorato, P.; Malgieri, M.; Ambrosis, A.D. Measuring the hydrogen Balmer series and Rydberg’s constant with a homemade spectrophotometer. Eur. J. Phys. 2015, 36, 058001. [Google Scholar] [CrossRef][Green Version]
- Rosi, T.; Malgieri, M.; Onorato, P.; Oss, S. What are we looking at when we say magenta? Quantitative measurements of RGB and CMYK colours with a homemade spectrophotometer. Eur. J. Phys. 2016, 37, 065301. [Google Scholar] [CrossRef]
- Haglund, J. Thermal Cameras in Science Education; Number v.26 in Innovations in Science Education and Technology Series; Springer International Publishing: Cham, Switzerland, 2022; ISSN 2213-2236. [Google Scholar] [CrossRef]
- Vollmer, M.; Möllmann, K.P. Infrared Thermal Imaging: Fundamentals, Research and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar] [CrossRef]
- Cheron. Transferts Thermiques: Résumé de Cours, problèMes Corrigés; Ellipses Marketing: Paris, France, 1999. [Google Scholar]
- PASCO. 2025. Available online: https://www.pasco.com/ (accessed on 28 February 2025).
- de Izarra, C.; Gitton, J.M. Calibration and temperature profile of a tungsten filament lamp. Eur. J. Phys. 2010, 31, 933–942. [Google Scholar] [CrossRef]
- Onorato, P.; Rosi, T.; Tufino, E.; Caprara, C.; Malgieri, M. Quantitative experiments in a distance lab: Studying blackbody radiation with a smartphone. Eur. J. Phys. 2021, 42, 045103. [Google Scholar] [CrossRef]
- Pathare, S.; Lahane, R.; Sawant, S.; Patil, C. Power loss from hot tungsten filament. Phys. Educ. 2010, 27, 15. [Google Scholar]
- Prasad, B.S.N.; Mascarenhas, R. A laboratory experiment on the application of Stefan’s law to tungsten filament electric lamps. Am. J. Phys. 1978, 46, 420–423. [Google Scholar] [CrossRef]
- Deiber, A.; Kempf, O. Lampe à incandescence, corps noir, loi de Stefan et filtre passe-bas thermique. Bull. Union Phys. 2000, 827, 1595–1624. (In French) [Google Scholar]
- Durand, F. Étude d’une lampe a incandescence. Bull. Union Phys. 2000, 94, 1625–1633. [Google Scholar]
- Bouquet, F.; Dauphin, C.; Bernard, F.; Bobroff, J. Low-cost experiments with everyday objects for homework assignments. Phys. Educ. 2019, 54, 025001. [Google Scholar] [CrossRef][Green Version]
- Vieyra Software. Physics Toolbox. 2024. Available online: https://www.vieyrasoftware.net/physics-toolbox-sensor-suite (accessed on 28 February 2025).
- Shepardson, D.P.; Niyogi, D.; Choi, S.; Charusombat, U. Seventh grade students’ conceptions of global warming and climate change. Environ. Educ. Res. 2009, 15, 549–570. [Google Scholar] [CrossRef]
- Cook, J. Cranky Uncle Game. Melbourne Centre for Behaviour Change, University of Melbourne. 2025. Available online: https://crankyuncle.com/ (accessed on 28 February 2025).
- Center for Countering Digital Hate. The New Climate Denial 2024. 2024. Available online: https://counterhate.com/research/new-climate-denial/ (accessed on 28 February 2025).
- Climate Interactive. En-ROADS. 2025. Available online: https://www.climateinteractive.org/en-roads (accessed on 28 February 2025).
- Davide Faranda, Lucas Taligrot, N.C.A.T.F.R.R.C.P.C. ClimarisQ. 2025. Available online: https://climarisq.ipsl.fr/en/le-jeu/ (accessed on 28 February 2025).
- Chi, M.T.H.; Roscoe, R.D.; Slotta, J.D.; Roy, M.; Chase, C.C. Misconceived Causal Explanations for Emergent Processes. Cogn. Sci. 2011, 36, 1–61. [Google Scholar] [CrossRef]
- Jin, H.; Wei, X. Using Ideas from the History of Science and Linguistics to Develop a Learning Progression for Energy in Socio-ecological Systems. In Teaching and Learning of Energy in K—12 Education; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 157–173. [Google Scholar] [CrossRef]
- Lombrozo, T.; Carey, S. Functional explanation and the function of explanation. Cognition 2006, 99, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Anderson, C.W. A learning progression for energy in socio-ecological systems. J. Res. Sci. Teach. 2012, 49, 1149–1180. [Google Scholar] [CrossRef]
- Mohan, L.; Chen, J.; Anderson, C.W. Developing a multi-year learning progression for carbon cycling in socio-ecological systems. J. Res. Sci. Teach. 2009, 46, 675–698. [Google Scholar] [CrossRef]
- Rönnebeck, S.; Bernholt, S.; Ropohl, M. Searching for a common ground—A literature review of empirical research on scientific inquiry activities. Stud. Sci. Educ. 2016, 52, 161–197. [Google Scholar] [CrossRef]
- Fischer, H.; Fleming, S. Why metacognition matters in politically contested domains. Trends Cogn. Sci. 2024, 28, 783–785. [Google Scholar] [CrossRef]
- Osborne, J. Teaching Scientific Practices: Meeting the Challenge of Change. J. Sci. Teach. Educ. 2014, 25, 177–196. [Google Scholar] [CrossRef]
- PISA 2018 Assessment and Analytical Framework. OECD: Paris, France, 2019. [CrossRef]
- Roberts, R.; Gott, R.; Glaesser, J. Students’ approaches to open-ended science investigation: The importance of substantive and procedural understanding. Res. Pap. Educ. 2010, 25, 377–407. [Google Scholar] [CrossRef]
- Pedaste, M.; Mäeots, M.; Siiman, L.A.; de Jong, T.; van Riesen, S.A.; Kamp, E.T.; Manoli, C.C.; Zacharia, Z.C.; Tsourlidaki, E. Phases of inquiry-based learning: Definitions and the inquiry cycle. Educ. Res. Rev. 2015, 14, 47–61. [Google Scholar] [CrossRef]
- Lozano, R. Envisioning sustainability three-dimensionally. J. Clean. Prod. 2008, 16, 1838–1846. [Google Scholar] [CrossRef]
- Kassel, K.; Rimanoczy, I.; Mitchell, S.F. The Sustainable Mindset: Connecting Being, Thinking, and Doing in Management Education. Acad. Manag. Proc. 2016, 2016, 16659. [Google Scholar] [CrossRef]
- Kusumojanto, D.D.; Wibowo, A.; Kustiandi, J.; Narmaditya, B.S. Do entrepreneurship education and environment promote students’ entrepreneurial intention? The role of entrepreneurial attitude. Cogent Educ. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Anåker, A.; Spante, M.; Elf, M. Nursing students’ perception of climate change and sustainability actions—A mismatched discourse: A qualitative, descriptive exploratory study. Nurse Educ. Today 2021, 105, 105028. [Google Scholar] [CrossRef]
- Lorenzoni, I.; Nicholson-Cole, S.; Whitmarsh, L. Barriers perceived to engaging with climate change among the UK public and their policy implications. Glob. Environ. Chang. 2007, 17, 445–459. [Google Scholar] [CrossRef]
- Whitmarsh, L.; O’Neill, S.; Lorenzoni, I. Public engagement with climate change: What do we know and where do we go from here? Int. J. Media Cult. Politics 2013, 9, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Kumpu, V. What is Public Engagement and How Does it Help to Address Climate Change? A Review of Climate Communication Research. Environ. Commun. 2022, 16, 304–316. [Google Scholar] [CrossRef]
- Guigon, V.; Villeval, M.C.; Dreher, J.C. Metacognition biases information seeking in assessing ambiguous news. Commun. Psychol. 2024, 2, 122. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.C.; Kalof, L.; Dietz, T.; Guagnano, G.A. Values, Beliefs, and Proenvironmental Action: Attitude Formation Toward Emergent Attitude Objects1. J. Appl. Soc. Psychol. 1995, 25, 1611–1636. [Google Scholar] [CrossRef]
- Bamberg, S.; Rees, J.; Seebauer, S. Collective climate action: Determinants of participation intention in community-based pro-environmental initiatives. J. Environ. Psychol. 2015, 43, 155–165. [Google Scholar] [CrossRef]
- Kormos, C.; Gifford, R. The validity of self-report measures of proenvironmental behavior: A meta-analytic review. J. Environ. Psychol. 2014, 40, 359–371. [Google Scholar] [CrossRef]
- Ockwell, D.; Whitmarsh, L.; O’Neill, S. Reorienting Climate Change Communication for Effective Mitigation: Forcing People to be Green or Fostering Grass-Roots Engagement? Sci. Commun. 2009, 30, 305–327. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmoiraghi, A.; Zamboni, A.; Toffaletti, S.; Di Mauro, M.; Malgieri, M.; Fiorello, C.; Onorato, P.; Oss, S. Core of Sustainability Education: Bridging Theory and Practice in Teaching Climate Science to Future Mathematics and Physics Teachers. Sustainability 2025, 17, 5120. https://doi.org/10.3390/su17115120
Salmoiraghi A, Zamboni A, Toffaletti S, Di Mauro M, Malgieri M, Fiorello C, Onorato P, Oss S. Core of Sustainability Education: Bridging Theory and Practice in Teaching Climate Science to Future Mathematics and Physics Teachers. Sustainability. 2025; 17(11):5120. https://doi.org/10.3390/su17115120
Chicago/Turabian StyleSalmoiraghi, Alessandro, Andrea Zamboni, Stefano Toffaletti, Marco Di Mauro, Massimiliano Malgieri, Camilla Fiorello, Pasquale Onorato, and Stefano Oss. 2025. "Core of Sustainability Education: Bridging Theory and Practice in Teaching Climate Science to Future Mathematics and Physics Teachers" Sustainability 17, no. 11: 5120. https://doi.org/10.3390/su17115120
APA StyleSalmoiraghi, A., Zamboni, A., Toffaletti, S., Di Mauro, M., Malgieri, M., Fiorello, C., Onorato, P., & Oss, S. (2025). Core of Sustainability Education: Bridging Theory and Practice in Teaching Climate Science to Future Mathematics and Physics Teachers. Sustainability, 17(11), 5120. https://doi.org/10.3390/su17115120








